Abstract
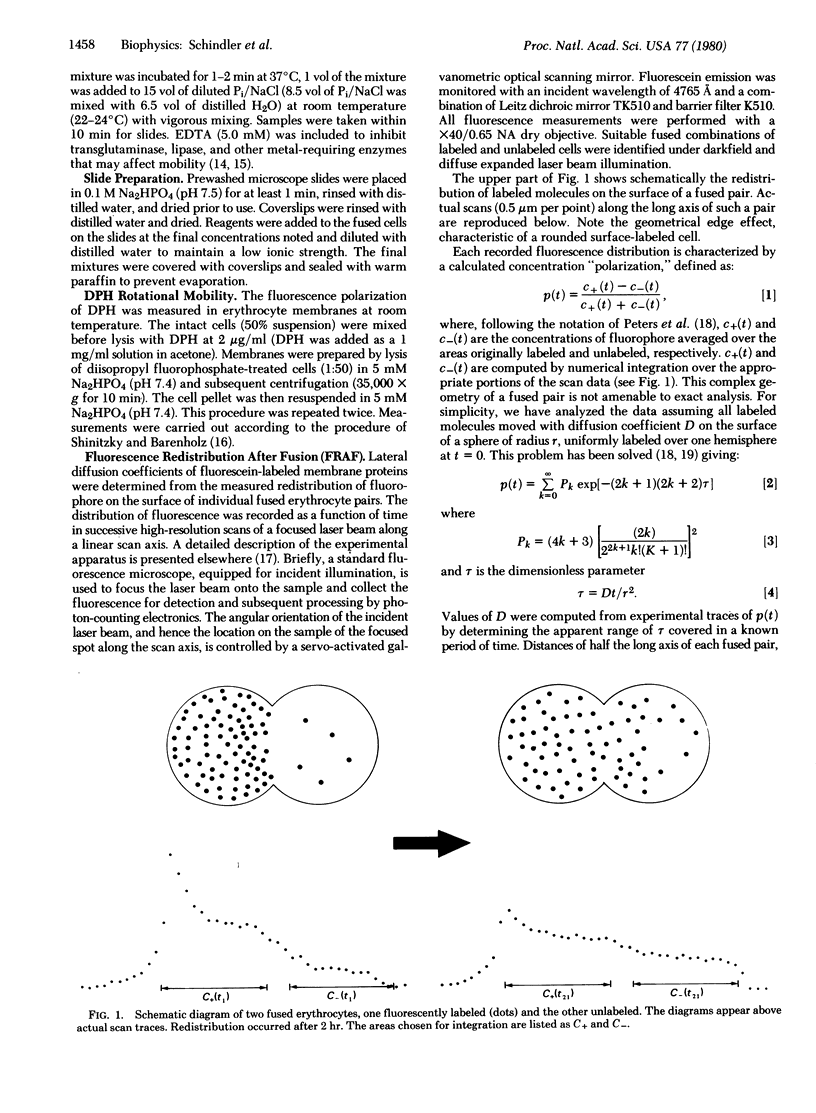
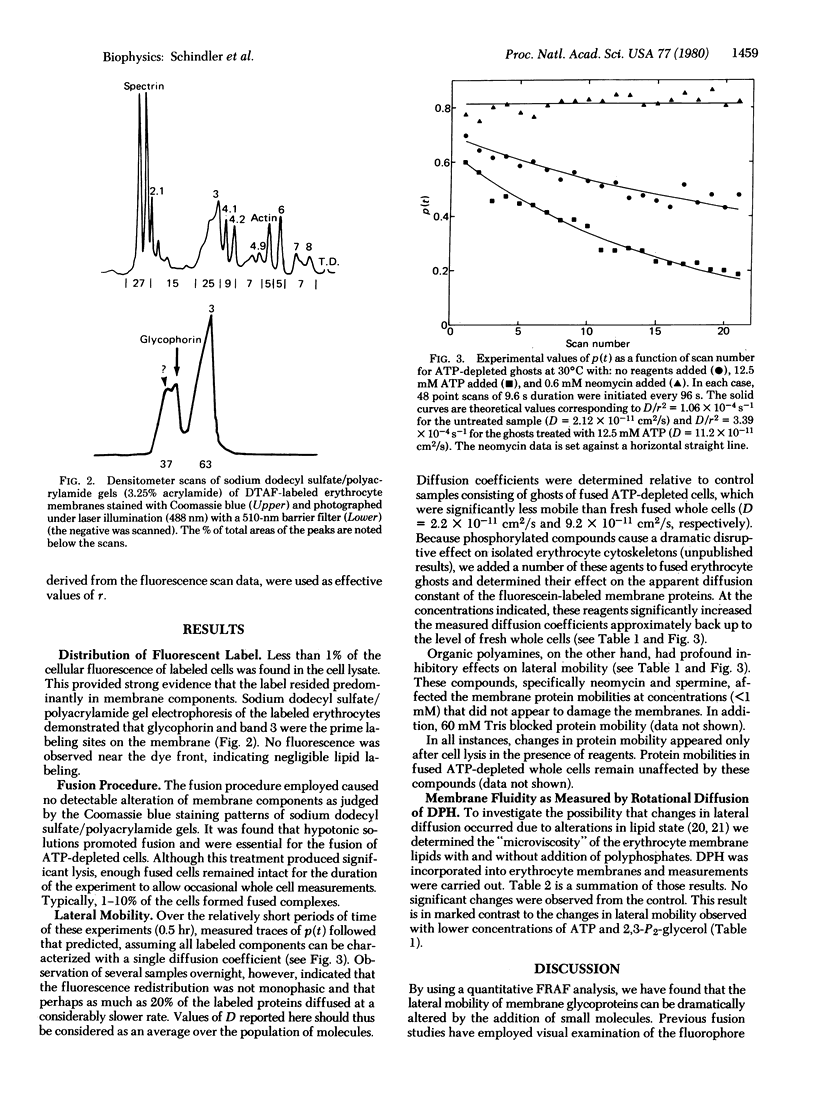
The lateral mobility of fluorescein-labeled membrane glycoproteins was measured in whole unlysed erythrocytes and erythrocyte ghosts by the technique of “fluorescence redistribution after fusion.” Measurements were made on polyethylene glycol-fused cell pairs in which only one member of the couplet was initially fluorescently labeled. Diffusion coefficients were estimated from the rate of fluorescence redistribution determined from successive scans with a focused laser beam across individual fused pairs. This technique allows for the analysis of diffusion within cell membranes without the possible damaging photochemical events caused by photobleaching. It was found that lateral mobility of erythrocyte proteins can be increased by the addition of polyphosphates (i.e., ATP and 2,3-diphosphoglycerate) and decreased by the addition of organic polyamines (i.e., neomycin and spermine). This control is exerted by these molecules only when they contact the cytoplasmic side of the membrane and is not dependent upon high-energy phosphates. Microviscosity experiments employing diphenylhexatriene demonstrated no changes in membrane lipid state as a function of these reagents. Our results, in conjunction with data on the physical interactions of cytoskeletal proteins, suggest that the diffusion effector molecules alter the lateral mobility of erythrocyte membrane proteins through modifications of interactions in the shell, which is composed of spectrin, actin, and component 4.1.
Keywords: fluorescence redistribution after fusion (FRAF); glycoprotein mobility; cytoskeleton; spermine; 2,3-diphosphoglycerate
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Wight A., Webb W., Horwitz A. Influence of membrane lipids on acetylcholine receptor and lipid probe diffusion in cultured myotube membrane. Biochemistry. 1978 Aug 22;17(17):3604–3609. doi: 10.1021/bi00610a029. [DOI] [PubMed] [Google Scholar]
- Buckley J. T., Hawthorne J. N. Erythrocyte membrane polyphosphoinositide metabolism and the regulation of calcium binding. J Biol Chem. 1972 Nov 25;247(22):7218–7223. [PubMed] [Google Scholar]
- Bunn H. F., Ransil B. J., Chao A. The interaction between erythrocyte organic phosphates, magnesium ion, and hemoglobin. J Biol Chem. 1971 Sep 10;246(17):5273–5279. [PubMed] [Google Scholar]
- Cherry R. J., Bürkli A., Busslinger M., Schneider G., Parish G. R. Rotational diffusion of band 3 proteins in the human erythrocyte membrane. Nature. 1976 Sep 30;263(5576):389–393. doi: 10.1038/263389a0. [DOI] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edidin M. Rotational and translational diffusion in membranes. Annu Rev Biophys Bioeng. 1974;3(0):179–201. doi: 10.1146/annurev.bb.03.060174.001143. [DOI] [PubMed] [Google Scholar]
- Elgsaeter A., Shotton D. M., Branton D. Intramembrane particle aggregation in erythrocyte ghosts. II. The influence of spectrin aggregation. Biochim Biophys Acta. 1976 Feb 19;426(1):101–122. doi: 10.1016/0005-2736(76)90433-8. [DOI] [PubMed] [Google Scholar]
- Fairbanks G., Avruch J., Dino J. E., Patel V. P. Phosphorylation and dephosphorylation of spectrin. J Supramol Struct. 1978;9(1):97–112. doi: 10.1002/jss.400090110. [DOI] [PubMed] [Google Scholar]
- Fowler V., Branton D. Lateral mobility of human erythrocyte integral membrane proteins. Nature. 1977 Jul 7;268(5615):23–26. doi: 10.1038/268023a0. [DOI] [PubMed] [Google Scholar]
- Frye L. D., Edidin M. The rapid intermixing of cell surface antigens after formation of mouse-human heterokaryons. J Cell Sci. 1970 Sep;7(2):319–335. doi: 10.1242/jcs.7.2.319. [DOI] [PubMed] [Google Scholar]
- Hanski E., Rimon G., Levitzki A. Adenylate cyclase activation by the beta-adrenergic receptors as a diffusion-controlled process. Biochemistry. 1979 Mar 6;18(5):846–853. doi: 10.1021/bi00572a017. [DOI] [PubMed] [Google Scholar]
- Huang H. W. Mobility and diffusion in the plane of cell membrane. J Theor Biol. 1973 Jul;40(1):11–17. doi: 10.1016/0022-5193(73)90161-6. [DOI] [PubMed] [Google Scholar]
- Johnson M., Edidin M. Lateral diffusion in plasma membrane of mouse egg is restricted after fertilisation. Nature. 1978 Mar 30;272(5652):448–450. doi: 10.1038/272448a0. [DOI] [PubMed] [Google Scholar]
- Keen J. H., Willingham M. C., Pastan I. H. Clathrin-coated vesicles: isolation, dissociation and factor-dependent reassociation of clathrin baskets. Cell. 1979 Feb;16(2):303–312. doi: 10.1016/0092-8674(79)90007-2. [DOI] [PubMed] [Google Scholar]
- Koppel D. E. Fluorescence redistribution after photobleaching. A new multipoint analysis of membrane translational dynamics. Biophys J. 1979 Nov;28(2):281–291. doi: 10.1016/S0006-3495(79)85176-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorand L., Weissmann L. B., Epel D. L., Bruner-Lorand J. Role of the intrinsic transglutaminase in the Ca2+-mediated crosslinking of erythrocyte proteins. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4479–4481. doi: 10.1073/pnas.73.12.4479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loyter A., Ben-Zaquen R., Marash R., Milner Y. Dephosphorylation of human erythrocyte membranes induced by sendai virus. Biochemistry. 1977 Aug 23;16(17):3903–3909. doi: 10.1021/bi00636a028. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L., Painter R. G. Anionic sites of human erythrocyte membranes. II. Antispectrin-induced transmembrane aggregation of the binding sites for positively charged colloidal particles. J Cell Biol. 1973 Nov;59(2 Pt 1):395–406. doi: 10.1083/jcb.59.2.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E. A., Cherry R. J. Influence of temperature and cholesterol on the rotational diffusion of band 3 in the human erythrocyte membrane. Biochemistry. 1979 Aug 7;18(16):3457–3465. doi: 10.1021/bi00583a004. [DOI] [PubMed] [Google Scholar]
- Nigg E., Cherry R. J. Dimeric association of band 3 in the erythrocyte membrane demonstrated by protein diffusion measurements. Nature. 1979 Feb 8;277(5696):493–494. doi: 10.1038/277493a0. [DOI] [PubMed] [Google Scholar]
- Peters R., Peters J., Tews K. H., Bähr W. A microfluorimetric study of translational diffusion in erythrocyte membranes. Biochim Biophys Acta. 1974 Nov 15;367(3):282–294. doi: 10.1016/0005-2736(74)90085-6. [DOI] [PubMed] [Google Scholar]
- Schacht J. Purification of polyphosphoinositides by chromatography on immobilized neomycin. J Lipid Res. 1978 Nov;19(8):1063–1067. [PubMed] [Google Scholar]
- Schindler M., Osborn M. J., Koppel D. E. Lateral mobility in reconstituted membranes--comparisons with diffusion in polymers. Nature. 1980 Jan 24;283(5745):346–350. doi: 10.1038/283346a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schramm M., Orly J., Eimerl S., Korner M. Coupling of hormone receptors to adenylate cyclase of different cells by cell fusion. Nature. 1977 Jul 28;268(5618):310–313. doi: 10.1038/268310a0. [DOI] [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P. DNase-I-dependent dissociation of erythrocyte cytoskeletons. J Cell Biol. 1979 Apr;81(1):266–270. doi: 10.1083/jcb.81.1.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheetz M. P., Koppel D. E. Membrane damage caused by irradiation of fluorescent concanavalin A. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3314–3317. doi: 10.1073/pnas.76.7.3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinitzky M., Barenholz Y. Dynamics of the hydrocarbon layer in liposomes of lecithin and sphingomyelin containing dicetylphosphate. J Biol Chem. 1974 Apr 25;249(8):2652–2657. [PubMed] [Google Scholar]
- Shotton D., Thompson K., Wofsy L., Branton D. Appearance and distribution of surface proteins of the human erythrocyte membrane. An electron microscope and immunochemical labeling study. J Cell Biol. 1978 Feb;76(2):512–531. doi: 10.1083/jcb.76.2.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singer S. J., Ash J. F., Bourguignon L. Y., Heggeness M. H., Louvard D. Transmembrane interactions and the mechanisms of transport of proteins across membranes. J Supramol Struct. 1978;9(3):373–389. doi: 10.1002/jss.400090308. [DOI] [PubMed] [Google Scholar]
- Triplett R. B., Carraway K. L. Proteolytic digestion of erythrocytes, resealed ghosts, and isolated membranes. Biochemistry. 1972 Jul 18;11(15):2897–2903. doi: 10.1021/bi00765a024. [DOI] [PubMed] [Google Scholar]


