Abstract
The Actinomyces oris type-1 pili are important for the initial formation of dental plaque by binding to salivary proteins that adhere to the tooth surface. Here we present the X-ray structure of FimP, the protein that is polymerized into the type-1 pilus stalk, assisted by a pili-specific sortase. FimP consists of three tandem IgG-like domains. The middle and C-terminal domains contain one autocatalyzed intramolecular isopeptide bond each, a feature used by Gram-positive bacteria for stabilization of surface proteins. While the N-terminal domain harbours all the residues necessary for forming an isopeptide bond, no such bond is observed in the crystal structure of this unpolymerized form of FimP. The monomer is further stabilized by one disulfide bond each in the N- and C-terminal domains as well as by a metal-coordinated loop protruding from the C-terminal domain. A lysine, predicted to be crucial for FimP polymerization by covalent attachment to a threonine from another subunit, is located at the rim of a groove lined with conserved residues. The groove may function as a docking site for the sortase-FimP complex. We also present sequence analyses performed on the genes encoding FimP as well as the related FimA, obtained from clinical isolates.
Introduction
Pili are hair-like organelles on the surface of bacteria. They are essential for functions such as biofilm formation, host-pathogen interactions and attachment to surfaces. Gram-negative pili are well studied both regarding structure and function [1], [2] whereas less is known about the structure, function and bioassembly of Gram-positive pili, even though they were first described decades ago [3]. However, during the last few years Gram-positive pilin structures from C. diphtheriae [4], Actinomyces oris [5], Streptococcus pyogenes [6], [7], [8], Streptococcus pneumoniae [9], [10], [11], [12], Streptococcus agalactiae [13], [14] and Bacillus cereus [15] have been described. In short the Gram-positive pili are built up from multiple copies of covalently linked major pilin proteins, resulting in a shaft. In addition, some pili, but not all, have minor pilin proteins incorporated into the stalk. In general, an adhesin is positioned at the tip. The recent advances in structure and function of Gram-positive pili are excellently reviewed by Kang and Baker [16].
Gram-positive proteins that function as building blocks for pili polymerization share some common characteristics. There is a signal peptide located in the N-terminus and an LPXTG motif in the C-terminus, followed by a transmembrane segment. The LPXTG motif is a sorting signal recognized by a sortase (a cysteine transpeptidase) that cleaves the protein between the threonine and the glycine in the motif. In the next step the threonine is covalently attached either to the cell-wall peptidoglycan if the sortase is a housekeeping sortase or to a lysine of a central pilin motif (WXXXVXVYPK) [17] of an identical pilin protein if a polymerization reaction is being catalyzed. The covalent polymerization of pilin proteins is performed by pili-specific sortases. The mechanism underlying the incorporation of auxiliary proteins into the fimbria is still not fully understood [18], [19], [20].
Dental plaque is a microbial biofilm built up from several hundreds of different bacterial species [21]. Actinomyces spp together with streptococci are among the first colonizers of the oral biofilm and promote further biofilm formation by their interaction with a wide variety of proteins and carbohydrates on microorganisms and host cells, or from saliva. A. oris (previously Actinomyces naeslundii genospecies 2 [22]) can express two different types of pili: type-1 and type-2. Type-1 pili mediate the first attachment to host salivary proline-rich proteins (PRPs) that coat the tooth, whereas type-2 pili mediate attachment to carbohydrate structures on oral streptococci [23], [24] and host cells [25]. The two types of pili are encoded by two separate gene clusters. Each gene cluster contains three genes that encode a large putative adhesin, the pilus shaft protein and the pili-specific sortase. The encoded pilin proteins are as follows: FimQ, FimP and SrtC-1 for type-1 and FimA, FimB and SrtC-2 for type-2 [26], [27]. The pilus shaft proteins FimP and FimA are 28% identical in sequence and are very similar in size. The sortases SrtC-1 and SrtC-2 share 42% sequence identity within the enzymatic domain. In contrast, the putative adhesins differ in both size and sequence (1413 residues for FimQ and 976 residues for FimB). This may reflect their differences in binding specificity.
Intriguingly, it was recently shown for type-2 pili that the pili stalk alone (FimA) is involved in the co-aggregation reaction with carbohydrates [28] which leaves the function of FimB unclear. However, in a similar study on the type-1 pili it was shown that the presumed adhesin, FimQ, did indeed interact with PRPs and that the shaft protein FimP appeared not to be involved in this interaction [29]. To unravel some of the basics of the molecular function of these pili it is necessary to study the molecular organization of the participating proteins. Recently the crystal structure of the carboxy-terminal fragment of A. oris FimA was presented [5] as well as the crystal structure of the FimP-specific sortase SrtC-1 [30]. To gain more insight into the structure and function of the A. oris type-1 pili, we have solved the structure of the FimP shaft protein, refined to 1.6 Å resolution and analyzed the conserved and polymorphic FimP and FimA amino acid variations among clinical isolates.
Results and Discussion
Structure Determination
A construct comprising residues 31–491 of FimP (FimP31–491) from A. oris strain T14V was expressed in E. coli, purified and crystallized. The N-terminal signal peptide, the C-terminal transmembrane helix and the cell-wall anchoring motif LPLTG were not included in the construct (Fig. 1). Phases were experimentally determined using single wavelength anomalous dispersion (SAD) of a selenomethionine (SeMet) labeled triple mutant, FimP-3M, in which three isoleucines (Ile-121, Ile-204 and Ile-347) were exchanged for methionines [31]. SAD data were collected to 2.0 Å resolution and an initial model was built. The model was further refined against a native data set to 1.6 Å. The asymmetric unit contains one molecule of FimP31–491. The final model is well ordered with an overall B-factor of 18.7 Å2 (Table 1). The refined model comprises residues 35–490. No or weak electron density was observed for the loop residues 57–63 and 70–72. In addition, four metal ions and 833 water molecules were included.
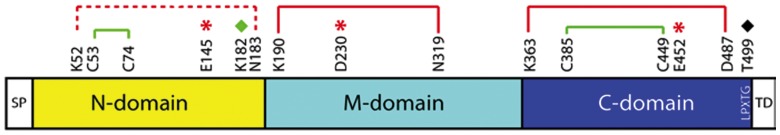
Figure 1. Domain architecture of FimP.
The FimP protein is comprised of a signal peptide (SP), an N-terminal domain, a middle domain and a C-terminal domain followed by an LPXTG motif and a transmembrane domain (TD). Residues involved in isopeptide and disulfide bonds are illustrated with bars and stars, in red and green, respectively. A lysine and a threonine involved in pili polymerization are illustrated with a green and black diamond respectively.
Table 1. Data collection, refinement and model quality statistics for FimP.
| Native FimP | SeMet FimP-3M | |
| Data collection | ||
| Space group | P21212 | P21212 |
| Cell dimensions a, b, c(Å) | 77.24, 176.59, 40.12 | 76.27, 168.13, 39.76 |
| Wavelength (Å) | 0.9334 | 0.97918 |
| Resolution (Å) | 46.82–1.6 | 45.16–2.0 |
| Highest resolution shell(Å) | 1.69–1.6 | 2.11–2.0 |
| Total reflectionsa | 382619 (27906) | 220706 (6864) |
| Unique reflectionsa | 71266 (9922) | 35477 (1257) |
| I/σ (I)a | 21.4 (5.8) | 29.9 (15.7) |
| Rsym(%)a, b | 5.9 (16.1) | 4.0 (9.2) |
| Completeness (%)a | 97.5 (95.1) | 99.8 (99.4) |
| Overall redundancy | 5.4 (2.8) | 6.2 (6.3) |
| Refinement | ||
| No. reflections in working set | 67601 | |
| No. reflections in test set | 3593 | |
| Rwork/Rfree (%)c | 16.93/19.57 | |
| Average B-factors (Å2) | ||
| Wilson plot | 20.4 | |
| Protein | 18.7 | |
| Water | 30.3 | |
| Metal ions | 22.1 | |
| No. protein atoms | 3419 | |
| No. metal ions | 4 | |
| No. water molecules | 833 | |
| RMSD from ideal | ||
| Bond lengths (Å) | 0.12 | |
| Bond angles (°) | 1.432 | |
| Ramachandran plot | ||
| Preferred, allowed,outliers (%) | 95.9/3.2/0.9 |
Values in parentheses indicate statistics for the highest resolution shell.
R sym = Σhkl Σi |I i (hkl)−<I(hkl)>|/Σhkl Σi I i (hkl), where Ii(hkl) is the intensity of the ith observation of reflection hkl and <I(hkl)> is the average over of all observations of reflection hkl.
R work = Σ | |F obs|−| F calc| |/Σ | F obs|, where F obs and F calc are the observed and calculated structure factor amplitudes, respectively. R free is R work calculated using 5% of the data, randomly omitted from refinement.
Overall Structure of FimP
FimP is an elongated protein, approximately 105 Å long and 35 Å wide, folded into three IgG-like domains: the N-terminal (N), middle (M) and C-terminal (C) domains (Fig. 2). The IgG-folds are of the CnaA- (M-domain) or the CnaB- (the N- and C-terminal domains) types. These IgG-like folds are extensively found in cell surface adhesins [32]. The M-domain (187–355) and the C-domain (356–490) are rigidly connected in line via a shared strand whereas the N-domain (35–186) and M-domain are connected via a hinge. The mobility of the hinge is reflected by the slight alternation in N-domain position, observed when comparing the native structure and the SeMet-labeled FimP-3M structures. The difference in N-domain rotation is also reflected by the difference in unit cell dimensions, where the b-axis is approximately 5% shorter in the SeMet structure than in the native structure. The shift in the N-domain positions may be caused by one of the introduced (seleno)methionines, I347M. Residue 347 is located at the interface between the domains and a change from isoleucine to selenomethionine can indeed alter the interaction properties. In the FimP-3M structure 165 contacts are observed between the N- and M-domains, compared to 213 in the native structure as calculated with the program CONTACTS in the CCP4 suite [33]. The selenomethionine itself at position 347 has 20 contacts whereas the native isoleucine has 39. The other mutants, I121M and I347M, do not cause any changes.
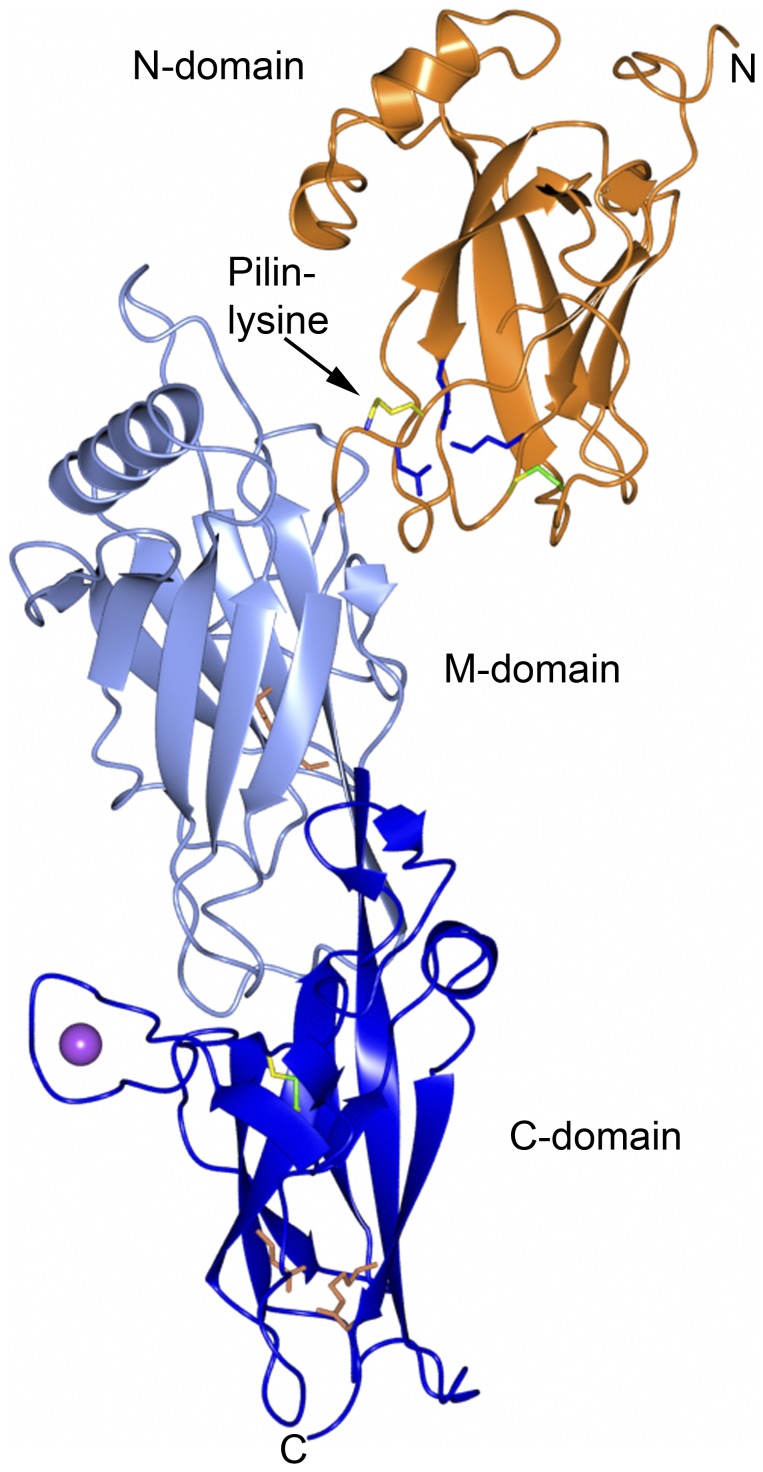
Figure 2. Overall structure of FimP.
Ribbon representation of FimP31–491. The N-, M- and C-domains are in coral, light blue, and blue respectively. The residues forming isopeptide bonds in the M- and C-domains are shown as coral stick models and the disulfides in the N- and C-domains as green sticks. The lysine in the pilin motif is shown as a stick model in yellow and blue. The Ca2+ ion bound to the C domain is depicted as a sphere in magenta. Residues in the N-domain putatively involved in isopeptide bond formation are shown in blue. N- and C-termini are indicated.
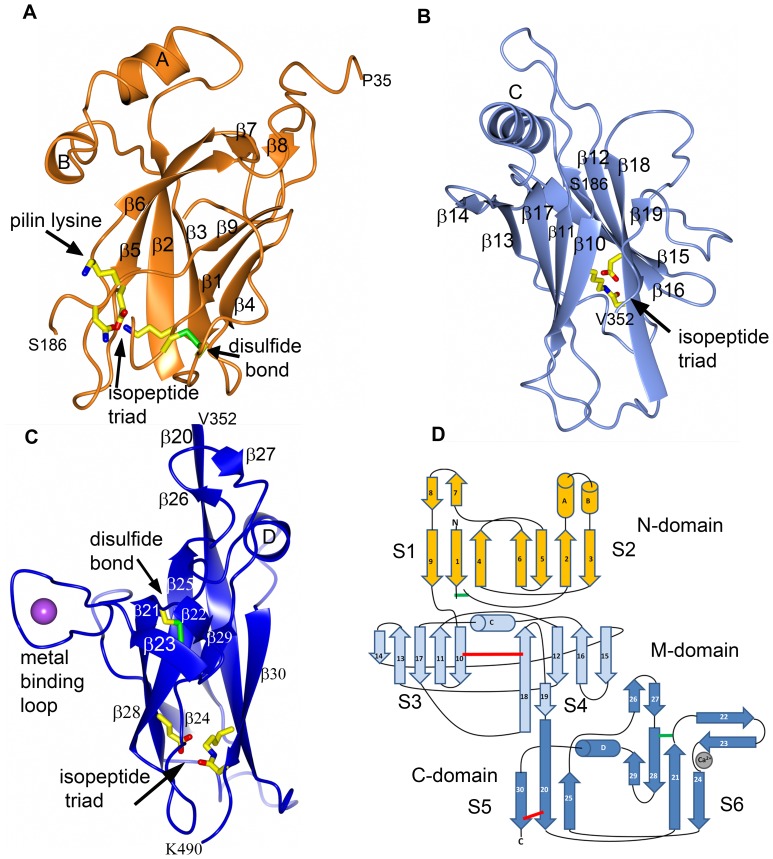
The N-domain comprises a β-sandwich of one three-stranded, mixed β-sheet (S1) and one four-stranded (S2) anti-parallel sheet. Two helices (HA and HB) pack against the S2 sheet. In addition, two short anti-parallel β-strands, β7-β8, connected by a long loop, are located perpendicular to the β-sandwich (Fig. 3a and 3d).
Figure 3. Domains and topology diagram.
Each domain (N-, M- and C-) with labeled secondary structure. A: N-domain in coral, B: M-domain in light blue, C: C-domain in blue. The Ca2+ ion is depicted as a magenta sphere. D: Topology diagram of FimP with domains colored as in A–C. The isopeptides are depicted as red, and disulfides as green, bars. The Ca2+ ion coordinated by the loop is shown as a grey sphere.
The M-domain comprises a β-sandwich of two five-stranded anti-parallel sheets, S3 and S4. A helix (HC) is wedged in between the upper part of the sandwich. Below the S4 sheet two anti-parallel strands, β26 and β27, are located. A 20-residue long loop that connects β18 with β19 packs against the S4 sheet. The continuation of β19, β20 connects the M- and C-domains (Fig. 3b and 3d).
The C-domain, like the N-domain, consists of a β-sandwich of one mixed, three-stranded and one four-stranded β-sheet, S5 and S6, respectively. A short helix (HD), is located at the top of the S5 sheet. A long segment connects strands β21 and β24 and contains a β-hairpin (β22/β23) packed against the S6 sheet, as well as a long loop that coordinates a Ca2+ ion (Fig. 3c and 3d).
All Three FimP Domains are Stabilized with Covalent Bonds
The presence of intramolecular isopeptide bonds in Gram-positive surface proteins was first described for the S. pyogenes pilin Spy0128 [6] and these bonds are now considered a widespread feature among Gram-positive surface proteins. Intramolecular isopeptide bonds are used for increasing the stability of the surface exposed protein, both regarding the sensitivity to proteases and mechanical force. Generally, a covalent amide bond is formed between the NZ atom of a lysine and the CG atom of an asparagine or an aspartic acid, assisted by the presence of a close acidic residue in an hydrophobic environment.
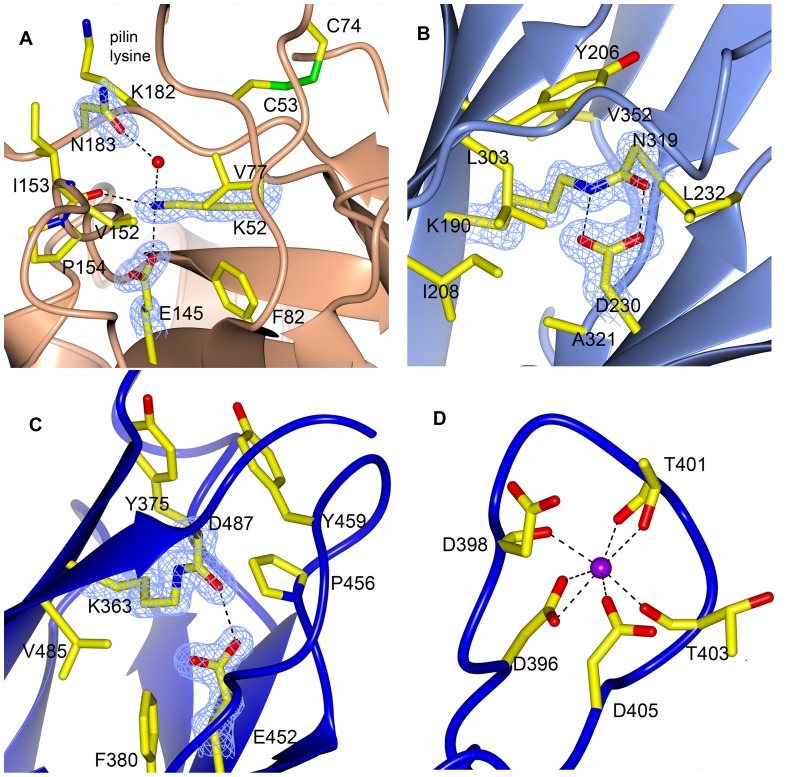
Accordingly, isopeptide bonds are also found in FimP31–491. The M-domain is stabilized by a bond between Lys-190 and Asn-319, linking two strands that run anti-parallel to each other (Fig. 4b). The strands originate from the S3 and S4 sheets respectively. The formation of the isopeptide bond is catalyzed by Asp-230 located in the S4 sheet. Asp-230 forms bidentate hydrogen bonds with the amide hydrogen and the carbonyl oxygen of the isopeptide bond. The linkage stacks with the aromatic Tyr-206 and is surrounded by additional hydrophobic residues (Ile-208, Leu-303, Ala-321, Leu-232 and Val-352).
Figure 4. Stabilizing isopeptide bonds (formed and unformed) and a metal binding loop.
A: The putative isopeptide residues in the N-domain, Lys-52, Asn-183 and Glu-145, do not form an isopeptide bond in the crystal structure. B: The M-domain isopeptide bond formed between Lys-190 and Asn-319 with the catalytic Asp-230. Asp-230 forms a bidentate hydrogen bond with the isopeptide bond. C: The C-domain isopeptide bond formed between Lys-363 and Asp-487 with the catalytic Glu-452. Glu-452 forms one hydrogen bond with the isopeptide bond carbonyl oxygen. D: A Ca2+ ion is coordinated by five residues of a loop that protrudes from the C-domain. Residues involved in isopeptide bond formation are represented as stick models, colored by atom type in a simulated annealing, omit Fo-Fc maps contoured at 4σ. Hydrogen bonds are shown as broken lines. Surrounding hydrophobic residues are shown as stick models.
The C-domain also contains one isopeptide bond, formed between Lys-363 and Asp-487 (Fig. 4c). In this domain the bond links the first and last β-strands of the domain, β20 and β30, that run parallel to each other in the S5 sheet. The formation of the bond is assisted by the presence of Glu-452, previously described as the E-box motif. The glutamic acid forms only one hydrogen bond to the carbonyl oxygen of the isopeptide crosslink. Similar to the M-domain, the bond is surrounded by aromatic and hydrophobic residues, such as Tyr-375, Phe-380, Pro-456, Tyr-459 and Val-485.
There is no isopeptide bond observed in the N-domain but when superimposing the C-domain with the N-domain it is obvious that the N-domain also has all the necessary amino acids, Lys-52, Asn-183 and Glu-145, to form an isopeptide linkage. However, in our crystal structure, Asn-183 is located on the hinge between the N- and M-domains, and its CG atom is too far away from the lysine NZ atom for a bond to form, although the two side chains are connected via a water molecule (Fig. 4a). Instead the residue following the lysine, Cys-53, forms a disulfide bridge with Cys-74, thereby contributing to the stability of the S1 sheet by other means. If formed, the isopeptide bond in the N-domain would link the two parallel strands of the three-stranded sheet, similar to the bond in the C-domain. Interestingly, the Asn-183 residue expected to form the isopeptide bond in the N-domain, is positioned next to Lys-182, the lysine that is crucial for formation of the intermolecular bond with Thr-499 of another FimP subunit upon pili polymerization.
The presence of the two isopeptide bonds was evident from the continuous electron density between the lysines and the asparagine (in the M-domain) or the aspartic acid (in the C-domain). Their existence was also confirmed by ESI-TOF-MS. Site directed mutagenesis was applied to generate two mutants, D230A and E452A, each replacing the catalytic residue of the isopeptide triads with alanine. Mass analyses of the mutant and wild type proteins confirmed the approximate mass losses of NH3 (M-domain), H2O (C-domain) or both (wild type) upon isopeptide bond formation compared to the masses calculated from the primary FimP sequence (Table 2). Thus the mutants, where the respective catalytic residue has been removed, form one isopeptide bond, whereas in the native protein two isopeptide bonds are formed. Accordingly, the ESI-TOF-MS experiments also verify that a third isopeptide bond is not present in the unpolymerized recombinant form.
Table 2. Mass determination of wild type and mutant FimP31–491 proteins.
| Protein | Average mass (Da) | Difference (Da) | |
| Calculated from sequence | Observed by MSa | calculated-observed | |
| FimP31–491 | 53067 | 53033 | −34 |
| FimP31–491 D230A | 53023 | 53006 | −17 |
| FimP31–491 E452A | 53009 | 52993 | −16 |
MS, ESI-TOF mass spectrometry.
Not all Gram-positive bacteria use disulfide bonds to stabilize secreted proteins although it is a common tool used by Actinobacteria [34]. In agreement with that, we observe that the FimP shaft protein is stabilized by two disulfide bonds, one in the N-domain and one in the C-domain, the domains that share the CnaB fold. In the N-domain, C53 and C74 form a disulfide bond between the start and the end of loop β1-β2, of which the C53 is directly positioned after the lysine putatively involved in isopeptide bond formation, as discussed above (Fig. 3a,d 4a). The loop segment connected by the disulfide is the most flexible in the FimP31–491 structure and interpretable electron density is missing for residues 57–63 and 70–72.
In the C-domain a disulfide bond between C385 and C449 joins the S6 sheet of the β-sandwich and the β22-β23 β-hairpin, the structural segment that is followed by the protruding metal-coordinating loop. (Fig. 3c, d).
Metal-binding Sites
Four metal ions are found in the FimP31–491 structure and they are modeled as calcium due to the high concentration of calcium in the crystallization conditions. Three of them are likely to be present due to the crystallization solution; one is located between symmetry-related molecules and the other two are coordinated by only three protein atoms each. The fourth metal seems to have a structural role and is coordinated by a long loop in the C-domain, protruding from the S6 sheet. This metal is coordinated by seven oxygen atoms: Asp-396 (OD1 and OD2), Asp-398 (O), Thr-401 (OG1 and O), Thr-403 (O) and Asp-405 (OD2) (Figure 4d). The distances between the metal and the seven coordinating oxygen atoms in the loop refine to an average of 2.43 Å which is more consistent with Ca2+ (2.33–2.39Å) than to for instance Mg2+ (2.05–2.26Å) [35]. Moreover the metal coordinated by the loop refines to a B-factor of 23.5 Å2 with is similar to the surrounding oxygen atoms that are refined to an average of 26.4 Å2.
Comparison with FimA and Other Pilin Structures
A structure similarity search of the FimP31–491 structure in the Protein Data Bank using the DALI server [36] found several Gram-positive surface proteins as structural relatives. SpaA from C. diphteriae (PDB 3HR6, Z-score of 24.8 [4]), which also contains three IgG-like domains, was identified as the closest structural relative. Separate searches performed with the N-domain alone and with the M- and C-domains together gave the same hit. The N-domain search resulted in a Z-score of 14.9 and an root mean square deviation (RMSD) of 1.9 Å on 115 aligned Cα residues compared to SpaA. When the M- and C-domains were used, the Z-score was 21.6 and the RMSD 3.3 Å on 266 aligned Cα atoms. SpaA and and FimP31–491 are very similar in structure and share the same topology of the β-sheets. The SpaA and the FimP31–491 structures also share the positions of the isopeptide bonds in the M- and C-domains. The differences between the two structures are mainly the positions of the bound metal ions. Their respective structural metal ion is located in similar, but not identical, areas of the molecules close to the interface between the M- and C-domains, but coordinated by loops from different domains. In FimP, the metal-coordinating loop belongs to the C-domain and protrudes from the core of the molecule, whereas the metal-binding loop in SpaA originates from the M-domain and is more involved in domain-domain interactions. Both FimP and SpaA have a disulfide bond in their respective C-domain, located in similar positions, however the presence of a disulfide bond in the N-domain is unique for FimP. Neither FimP nor SpaA has an isopeptide bond in the N-domain, although FimP has all the residues needed to form such a bond and SpaA does not. Other DALI hits, with somewhat lower Z-scores, were the C-terminal fragment of FimA from A. oris (PDB code 3QDH, Z-score, 19.9 [5]) that could be superposed onto FimP31–491 with an RMSD of 2.8 Å on 235 aligned Cα atoms. Similarly, the pilus protein GBS80 from S. agalactiae can be superimposed on the FimP with an RMSD of 3.2 Å on 246 aligned Cα atoms (PDB code 3PF2, Z-score 15.9 [13]). These are all Gram-positive pilin proteins consisting of three IgG-like domain (only M- and C domains are present in the models of FimA and GBS80).
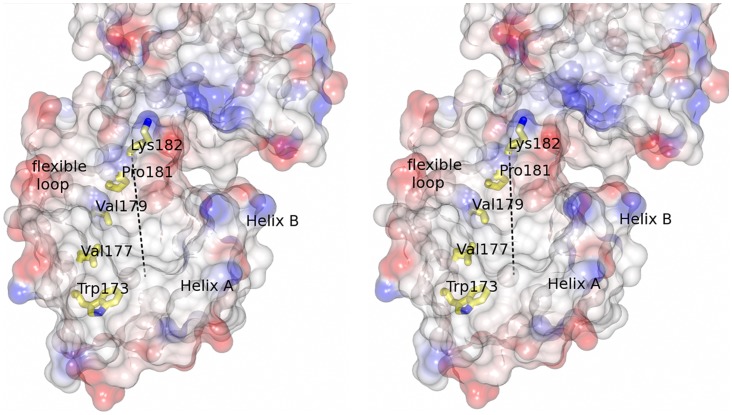
The Pilin Motif is Located in a Groove
In FimP, the lysine of the pilin motif (WNYNVHVYPK) is exposed on the hinge that connects the N- and M-domains, and is thereby available for the sortase mediated pili polymerization reaction (Fig. 3a, 4a and 5). The pilin lysine, Lys-182, is located at the end of a mostly non-polar groove formed between helix A, the β-sandwich, and the mobile loop of the N-domain. The groove is lined by the pilin-motif conserved residues Trp-73, Val-177, Val-179, and Pro-181 with the pilin lysine Lys-182 located at the rim of the channel. The conserved Tyr-180 stacks with His-51 and forms a hydrogen bond with the main chain amide of Asp-69, located close to the mobile loop. The isopeptide triad (Lys-52, Asn-183, Glu-145) is positioned within the groove however the isopeptide bond is not observed in the crystal structure as discussed above.
Figure 5. The pilin motif forms a groove.
Stereo representation of the pilin motif residues lining a cleft that runs through the N-domain. Lys-182, involved in polymerization of FimP subunits, is localized at the rim of the cleft. The domain is presented as a semi-transparent electrostatic surface, colored in red and blue according to negative and positive electrostatic potential, respectively. The residues in the pilin motif are shown as stick models. The groove is highlighted with a dashed line.
The recent structures of the S. pneumoniae pilin RrgB give an explanation of the function of the N-domain cleft and what dictates its isopeptide bond formation. When RrgB was crystallized in a form devoid of the sorting motif, an isopeptide bond in the N-terminal domain was not formed [10]. However, when a longer RrgB form was crystallized, its sorting motif was shown to interact with the pilin lysine of a neighboring pilin leading to a conformational change in the N-terminal domain and the subsequent formation of the covalent isopeptide bond [11].
In concurrence with the RrgB structures we propose a similar role for the FimP N-terminal groove; that the cleft and mobile loop (residues 56–64) function as recognition and interaction sites for the sortase-FimP complex during pili polymerization and that a preformed isopeptide bond (juxtaposed to the pilin Lys-182) results in a protein too rigid to properly present the pilin lysine to the sortase. However, during pili polymerization more strain may be imposed on the flexible hinge between the N- and M-domains and Asn-183 and Lys-52 may come close enough to form a bond. Indeed, in the BcpA pilin the N-domain isopeptide bond is observed only after BcpA polymerization [15].
In the quest for new drug candidates against pili-bearing Gram-positive bacteria, the N-domain groove may constitute a target for the development of pili inhibiting peptides or chemicals, with the sorting signal as the original template.
Structural Comparison with FimA
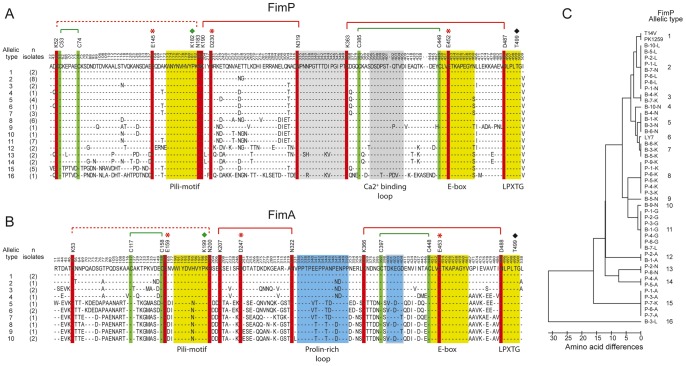
Actinomyces spp can express two different forms of pili, encoded by separate gene clusters. The respective shaft proteins, FimP and FimA, are similar in terms of size and sequence. FimP consists of 533 residues and FimA of 535. A sequence analysis reveals that the major features; the pilin motif, the number of cysteines, the residues involved in isopeptide bond formation and the LPXTG motif are strictly conserved. Thus FimP and FimA are expected to have very similar structures. On the other hand, some differences are expected to be found, e.g., in the organization of loops and metal binding (Fig. 6).
Figure 6. Sequence analyses of FimP and FimA among A. oris isolates.
A: Sequence alignment of FimP (n = 48) with fully conserved isopeptide bond triads (red), disulfide bonds (green), a conserved metal binding loop (grey) and pilin-, E-box- and LPLTG motifs in yellow. B: Sequence alignment of FimA (n = 14) with fully conserved isopeptide bond triads (red), disulfide bonds (green), a conserved proline-rich loop (blue) and pilin-, E-box- and LPLTG motifs in yellow. In addition, in A and B, polymorphic amino acid residues are shown (single letter codes). The top lines represent the consensus sequence and amino acid positions based on FimP and FimA respectively of reference strain T14V. C: Neighboring joining tree with sixteen allelic or sequence fimP types among A. oris isolates (n = 48) due to the single amino acid variations.
Limited proteolysis experiments digesting FimA and FimP with trypsin gave different results which indicate some differences in exposure of certain regions (data not shown). Trypsin treatment of FimP generated a cut at Lys-62, located in the mobile loop of the N-domain whereas FimA (from A. naeslundii strain 12104) was cut at the pilin motif lysine as well as in the C-domain (Lys-372). Mishra et al [5] reported that FimA from A. oris strain T14V is similarly cut at the pilin lysine. This difference in sensitivity to trypsin implies that FimP is more rigid than FimA in which the pilin motif is more exposed.
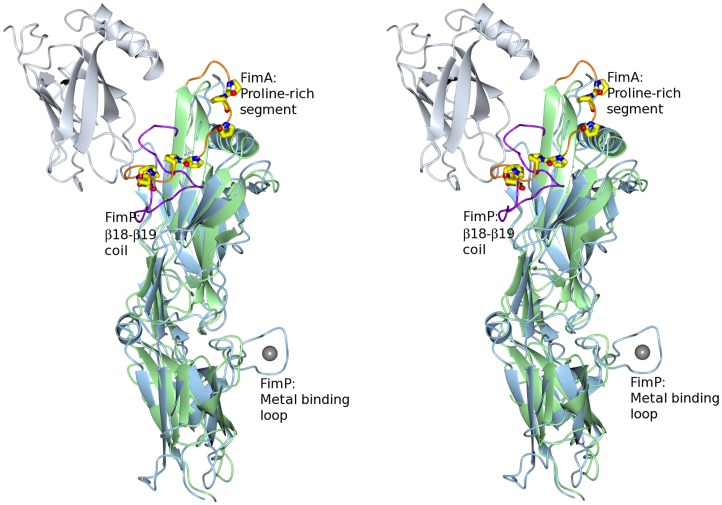
A structural comparison between FimP31–491 and FimA (M- and C-domains) revealed that the major differences between the two structures are the loops connecting the β-strands. The most prominent difference is the segments that pack against the S4 sheet of their respective M-domains. In FimA this constitutes a proline-rich segment (332PPTPETPPTDPENPP347) that runs diagonally over half the sheet. In FimP the equivalent segment is a long, coiled region, located between β18 and β19, that folds over the whole S4 sheet like a clamp (Fig. 7).
Figure 7. Superposition of FimP and FimA.
The FimP crystal structure superimposed onto the corresponding structure of FimA (M- and C domains). The FimP structure is depicted in light blue and FimA in green. The proline-rich segment in FimA is coloured in orange with the prolines as stick models. The equivalent loop in FimP is colored in purple. The metal ion coordinated by a FimP loop is depicted in grey. The N-terminal domain of FimP is colored in light grey. The figure is shown in stereo.
Another distinct structural difference is the FimP metal-binding loop that protrudes from the C-domain like a knob. This loop has no equivalent in FimA. Such a significant structural element is likely to be available for recognition by certain microbes of the biofilm. In analogy, the surface adhesin SspB of the oral bacteria Streptococcus gordonii exhibits a surface-exposed helix, stabilized by a metal ion [37]. This helix is solely recognized by the periodontal pathogen Porphyromonas gingivalis [38]. Likewise, the proline-rich segment in FimA may function as a recognition site for other bacteria-bacteria interactions. However, to further unravel the complex mechanisms that control colonization and formation of the oral biofilm, extensive structural and functional studies have to be performed.
Sequence comparison of the FimP and FimA N-domains indicates different positions of the disulfide bridges. In FimP the first cysteine of the N-domain is located directly after the presumed isopeptide forming lysine and participate in a disulfide bond that unites the ends of a flexible loop. Also in FimA, the presumed disulfide bond is located near the putative isopeptide bond but with a different organization where its second cysteine is located directly before the catalytic glutamic acid. When the FimA sequence is applied on the FimP template, the first cysteine is located in the coil after the two helices A and B and the second in the S2 sheet, next to the isopeptide bond glutamic acid, as discussed. Assuming that a disulfide bridge is formed also in FimA, we suggest that its S2 sheet is more occluded due to tighter packing with the N-domain helices. The overall appearance of the N-domains may therefore be quite different, which indeed is reflected by their different sensitivity to trypsin. The structural differences are consistent with the differential functional roles of the two pili. The evolution and adaptation of type-1 (FimP) and type-2 (FimA) pili to different intraoral niches and tropisms, suggest their pilin proteins to be organized and function more differently than assumed from their co-presence in the Actinomyces genus. The metal binding loop, proline-rich motifs or differential N-domain structures could accordingly participate in various intergeneric bacteria-bacteria adhesion (co-aggregation) or host-bacteria adhesion partnerships. Notably, Actinomyces has multiple co-aggregation partners and hosts, therefore their pilin proteins are expected to possess multiple binding activities.
Conserved or Polymorphic FimP and FimA Features among Clinical A. oris Isolates
Sequencing of the fimP gene from six A. oris reference strains (T14V, PK1259, P-1-N, P-8-L, LY7 and P-1-K) expressing FimP pili of defined binding profiles [39], [40] and clinical isolates (n = 42) revealed a highly conserved (97% identity/98% similarity) sequence (Fig. 6a). All three isopeptide bond triads, the cysteine bridges, pilin and LPLTG motifs were fully, and the metal binding loop highly, conserved among the strains (n = 48). The variable or polymorphic amino acid sites (19%), which localized generally over the domains, loops and β-strands without any apparent clustering or patterning, generated a total of sixteen allelic or sequence types (Fig. 6c).
FimP was also compared to FimA, deduced from fimA from A. oris isolates (n = 14). The FimP and FimA proteins showed 31% identity/45% similarity and fully conserved isopeptide bond triads, number of cysteines, pilin and LPLTG motifs. The metal binding loop was proved to be unique for FimP and the proline-rich segment unique for FimA. In addition, FimA from A. oris (n = 14) and A. naeslundii (n = 17) were highly related (64% identity/76% similarity) with fully conserved isopeptide bond triads, position of cysteines, pilin and LPLTG motifs as well as proline residues in the proline-rich segment. The sequence alignment of FimA from A. oris is presented in Fig. 6b.
Materials and Methods
Cloning, Purification and Crystallization
FimP31–491was cloned from A. oris strain T14V, expressed and crystallized as described [31]. In short, N-terminal 6His-tagged FimP was purified by nickel-affinity chromatography followed by size-exclusion chromatography. The protein was concentrated to 92 mg/ml in 20 mM Tris-HCl, pH 8.0. Selenomethionine (SeMet)-substituted protein was obtained after mutating three isoleucines to methionines (FimP-3M) [31]. The protein was subjected to in situ proteolysis with 1% (w/w) α-chymotrypsin immediately before crystallization set up. Crystals of the native protein were obtained in 100 mM sodium acetate pH 5.0, 100 mM CaCl2 and 20% PEG4000. Crystals of the SeMet containing protein were obtained in the same conditions after seeding with the native crystals.
Generation of Isopeptide Bond Mutants
Generation of the mutants D230A and E452A was performed using the overlap extension PCR technique [41]. In short, for each mutant a first round of PCR generated two overlapping PCR fragments. In the second PCR step the two fragments were hybridized and amplified. The final PCR products were ligated into an expression vector as described [31]. The mutant proteins were purified as the native protein.
Data Collection and Structure Determination
Crystals were flash-cooled in liquid nitrogen after a 30 s soak in the crystallization solution supplemented with 20% glycerol. X-ray diffraction data of the native crystals were collected at beamline ID14-1 and of the SeMet crystals at beamline ID-23 at the European Synchrotron Radiation Facility, ESRF, in Grenoble, France to 1.6 and 2.0 Å resolution respectively. Data were processed with XDS [42] and scaled with SCALA from the CCP4 program suit [33]. The SeMet containing structure was solved with SAD-phasing using AutoRickshaw [43]. Density modification and automatic model building were performed using AutoRickshaw and ArpWarp [44] and resulted in a readily interpretable map. For refinement, 5% of the reflections were removed for the calculation of Rfree. The model was further built using rounds of manual building in COOT [45] and refinement using phenix.refine [46]. The first refinement step included simulated annealing starting at 5000 K.
The native structure was solved with MOLREP [47] using the FimP SeMet structure as the search model. Due to movements of the N-domain, only residues 190–490 were placed in the molecular replacement solution and residues 35–189 were built manually. The native structure was refined as the SeMet structure. The quality of the model was analyzed with WHATCHECK [48]. Ramachandran statistics were obtained using COOT [45]. Crystallographic statistics are presented in Table 1.
The X-ray coordinates and structure factors have been deposited in the Protein Data Bank under accession codes 3UXF.
Mass Spectrometry Analyses
Buffer solutions of FimP, FimP-D230A, and FimP-E452A were exchanged for water by dialysis. Accurate molecular masses were determined by ESI-TOF mass spectrometry at Proteomics Karolinska (PK) Institute, Stockholm, Sweden.
Limited Proteolysis and N-terminal Sequencing of FimA and FimP Fragments
Purified recombinant FimP (A. oris strain T14V) and FimA (A. naeslundii strain 12104) in 20 mM CaCl2, 50 mM Tris-HCl pH 8.0, 1% glycerol and 2 mM DTT were incubated with trypsin at a ratio 1∶100 trypsin: FimP/FimA at 293 K for 1 h. The cleavage products were separated on by SDS-PAGE and transferred to a PVDF membrane. N-terminal sequencing of the cleavage products was performed.
Sequencing of FimP and FimA from A. oris and A. naeslundii Strains
The fimP genes were sequenced from A. oris clinical isolates (n = 42) and reference strains (n = 6; T14V, PK1259, P-1-N, P-8-L, LY7 and P-1-K). The fimP genes were amplified by PCR using purified genomic DNA and the Ready-To-Go PCR polymerase kit (GE healthcare).
The fimA genes were sequenced from A. oris (n = 14) and A. naeslundii (n = 17) isolates. The fimA genes were amplified by PCR from purified DNA using the Iproof High-Fidelity enzyme (BioRad). Purified fragments were cloned into Zero Blunt® TOPO® PCR Cloning Kit (Invitrogen) and sequenced. All sequencing was performed by Eurofins MWG Operon. The fimP (corresponding to amino acids 31–516) and fimA (corresponding to the full length protein) sequences were assembled and analyzed using the CodonCode Aligner and MEGA5 software. Identity/similarity values were generated using the NCBI web site.
Acknowledgments
We are grateful for access to the beamlines ID23-1 and ID14-1, ESRF, Grenoble and for the support of the beamline staff.
Funding Statement
This work was supported by the Swedish Research Council, The County Council of Västerbotten and foundations from Umeå University, Sweden. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Kline KA, Dodson KW, Caparon MG, Hultgren SJ (2010) A tale of two pili: assembly and function of pili in bacteria. Trends Microbiol 18: 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Geibel S, Waksman G (2011) Crystallography and electron microscopy of chaperone/usher pilus systems. Adv Exp Med Biol 715: 159–174. [DOI] [PubMed] [Google Scholar]
- 3. Yanagawa R, Otsuki K, Tokui T (1968) Electron microscopy of fine structure of Corynebacterium renale with special reference to pili. Jpn J Vet Res 16: 31–37. [PubMed] [Google Scholar]
- 4. Kang HJ, Paterson NG, Gaspar AH, Ton-That H, Baker EN (2009) The Corynebacterium diphtheriae shaft pilin SpaA is built of tandem Ig-like modules with stabilizing isopeptide and disulfide bonds. Proc Natl Acad Sci U S A 106: 16967–16971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Mishra A, Devarajan B, Reardon ME, Dwivedi P, Krishnan V, et al. (2011) Two autonomous structural modules in the fimbrial shaft adhesin FimA mediate Actinomyces interactions with streptococci and host cells during oral biofilm development. Mol Microbiol 81: 1205–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kang HJ, Coulibaly F, Clow F, Proft T, Baker EN (2007) Stabilizing isopeptide bonds revealed in gram-positive bacterial pilus structure. Science 318: 1625–1628. [DOI] [PubMed] [Google Scholar]
- 7. Pointon JA, Smith WD, Saalbach G, Crow A, Kehoe MA, et al. (2010) A highly unusual thioester bond in a pilus adhesin is required for efficient host cell interaction. J Biol Chem 285: 33858–33866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Linke C, Young PG, Kang HJ, Bunker RD, Middleditch MJ, et al. (2010) Crystal structure of the minor pilin FctB reveals determinants of Group A streptococcal pilus anchoring. J Biol Chem 285: 20381–20389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Izore T, Contreras-Martel C, El Mortaji L, Manzano C, Terrasse R, et al. (2010) Structural basis of host cell recognition by the pilus adhesin from Streptococcus pneumoniae . Structure 18: 106–115. [DOI] [PubMed] [Google Scholar]
- 10. Paterson NG, Baker EN (2011) Structure of the full-length major pilin from Streptococcus pneumoniae: implications for isopeptide bond formation in gram-positive bacterial pili. PLoS One 6: e22095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. El Mortaji L, Contreras-Martel C, Moschioni M, Ferlenghi I, Manzano C, et al. (2012) The full-length Streptococcus pneumoniae major pilin RrgB crystallizes in a fibre-like structure, which presents the D1 isopeptide bond and provides details on the mechanism of pilus polymerization. Biochem J 441: 833–841. [DOI] [PubMed] [Google Scholar]
- 12. Spraggon G, Koesema E, Scarselli M, Malito E, Biagini M, et al. (2010) Supramolecular organization of the repetitive backbone unit of the Streptococcus pneumoniae pilus. PLoS One 5: e10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vengadesan K, Ma X, Dwivedi P, Ton-That H, Narayana SV (2011) A model for group B Streptococcus pilus type 1: the structure of a 35-kDa C-terminal fragment of the major pilin GBS80. J Mol Biol 407: 731–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krishnan V, Gaspar AH, Ye N, Mandlik A, Ton-That H, et al. (2007) An IgG-like domain in the minor pilin GBS52 of Streptococcus agalactiae mediates lung epithelial cell adhesion. Structure 15: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Budzik JM, Poor CB, Faull KF, Whitelegge JP, He C, et al. (2009) Intramolecular amide bonds stabilize pili on the surface of bacilli. Proc Natl Acad Sci U S A 106: 19992–19997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kang HJ, Baker EN (2012) Structure and assembly of Gram-positive bacterial pili: unique covalent polymers. Curr Opin Struct Biol 22: 200–207. [DOI] [PubMed] [Google Scholar]
- 17. Ton-That H, Schneewind O (2004) Assembly of pili in Gram-positive bacteria. Trends Microbiol 12: 228–234. [DOI] [PubMed] [Google Scholar]
- 18. Mandlik A, Das A, Ton-That H (2008) The molecular switch that activates the cell wall anchoring step of pilus assembly in gram-positive bacteria. Proc Natl Acad Sci U S A 105: 14147–14152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ton-That H, Marraffini LA, Schneewind O (2004) Sortases and pilin elements involved in pilus assembly of Corynebacterium diphtheriae. Mol Microbiol 53: 251–261. [DOI] [PubMed] [Google Scholar]
- 20. Hendrickx AP, Budzik JM, Oh SY, Schneewind O (2011) Architects at the bacterial surface - sortases and the assembly of pili with isopeptide bonds. Nat Rev Microbiol 9: 166–176. [DOI] [PubMed] [Google Scholar]
- 21. Paster BJ, Boches SK, Galvin JL, Ericson RE, Lau CN, et al. (2001) Bacterial diversity in human subgingival plaque. J Bacteriol 183: 3770–3783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Henssge U, Do T, Radford DR, Gilbert SC, Clark D, et al. (2009) Emended description of Actinomyces naeslundii and descriptions of Actinomyces oris sp. nov. and Actinomyces johnsonii sp. nov., previously identified as Actinomyces naeslundii genospecies 1, 2 and WVA 963. Int J Syst Evol Microbiol 59: 509–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cisar JO, Sandberg AL, Abeygunawardana C, Reddy GP, Bush CA (1995) Lectin recognition of host-like saccharide motifs in streptococcal cell wall polysaccharides. Glycobiology 5: 655–662. [DOI] [PubMed] [Google Scholar]
- 24. Cisar JO, Takahashi Y, Ruhl S, Donkersloot JA, Sandberg AL (1997) Specific inhibitors of bacterial adhesion: observations from the study of gram-positive bacteria that initiate biofilm formation on the tooth surface. Adv Dent Res 11: 168–175. [DOI] [PubMed] [Google Scholar]
- 25. Strömberg N, Karlsson KA (1990) Characterization of the binding of Actinomyces naeslundii (ATCC 12104) and Actinomyces viscosus (ATCC 19246) to glycosphingolipids, using a solid-phase overlay approach. J Biol Chem 265: 11251–11258. [PubMed] [Google Scholar]
- 26. Chen P, Cisar JO, Hess S, Ho JT, Leung KP (2007) Amended description of the genes for synthesis of Actinomyces naeslundii T14V type 1 fimbriae and associated adhesin. Infection & Immunity 75: 4181–4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yeung MK, Donkersloot JA, Cisar JO, Ragsdale PA (1998) Identification of a gene involved in assembly of Actinomyces naeslundii T14V type 2 fimbriae. Infection & Immunity 66: 1482–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mishra A, Wu C, Yang J, Cisar JO, Das A, et al. (2010) The Actinomyces oris type 2 fimbrial shaft FimA mediates co-aggregation with oral streptococci, adherence to red blood cells and biofilm development. Mol Microbiol. [DOI] [PMC free article] [PubMed]
- 29.Wu C, Mishra A, Yang J, Cisar JO, Das A, et al.. (2011) Dual function of a tip fimbrillin of Actinomyces in fimbrial assembly and receptor binding. J Bacteriol. [DOI] [PMC free article] [PubMed]
- 30. Persson K (2011) Structure of the sortase AcSrtC-1 from Actinomyces oris. Acta Crystallogr D Biol Crystallogr 67: 212–217. [DOI] [PubMed] [Google Scholar]
- 31. Persson K (2011) Crystallization of the fimbrial protein FimP from Actinomyces oris and of a triple Ile-to-Met mutant engineered to facilitate selenomethionine labelling. Acta Crystallogr Sect F Struct Biol Cryst Commun 67: 1207–1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Deivanayagam CC, Rich RL, Carson M, Owens RT, Danthuluri S, et al. (2000) Novel fold and assembly of the repetitive B region of the Staphylococcus aureus collagen-binding surface protein. Structure 8: 67–78. [DOI] [PubMed] [Google Scholar]
- 33. Collaborative Computational Project N (1994) The CCP4 suite: programs for protein crystallography. Acta Crystallogr D Biol Crystallogr 50: 760–763. [DOI] [PubMed] [Google Scholar]
- 34. Daniels R, Mellroth P, Bernsel A, Neiers F, Normark S, et al. (2010) Disulfide bond formation and cysteine exclusion in gram-positive bacteria. J Biol Chem 285: 3300–3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Harding MM (2006) Small revisions to predicted distances around metal sites in proteins. Acta Crystallogr D Biol Crystallogr 62: 678–682. [DOI] [PubMed] [Google Scholar]
- 36.Holm L, Kaariainen S, Wilton C, Plewczynski D (2006) Using Dali for structural comparison of proteins. Current Protocols in Bioinformatic Chapter 5: Unit 5 5. [DOI] [PubMed]
- 37. Forsgren N, Lamont RJ, Persson K (2010) Two intramolecular isopeptide bonds are identified in the crystal structure of the Streptococcus gordonii SspB C-terminal domain. Journal of Molecular Biology 397: 740–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Daep CA, Lamont RJ, Demuth DR (2008) Interaction of Porphyromonas gingivalis with oral streptococci requires a motif that resembles the eukaryotic nuclear receptor box protein-protein interaction domain. Infection & Immunity 76: 3273–3280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Drobni M, Jonasson A, Strömberg N, Johansson I (2006) Identification of a multiplicity of acidic proline-rich protein peptide epitopes among Actinomyces and Streptococcus with different protein ligand binding patterns. Umeå University dissertations ISBN 91-7264-111-8.
- 40. Drobni M, Li T, Kruger C, Loimaranta V, Kilian M, et al. (2006) Host-derived pentapeptide affecting adhesion, proliferation, and local pH in biofilm communities composed of Streptococcus and Actinomyces species. Infect Immun 74: 6293–6299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Heckman KL, Pease LR (2007) Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc 2: 924–932. [DOI] [PubMed] [Google Scholar]
- 42. Kabsch W (2010) Xds. Acta Crystallogr D Biol Crystallogr 66: 125–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Panjikar S, Parthasarathy V, Lamzin VS, Weiss MS, Tucker PA (2005) Auto-Rickshaw: an automated crystal structure determination platform as an efficient tool for the validation of an X-ray diffraction experiment. Acta Crystallogr D Biol Crystallogr 61: 449–457. [DOI] [PubMed] [Google Scholar]
- 44. Langer G, Cohen SX, Lamzin VS, Perrakis A (2008) Automated macromolecular model building for X-ray crystallography using ARP/wARP version 7. Nat Protoc 3: 1171–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Emsley P, Lohkamp B, Scott WG, Cowtan K (2010) Features and development of Coot. Acta Crystallogr D Biol Crystallogr 66: 486–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Afonine PV, Grosse-Kunstleve RW, Echols N, Headd JJ, Moriarty NW, et al. (2012) Towards automated crystallographic structure refinement with phenix.refine. Acta Crystallogr D Biol Crystallogr 68: 352–367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Vagin A, Teplyakov A (2010) Molecular replacement with MOLREP. Acta Crystallogr D Biol Crystallogr 66: 22–25. [DOI] [PubMed] [Google Scholar]
- 48. Hooft RW, Vriend G, Sander C, Abola EE (1996) Errors in protein structures. Nature 381: 272. [DOI] [PubMed] [Google Scholar]