Abstract
Background
The B-box (BBX) -containing proteins are a class of zinc finger proteins that contain one or two B-box domains and play important roles in plant growth and development. The Arabidopsis BBX gene family has recently been re-identified and renamed. However, there has not been a genome-wide survey of the rice BBX (OsBBX) gene family until now.
Methodology/Principal Findings
In this study, we identified 30 rice BBX genes through a comprehensive bioinformatics analysis. Each gene was assigned a uniform nomenclature. We described the chromosome localizations, gene structures, protein domains, phylogenetic relationship, whole life-cycle expression profile and diurnal expression patterns of the OsBBX family members. Based on the phylogeny and domain constitution, the OsBBX gene family was classified into five subfamilies. The gene duplication analysis revealed that only chromosomal segmental duplication contributed to the expansion of the OsBBX gene family. The expression profile of the OsBBX genes was analyzed by Affymetrix GeneChip microarrays throughout the entire life-cycle of rice cultivar Zhenshan 97 (ZS97). In addition, microarray analysis was performed to obtain the expression patterns of these genes under light/dark conditions and after three phytohormone treatments. This analysis revealed that the expression patterns of the OsBBX genes could be classified into eight groups. Eight genes were regulated under the light/dark treatments, and eleven genes showed differential expression under at least one phytohormone treatment. Moreover, we verified the diurnal expression of the OsBBX genes using the data obtained from the Diurnal Project and qPCR analysis, and the results indicated that many of these genes had a diurnal expression pattern.
Conclusions/Significance
The combination of the genome-wide identification and the expression and diurnal analysis of the OsBBX gene family should facilitate additional functional studies of the OsBBX genes.
Introduction
Transcription factors (TFs) are key regulatory proteins that can activate or repress the transcription of their target genes and thus play critical roles in the life-cycle of higher plants [1]. The rice genome (Oryza sativa L. ssp. japonica) encodes 2384 putative TFs that are grouped into 63 different gene families [2]. The B-box (BBX) proteins are a class of zinc finger TFs that contained one or two B-box domains with specialized tertiary structures that are stabilized though the binding of Zn ions [3], [4]. In addition, the BBX proteins are likely to be involved in DNA binding or protein-protein interactions [5]–[8]. Typically, the plant BBX proteins contain one or two B-box domains near the amino terminus, and some have a CCT (CONSTANS, CO-like and TIMING Of CAB1) domain near the carboxy terminus [3]. Initially, many of the Arabidopsis BBX genes were named as CO-Like (COL) because CO (CONSTANS) was the first identified BBX gene in Arabidopsis. Khanna et al. established a uniform terminology for the genomic identification of the 32 BBX members in Arabidopsis and renamed them as AtBBX genes [3], [9], [10].
The best-known AtBBX protein, CO, contains two B-box and one CCT domains, and plays an important role in the photoperiod regulation of flowering. CO promotes flowering under long-day (LD) conditions but has no effect on the flowering time under short-day (SD) conditions [5]. Unlike CO, the altered expression of BBX2 (COL1) and BBX3 (COL2) has little effect on the flowering time but the over-expression of BBX2 can shorten the period of two distinct circadian rhythms [11]. BBX4 (COL3) is a positive regulator of photomorphogenesis and promotes root growth [12]. BBX6 (COL5) works as an SD-specific inducer of flowering by promoting the expression of FT [13]. BBX7 (COL9) functions as an LD-specific repressor of flowering by reducing the expression of CO and FT [14]. A number of AtBBXs that only have two B-box domains have also been identified: BBX18 (DBB1a), BBX19 (DBB1b), BBX24 (STO) and BBX25 (STH1) are negative regulators of light signaling [3], [9], [10], [15]–[17], whereas BBX21 (STH2) and BBX22 (LZF1/STH3) are positive regulators [18]–[20]. BBX32, which contains only a single B-box, is also a modulator of light signaling [21].
In rice, only three BBX genes have been identified to date. The rice CO ortholog, Hd1, is a typical BBX protein that contains two B-box and one CCT domains. Hd1 has a parallel function in the regulation of flowering time, but promotes flowering under SD rather than LD conditions [22]. OsCO3 contains a single B-box and a CCT domain and modulates the photoperiodic flowering in rice [23]. The overexpression of OsCO3 in rice causes late flowering under SD conditions but does not affect the flowering time in Arabidopsis. OsCOL4 functions as a constitutive repressor of flowering under SD and LD conditions [24], [25].
Hence, it is timely to comprehensive analyze the BBX family in rice and provide a uniform nomenclature for this class of proteins. In this study, 30 rice BBX genes (OsBBXs) were identified through a genome-wide survey. The chromosomal location and phylogenetic relationship of the OsBBXs were performed. The expression profile of the OsBBX genes in the rice life-cycle was analyzed using Affymetrix GeneChip microarrays and confirmed by qPCR. The diurnal expressions of the OsBBX genes were analyzed through the Diurnal Project and qPCR. In addition, the expression analysis of the rice BBX genes under light/dark conditions and after phytohormone (NAA, KT and GA3) treatments was performed. To our knowledge, this is the first report that focuses on the family-level identification of the OsBBX genes and analyzes their expression patterns. These results therefore provide a solid base for future functional genomic studies on the BBX gene family in rice.
Materials and Methods
Sequence retrieval and gene family member identification
The BLAST search tools BLASTP and TBLASTN [26] were used to identify the putative OsBBX with the AtBBX22 (AT1G78600) protein sequence and the conserved sequence of B-box domain as queries against four databases: the MSU Rice Genome Annotation Project Database (Release 7 of MSU RGAP) (http://rice.plantbiology.msu.edu/) [27], the Rice Annotation Project Database (RAP-DB, Rice genome IRGSP/RAP build 5) (http://rapdb.dna.affrc.go.jp/) [28], [29], the National Center for Biotechnology Information (NCBI) (http://www.ncbi.nlm.nih.gov/) and the Knowledge-Based Oryza Molecular Biological Encyclopedia (KOME) (http://cdna01.dna.affrc.go.jp/cDNA/) [30]. The BLASTP and TBLASTN search parameters of these databases were set as follows: expect value <10 and 500 maximum target sequences. The domain search and putative function search with the key word B-box were used to search against the MSU RGAP. The SMART (http://smart.embl-heidelberg.de/) [31], [32], Pfam (http://pfam.sanger.ac.uk/) [33] and InterProScan (http://www.ebi.ac.uk/Tools/pfa/iprscan/) searches were used to confirm the presence of the B-box domain and to predict the domain composition of each OsBBX protein. Information about the transcript, chromosomal position, full-length cDNA and characteristics of the proteins were obtained from MSU RGAP. The gene structure of each gene was displayed using the Gene Structure Display Server (http://gsds.cbi.pku.edu.cn/) [34]. The available mutant lines for some of the OsBBX genes were identified in the RiceGE database (http://signal.salk.edu/cgi-bin/RiceGE).
Determining chromosomal localization and gene duplication
The distribution of the OsBBX genes on the chromosomes was drawn using the MapChart software [35] according to the physical positions of the OsBBX genes and modified manually with annotation. The OsBBX genes that are present on duplicated chromosomal segments were identified by segmental genome duplication of rice, which is available at MSU RGAP (http://rice.plantbiology.msu.edu/segmental_dup/index.shtml). The maximum distance permitted between the collinear gene pairs was 500 kb. The OsBBX genes that were separated by a maximum of five genes were considered as tandemly duplicated genes.
Phylogenetic analysis and sequence alignment
Multiple sequence alignments of the amino acid sequences were generated using ClustalW in MEGA 5.05 with the default parameters [36]. The obtained sequence alignments were used as the input for the neighbor-joining algorithm in MEGA 5.05 to construct the phylogenetic trees of these sequences. Bootstrap analysis was performed using 1000 replicates. The phylogenetic tree was also displayed and annotated using MEGA 5.05. The multiple sequence alignment that was generated using ClustalW was modified manually, and the conserved sequences were annotated according to the results obtained from the SMART and Pfam searches. The logos of the conserved motifs were generated by submitting the sequence alignments to WebLogo [37].
Expression profile analysis, phytohormone and light/dark treatments
The original expression data for the OsBBX genes were extracted from the CREP database, which was developed by our lab (GSE19024) [38]. Thirteen vegetative and eleven reproductive samples of different developmental stages that cover the whole life-cycle of rice cultivar Zhenshan 97 (ZS97) were used for the OsBBX expression profile analysis that was performed in this study (Table S1). The unique probe set for each OsBBX gene was chosen according to the criterion mentioned in a previous study [39].
The quantile normalization and summarization of the origin microarray data were performed following the method described by Wang et al [38]. The average signal value of the replicates for each probe set was used for the analysis. The expression value of each probe set was logarithmized and transformed by Z-score. The relative expression values (Z-score) were used for hierarchical cluster analysis using the method of complete linkage based on the Euclidean distances by R-2.9.0 (http://www.r-project.org/). For phytohormone and light/dark treatments, the expression values was logarithmized and used for hierarchical cluster analysis by complete linkage method.
The samples used for the qPCR verification of the whole life-cycle and light/dark treatments were the same samples that were used for the Affymetrix microarrays. In the phytohormone treatments, germinating ZS97 seeds were sown in the greenhouse. The seedlings were treated with 100 µM gibberellin (GA3), auxin (NAA) or cytokinin (KT) at the trefoil stage, and samples that were collected after treatment for 5, 15, 30 and 60 min were pooled for each hormone treatment.
qPCR analysis
The TRIzol reagent (Invitrogen, CA, USA) was used to extract the total RNA according to the manufacturer's instructions. A total of 3 µg RNA was treated with DNase I (Invitrogen, CA, USA) to eliminate any contaminating DNA. The first-strand cDNA was reverse transcribed from the total RNA using the M-MLV reverse transcriptase (Invitrogen, CA, USA). The primers used for the qPCR are listed in Table S2. The rice Ubiquitin (LOC_Os03g13170) gene was used as the internal control. The qPCR was performed in a total volume of 25 µl containing 2 µl of the reverse-transcribed product cDNA, 0.2 µM of each primer, 12.5 µl of SYBR Premix Ex Taq and 0.5 µl of ROX Reference Dye II (Takara, Japan) using the Applied Biosystems 7500 Real-Time PCR System. The data were analyzed using the relative quantification method [40].
Diurnal expression patterns and cis-element analysis
A preliminary analysis of the diurnal expression pattern of the OsBBX genes was obtained from the Diurnal Project (http://diurnal.cgrb.oregonstate.edu/) [41], [42]. Two treatments were chosen for the analysis: light/thermocycles (LDHC): exposed to 12 h light (L), and 12 h dark (D) cycles with a high day temperature of 31°C and a low night temperature of 20°C. Plants in the LLHH (LDHC) group were exposed to the LDHC treatment followed by a two-day exposure to continuous light at a high temperature (31°C). qPCR was used to validate the diurnal expression patterns of several OsBBX genes. The samples used for the qPCR analysis were treated as in a previous study [43]. Young leaves were harvested from three different plants as three biological replications for each treatment. The samples were collected in 4 h intervals, starting at 8:30 for a total of 24 h. The qPCR was performed following the protocol that was described above.
To identify the putative cis-regulatory elements that exist in the OsBBX genes, the genomic sequences that are 1000 bp upstream from the translational start codons were used to search the PlantCARE database (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) [44].
Results
The BBX gene family members in rice
To identify the candidate BBX genes in the rice genome, we searched four databases: MSU RGAP, RAP-DB, NCBI and KOME. After removing the repeated sequences and different transcripts of the same gene, we identified 60 putative OsBBX genes. A domain search (PF00643) and putative function search of the MSU RGAP database only identified 30 putative OsBBX genes. The protein sequences of these genes were used for SMART, Pfam and InterProScan searches to confirm the presence of the B-box domain in each sequence. A total of 30 genes were confirmed to encode BBX proteins in rice. The B-box TFs that belong to the previous CO-like family also have been identified and reported in PlantTFDB (http://planttfdb.cbi.edu.cn/) (15 distinct B-box TFs and six repeated B-box TFs) [45], PlnTFDB (http://plntfdb.bio.uni-potsdam.de/v3.0/) (17 distinct B-box TFs and six repeated B-box TFs) [46], GramineaeTFDB (http://gramineaetfdb.psc.riken.jp/) (25 members with two incorrectly assigned members) [47] and Grassius (http://grassius.org/) (eight members) databases [48], [49]. The results of this study were compared with these databases and no new member was identified. For convenience, the 30 OsBBX genes were named as OsBBX1 to OsBBX30 according to their positions on the rice chromosomes. The detailed information on the RGAP Loci, RAP Os IDs, full-length cDNAs, transcripts, chromosomal positions, characteristics of the corresponding proteins, mutant lines and the original names of the 30 OsBBX genes are listed in Table 1. The gene structure of each OsBBX gene is shown in Figure S1.
Table 1. The detailed information of OsBBX members.
| Name | RGAP LOC | RAP Os ID | Originalname | flcDNA | Genomic position | Gene orientation | CDS length | Protein length | Mutant lines |
| OsBBX1 | LOC_Os01g10580 | Os01g0202500 | OsDBB3c | AK242176 | 5638769–5640495 | Upward | 1074 | 357 | 2C-30193, 2A-20608 |
| OsBBX2 | LOC_Os02g07930 | Os02g0176000 | N/A | AK241477 | 4149767–4151241 | Downward | 669 | 222 | 2A-50063, 2A-70002 |
| OsBBX3 | LOC_Os02g08150 | Os02g0178100 | OsF | AK109630 | 4315272–4316958 | Upward | 1050 | 349 | N/A |
| OsBBX4 | LOC_Os02g39360 | Os02g0606200 | OsSTH | AK104293, AK061044, CT830238, AB001885 | 23753345–23754688 | Upward | 816 | 271 | 3A-06908, 1B-23515, RdSpm2065A_3.1, K-05221 |
| OsBBX5 | LOC_Os02g39710 | Os02g0610500 | OsCOL4/OsD | AK100097, AK058536 | 23983881–23985424 | Upward | 999 | 332 | GSNU_Ds48, 1C-02709 |
| OsBBX6 | LOC_Os02g43170 | Os02g0646200 | N/A | AK071507, CT832305 | 26021916–26023624 | Upward | 810 | 269 | 1C-09019, 4A-50785, 2D-21119, 3C-00699 |
| OsBBX7 | LOC_Os02g49230 | Os02g0724000 | OsN | CT832281, CT832280, CT832282, CT832279, AK071630, AB001888 | 30088390–30093805 | Upward | 1443 | 480 | 1B-01905, 03Z11BI26, T28979T, T29560T, K-02893, 3A-03271, H0027_1_204_1A |
| OsBBX8 | LOC_Os02g49880 | Os02g0731700 | OsK | AK120314, AK072346 | 30466919–30469904 | Downward | 1359 | 452 | GSNU_Ds56, DAI1F05 |
| OsBBX9 | LOC_Os03g22770 | Os03g0351100 | OsP | AK101822, AK099864, AB001882 | 13151678–13154262 | Upward | 1212 | 403 | GSNU_Ds123 |
| OsBBX10 | LOC_Os03g50310 | Os03g0711100 | OsJ | AK120563 | 28680012–28682770 | Upward | 1266 | 421 | 1A-21121, SAO6E12, |
| OsBBX11 | LOC_Os04g41560 | Os04g0493000 | OsSTO | AK061664, AB001883 | 24462763–24464258 | Downward | 774 | 257 | 3A-52431, 3D-01977, 1A-23305 |
| OsBBX12 | LOC_Os04g42020 | Os04g0497700 | OsC | AK121024, AK059443 | 24704844–24706348 | Upward | 1002 | 333 | N/A |
| OsBBX13 | LOC_Os04g45690 | Os04g0540200 | N/A | AK061333, CT830169 | 26842136–26844370 | Upward | 753 | 250 | 2A-50066, 1D-04450 |
| OsBBX14 | LOC_Os05g11510 | Os05g0204600 | OsDBB3b | AK106865 | 6514687–6517221 | Upward | 1137 | 378 | T00477T, 05NPBLD37 04Z11RN03 |
| OsBBX15 | LOC_Os06g01340 | N/A | N/A | N/A | 208204–209107 | Upward | 672 | 223 | 1C-06536, M0022530 RDs501_5.2, RDs686_5.1 |
| OsBBX16 | LOC_Os06g05890 | Os06g0152200 | OsDBB3a | AK072231, AK104083, AB001886 | 2694461–2698504 | Upward | 1083 | 360 | 3B-00658, 3A-07296 1A-06932 |
| OsBBX17 | LOC_Os06g15330 | Os06g0264200 | OsL | AK287836, AK241249 | 8703832–8705950 | Upward | 1347 | 448 | DAK3H04 |
| OsBBX18 | LOC_Os06g16370 | Os06g0275000 | Hd1/OsA | AK241018, AB041838 | 9335360–9337644 | Upward | 1188 | 395 | T09919T, T31488T, T40187T, T32475T, T09921T, T17365T, T34409T, T10771T |
| OsBBX19 | LOC_Os06g19444 | Os06g0298200 | OsM | AK072192, AK121630, AK098865, CT833045 | 11069109–11075722 | Downward | 1227 | 408 | 5A-00300, M0065138, NE2301 |
| OsBBX20 | LOC_Os06g44450 | Os06g0654900 | OsE | AK243265, CT837522 | 26842090–26843779 | Downward | 1116 | 371 | 4A-04189, 04Z11IK06 |
| OsBBX21 | LOC_Os06g45040 | Os06g0661200 | N/A | N/A | 27252386–27254042 | Upward | 825 | 274 | 3B-00549, 1A-15201, 05NPBMT81 |
| OsBBX22 | LOC_Os06g49880 | Os06g0713000 | N/A | AK105957, CT833308, CT831615, AB001884 | 30195099–30196607 | Upward | 927 | 308 | 2B-20503, 3A-51129 |
| OsBBX23 | LOC_Os07g47140 | Os07g0667300 | N/A | AK120101 | 28183868–28186917 | Downward | 1143 | 380 | 04Z11HI63, 05NPBNR43, FL047220 |
| OsBBX24 | LOC_Os08g08120 | Os08g0178800 | N/A | AK287608, AK063025 | 4609503–4611921 | Downward | 852 | 283 | NE1871 |
| OsBBX25 | LOC_Os08g15050 | Os08g0249000 | OsG | AK109938, CT833687 | 9097577–9098884 | Upward | 912 | 303 | N/A |
| OsBBX26 | LOC_Os08g42440 | Os08g0536300 | OsO | AK099722 | 26790110–26794467 | Downward | 1467 | 488 | 2C-60235, 1D-05321, 2C-20399, 3A-51217, 2D-30775, 3A-03017, 05NPBNP03, 05NPBNZ01 |
| OsBBX27 | LOC_Os09g06464 | Os09g0240200 | OsCO3/OsB | AK241570, CT835894, AB001887 | 3047020–3063571 | Downward | 1008 | 335 | 1C-03305, 1C-03306 |
| OsBBX28 | LOC_Os09g33550 | Os09g0509700 | N/A | AK120589 | 19783043–19786296 | Downward | 1416 | 471 | T28637T, NE4523_0_503_1A, T09425T, T09366T, FL007537 |
| OsBBX29 | LOC_Os09g35880 | Os09g0527900 | OsDBB1 | AK058794, AK122172, CT828272 | 20645916–20649512 | Downward | 636 | 211 | 3C-00717, 4A-01958 |
| OsBBX30 | LOC_Os12g10660 | Os12g0209200 | N/A | AK067820 | 5698772–5701407 | Upward | 633 | 210 | 2C-50216 |
Protein sequence and phylogenetic analysis of the OsBBX family
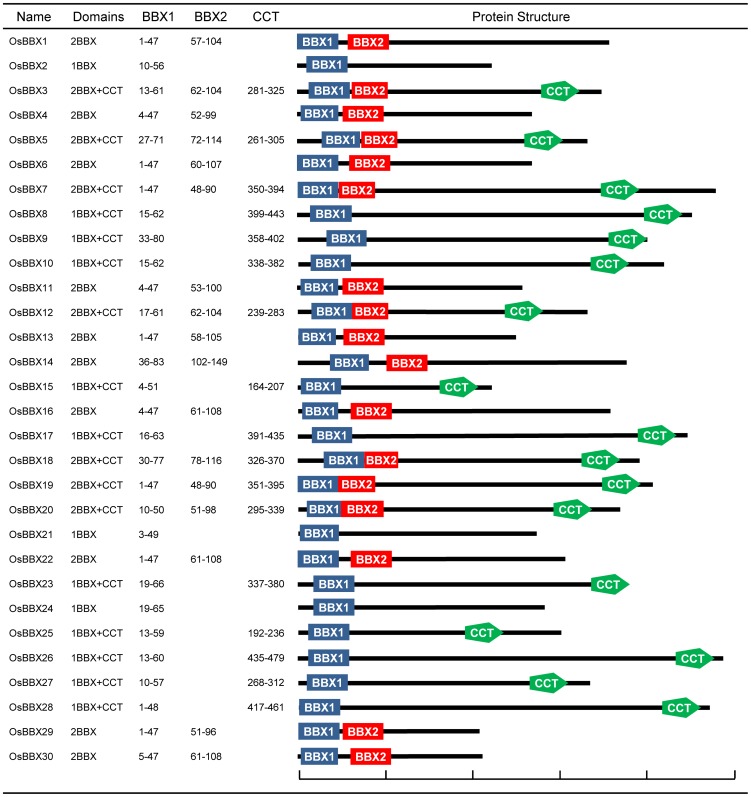
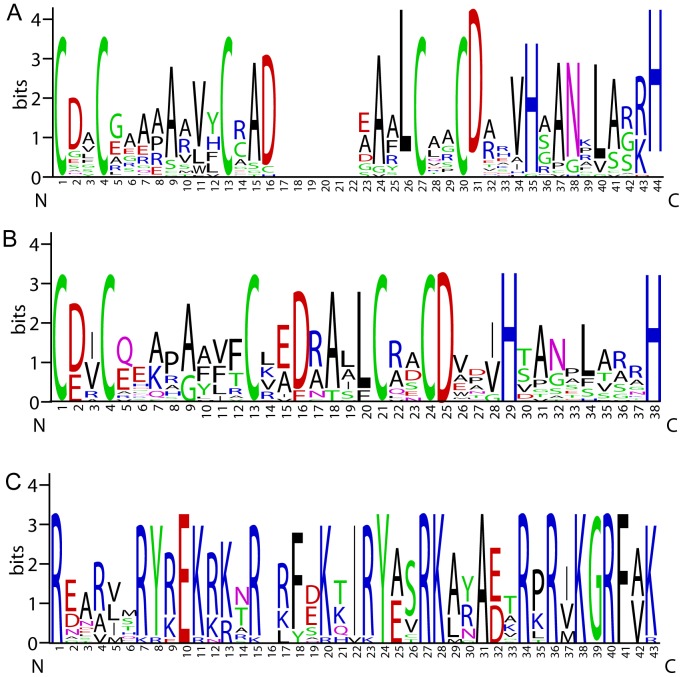
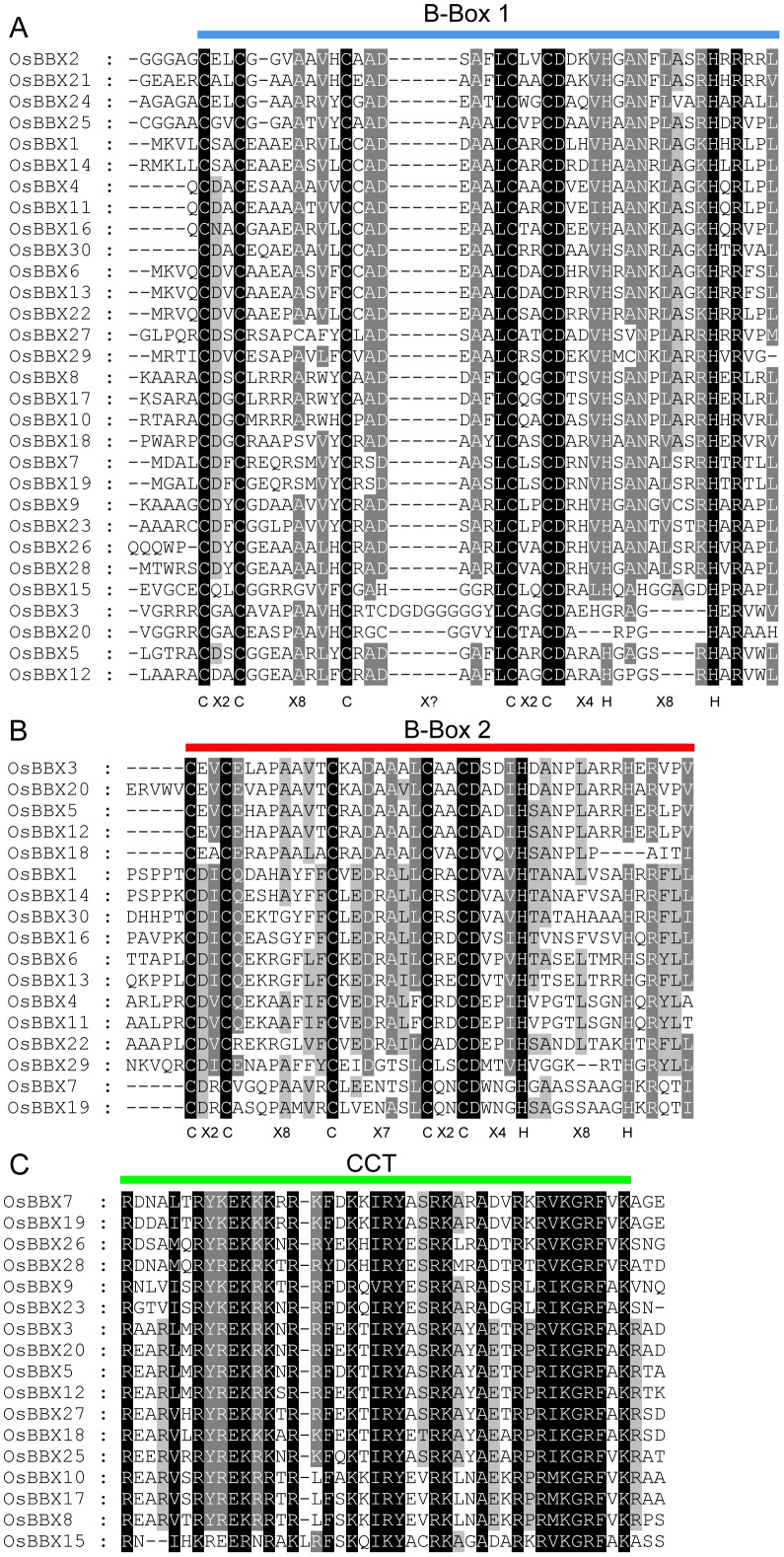
The OsBBX proteins vary widely in size, ranging from 210 to 488 amino acids in length. We identified seven OsBBXs that contain two B-box domains and a conserved CCT domain. Ten OsBBXs contained one B-box and one CCT domain. Three OsBBXs contained only one B-box domain, and 10 contained two B-box domains (Figure 1). The protein sequence alignment showed that the B-box 1 and B-box 2 domains of the OsBBXs have similar conserved sequences. The conserved sequence of B-box 1 is CDACGAAAAAVYCRADEAALCAACDAEVHAANKLARRH and the B-box 2 is CDVCEEAPAAVFCKEDRALLCRACDVDVHSANSLAARH. In addition, the Zinc finger domain in B-box 1 is in the form of C-X2-C-X8-C-X?-C-X2-C-X4-H-X8-H, whereas in B-box 2 is in the form of C-X2-C-X8-C-X7-C-X2-C-X4-H-X8-H. The CCT domain among the OsBBXs proteins is highly conserved. The logos of these domains in the OsBBXs proteins are shown in Figure 2, and their locations are illustrated in Figure 3.
Figure 1. Structures of the OsBBX proteins.
The name of each corresponding protein is shown on the left. The position of each domain is indicated in the figure. The length and order of the domains represent their actual location within each protein. The scale bar represents 100 amino acids.
Figure 2. The conserved domains in the OsBBX proteins.
Logos of the protein alignment of the B-box 1 (A), B-box 2 (B) and CCT domain (C) are shown. The x-axis represents the conserved sequences of the domain. Conservation of each residue across all proteins is indicated by the height of each letter. The y-axis is a scale of the relative entropy, which reflects the conservation rate of each amino acid.
Figure 3. Multiple sequence alignments of the domains of the OsBBXs.
Multiple sequence alignments of the B-box 1 (A), B-box 2 (B) and CCT (C) domains are shown. The identical amino acid, conserved amino acid and blocks of similar amino acid residues are shaded in black, charcoal gray and gray, respectively.
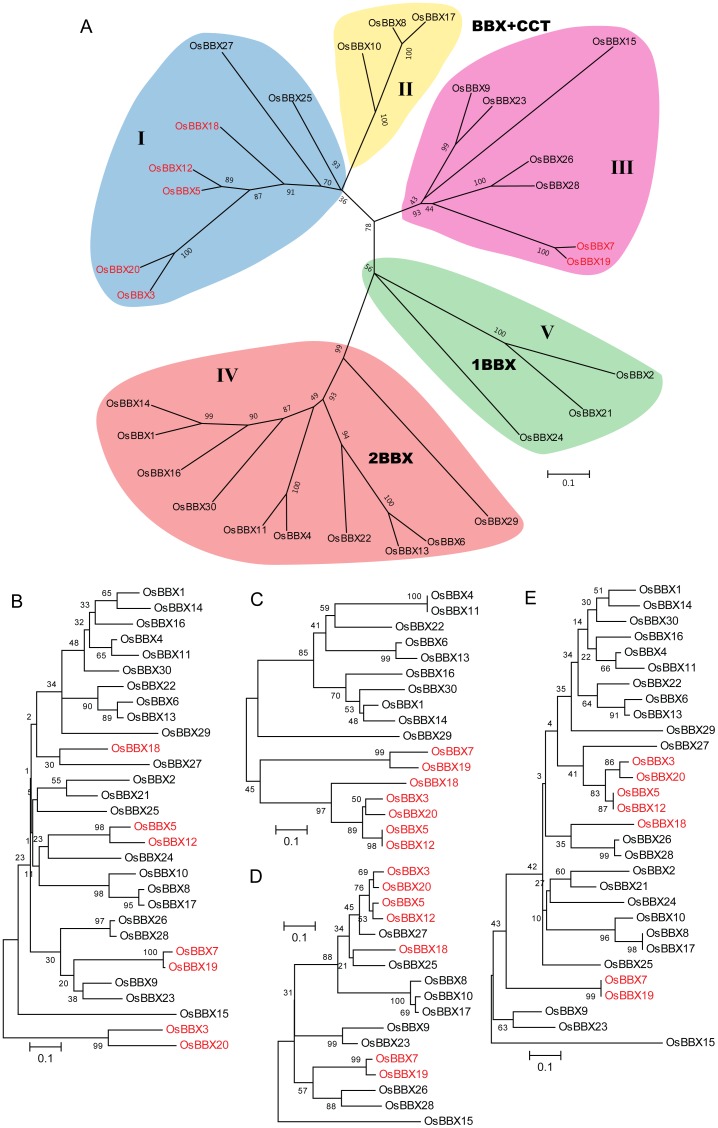
To examine in detail the phylogenetic relationship and functional divergence of the OsBBX members, the aligned 30 OsBBX protein sequences were used to construct an unrooted phylogenetic tree (Figure 4A). We also built the phylogenic tree including AtBBXs and OsBBXs as well as BBXs from maize, poplar and sorghum bicolor (Figure S4). Moreover, the aligned B-box 1 (Figure 4B), B-box 2 (Figure 4C), CCT (Figure 4D) and concatenated B-box and CCT (Figure 4E) domain sequences were also used for the phylogenetic analysis. The phylogenetic tree of OsBBX was further divided into 5 subfamilies based on our analysis and the previous study by Griffiths et al [24] (Figure 4). The members of subfamilies I, II and III are the OsBBXs that contain both B-box and CCT domains. We were unable to classify the OsBBXs that contain one B-box domain with a CCT domain and two B-box domains with a CCT domain into different subfamilies, which is different from the analysis of the phylogenetic tree of the AtBBXs. Rice, however, possesses two classes of BBXs that were not found in Arabidopsis. One class is in the subfamily I members, which contain a single B-box domain (OsBBX25 and OsBBX27). These two BBXs proteins evolved through the internal deletion of the second B-box domain [24]. The other class is in the subfamily III members, which includes five OsBBXs that have one B-box and one CCT domain, except that OsBBX7 and OsBBX19 possess the second B-box domains, which is different from the other members that contain two B-box domains. Subfamily II consists of three OsBBXs proteins with one B-box and one CCT domain. Subfamily IV contains 10 OsBBXs with two B-box domains, and subfamily V includes three OsBBXs with only one B-box domain.
Figure 4. Phylogenetic analysis of the OsBBX family.
The trees shown are based on the alignments of the protein sequences of the full length (A), the B-box 1 domain (B), B-box 2 domain (C), CCT domain (D) and concatenated B-box and CCT domains (E). The bootstrap values are indicated at each node. The scale bar represents 0.1 amino acid substitutions per site. The members marked in red contain two B-box and one CCT domains.
Chromosomal localization and gene duplication
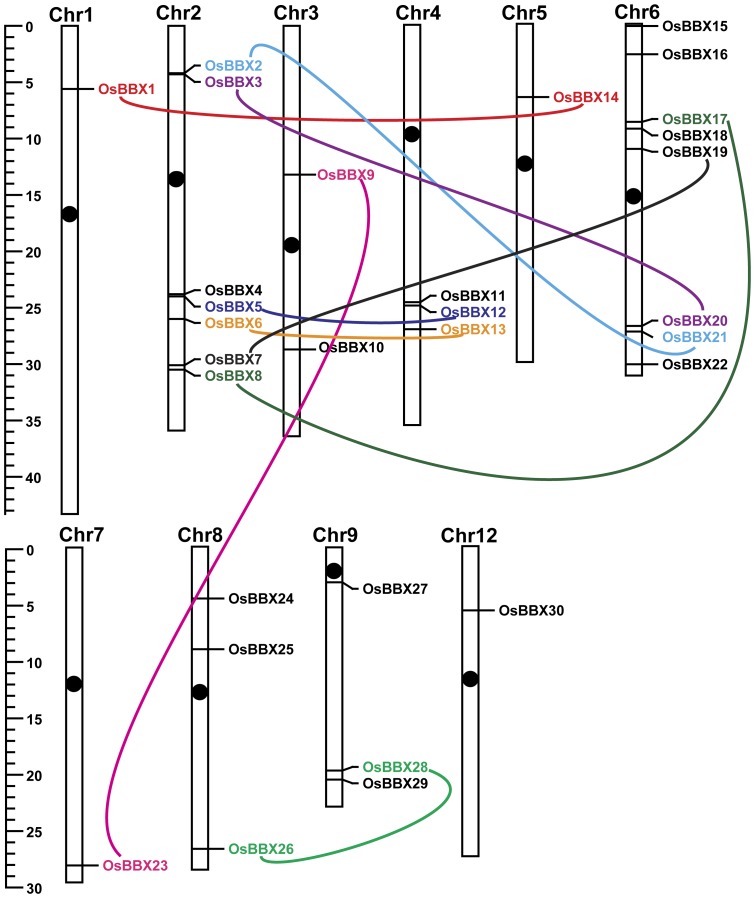
To determine the genomic distribution and the gene duplication of the BBX genes in rice, the approximate positions of the OsBBX genes were marked on the physical map of the rice chromosomes. According to the genomic localization, the 30 OsBBXs are distributed among all chromosomes except chromosomes 10 and 11. Only one OsBBX gene is found on chromosomes 1, 5, 7 and 12; seven genes are located on chromosome 2; two genes are localized on chromosome 3; three genes are found on chromosomes 4, 8 and 9, and eight genes are located on chromosome 6. The distribution of the OsBBX genes on the rice chromosomes is shown in Figure 5. Both the segmental and tandem duplications of the OsBBX gene family were analyzed. However, none of the OsBBXs are arranged in tandem. 18 OsBBXs are located in the duplicated segmental regions of rice chromosomes mapped by MSU RAGP (Figure 5).
Figure 5. Distribution of the OsBBX genes on the rice chromosomes.
The black ovals on the chromosomes indicate the position of the centromeres. The scale on the left is in megabases (Mb). The chromosome numbers are indicated at the top of each bar. The segmental duplicated genes are indicated in a different color and are connected by lines.
Expression profile of the OsBBX genes in the whole life-cycle of rice
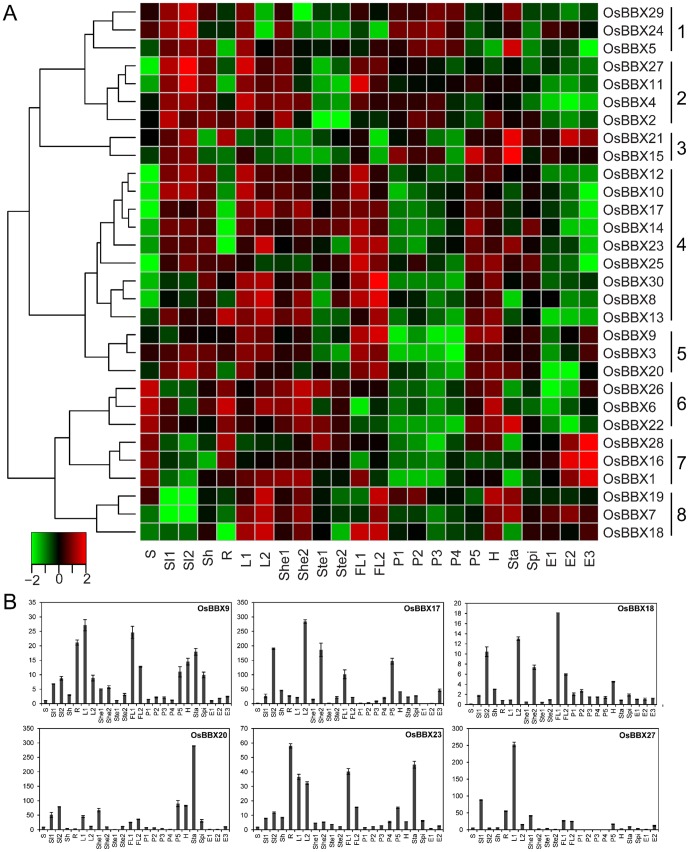
To analyze the expression profile of the OsBBX genes on a global scale, including a wide range of tissues at different developmental stages, we extracted the expression data of the OsBBX genes from the CREP database. All 30 OsBBX genes had at least one probe set. The average signal values of these genes from two or three biological replicates of each sample are shown in Table S3, and the hierarchical cluster analysis of their expression profile in ZS97 is illustrated in Figure 6A. Based on the hierarchical clustering, the expression profiles of the OsBBX genes can be divided into eight groups.
Figure 6. Expression profile of the OsBBX genes in the whole life-cycle of ZS97.
(A) Hierarchical cluster displaying the expression profiles of the 30 OsBBX genes. The tissues used for the expression profiling are indicated at the bottom of each column. A cluster dendrogram is shown on the left. The color key at the bottom represents the Z-score values transformed from log2-based expression values. The qPCR verification results of some of the OsBBX genes are shown (B). The x-axis of the qPCR results shows samples as listed in Table S1. The y-axis is the relative expression level of each gene compared to its expression level in the Endosperm 1 tissue. The error bars indicate the standard deviations of the independent biological replicates.
Group 1 consists of three genes (OsBBX29, 24 and 5) that show relative high expressions in seedlings, young panicles and stamen. Group 2 contains four genes (OsBBX27, 11, 4 and 2) with relative high expression in seedlings, leaves and sheath, but with low transcript accumulation in stem and endosperms. OsBBX21 and 15 in group 3 show relative high expression patterns in seedlings, panicles, stamen and endosperms. Nine genes (OsBBX12, 10, 17, 14, 23, 25, 30, 8 and 13) belong to group 4. These genes have relative high expression level in most vegetative tissues as well as mature panicle and hull. Group 5 (OsBBX9, 3 and 20) have relative high expression level in most tissues except developing young panicles and endosperms. Group 6 consists of three genes (OsBBX26, 6 and 22) which have relative high expression level in seed, most of the vegetative tissues, mature panicle, hull or stamen. All the genes in group 7 (OsBBX28, 16 and 1) have high expression level in seed, root and endosperms. The rest genes (OsBBX19, 7 and 18) belong to group 8 and have relative high expression in vegetative tissues like leaves, hull and endosperms. To validate the results of the microarray expression analysis, a qPCR analysis was performed for several representative genes. The expression patterns of the selected genes were in general agreement with the microarray data (Figure 6B).
Expression analysis of the OsBBX genes under light/dark treatment
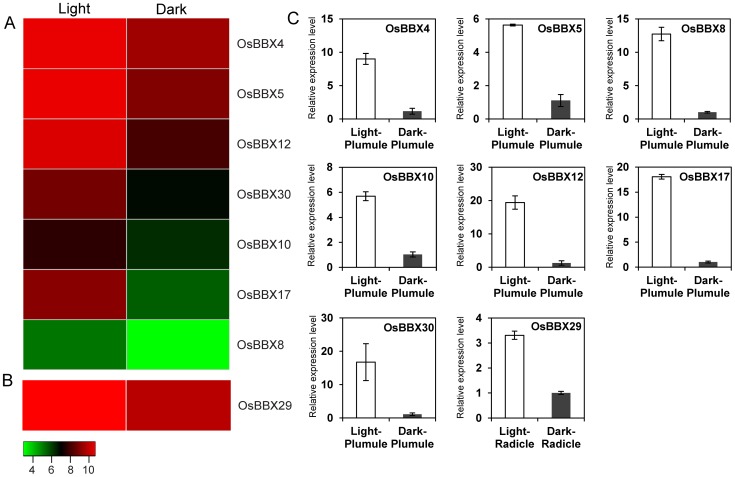
To investigate the possible light regulation of the OsBBX genes, the expression patterns of the OsBBX genes in the plumules and radicles under light/dark treatments were examined. Seven and one genes were differentially expressed in plumule and radicle, respectively (Figure 7A and 7B, Table S4). These eight genes (OsBBX4, 5/OsCOL4, 8, 10, 12, 17 and 30 in the plumule and OsBBX29 in the radicle) were up-regulated under light compared to darkness. The most responsive gene to light is OsBBX17, which is up-regulated 12-fold when the plumule is exposed to light compared to its level under dark conditions. In the plumule, the OsBBX8 and OsBBX12 genes are up-regulated nearly 5-fold under light conditions compare to under darkness. All the differentially expressed genes under light/dark treatments were verified by qPCR and are listed in Figure 7C.
Figure 7. The differentially expressed OsBBX genes are regulated by light and dark treatments.
(A) Differentially expressed genes in the plumule of 48 h after emergence with light/dark treatments. (B) Differentially expressed genes in the radicle of 48 h after emergence with light/dark treatments. The color key at the bottom represents the log2-based expression values. (C) The qPCR verification of all differentially expressed genes under the light/dark treatments. The x-axis indicates the treatments and tissues. The y-axis is the relative expression level of each gene. The error bars indicate the standard deviations of the independent biological replicates.
Diurnal expression pattern analysis
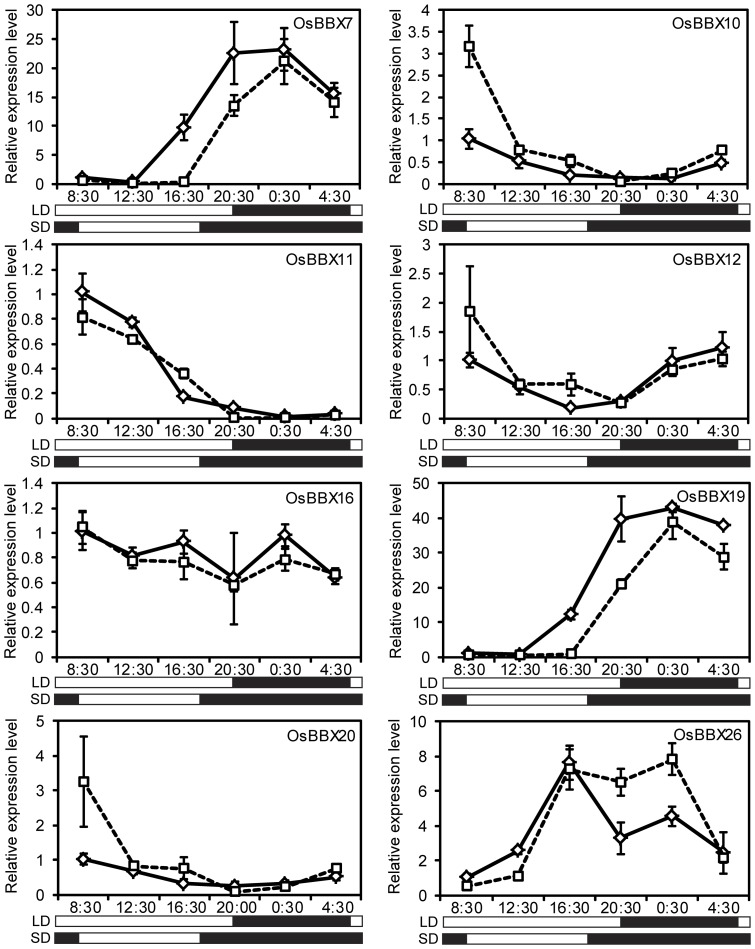
Some Arabidopsis BBX genes have shown diurnal expression patterns. Therefore, we verified the diurnal expression patterns of the OsBBX genes using data from the Diurnal Project. The expression patterns of 29 OsBBX genes can be found in the Diurnal Project. Among these genes, 25 have a diurnal expression pattern (Figure S2). Eight OsBBX genes with relative high expression levels were selected for validation by qPCR. The qPCR results confirmed that seven of the eight genes had a diurnal expression pattern (Figure 8). OsBBX7 and OsBBX19 exhibited the same expression pattern which belonged to a pair of segmental duplication genes. Under SD conditions, the expression of both of these genes immediately increased at the beginning of the night, exhibited a peak at midnight, rapidly dropped towards the end of the night and had their lowest expression value at dawn. Under LD conditions, both gene transcripts were much more abundant during the light period than during the dark period. The expression of both the OsBBX10 and OsBBX20 genes was higher under SD than under LD conditions and peaked in the early morning under both photoperiodic conditions. The level of OsBBX11 transcripts began to increase in the dark phase and peaked in the early morning under both photoperiodic conditions. The OsBBX12 gene exhibited the same diurnal expression pattern in both photoperiodic conditions. The transcript of OsBBX26 was much more abundant during the light period than during the dark period under LD conditions, whereas it was nearly the same under SD conditions. The OsBBX16 gene did not show an obvious diurnal expression pattern.
Figure 8. qPCR analysis of the diurnal expression patterns of the OsBBX genes.
The white and black bars at the bottom indicate the light and dark periods, respectively. The numbers above the bars indicate the time of the day. The y-axis shows the relative transcript levels of each gene. The error bars indicate the standard deviations of the independent biological replicates.
Response of the OsBBX genes to phytohormone treatments
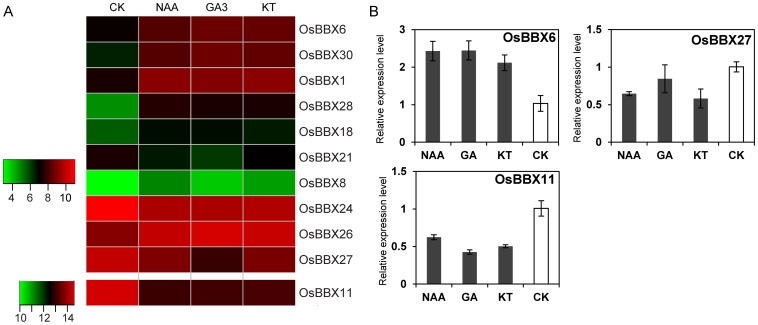
To survey the OsBBX genes in response to phytohormone treatment, we also studied the responsiveness of the OsBBX genes to different phytohormone treatments, including NAA (a member of the auxin family), GA3 (a gibberellin) and KT (a cytokinin) using microarray analysis (Figure 9A). Eleven genes showed differential expression that was more than two-fold under at least one phytohormone treatment. Seven genes (OsBBX1, 6, 11, 24, 26, 27/OsCO3 and 30) showed differential expression under the three phytohormone treatments, and three (OsBBX11, 24 and 27) of these genes were down-regulated. One gene (OsBBX8) was up-regulated under NAA and KT treatments, one gene (OsBBX21) was down-regulated under GA3 treatment, and two genes (OsBBX18 and OsBBX28) were up-regulated specifically in response to treatment with NAA. The fold-changes of the OsBBX genes that exhibited differential expression are shown in Table S4. We prepared new samples of rice plants that were exposed to phytohormone treatments and used these samples to verify the findings by qPCR; the results are shown in Figure 9B.
Figure 9. Expression profiles of the OsBBX genes in response to phytohormone treatments.
(A) Hierarchical cluster displaying the differentially expressed OsBBX genes under NAA, GA3 and KT treatments. The color key at the left represents the log2-based expression values. (B) The qPCR verification of some of the differentially expressed OsBBX genes. The x-axis indicates the treatment. The y-axis shows the relative expression level of each treatment compared to control. The error bars indicate the standard deviations of the independent biological replicates.
In silico cis-element analysis of the OsBBX genes
A total of 90 putative cis-elements in the OsBBX upstream genomic sequences were identified by the PlantCARE database. Except the common cis-acting element in the promoter, enhancer regions (CAAT-box) and core promoter element around −30 of transcription start (TATA-box), 18 others cis-elements were found in more than 10 of the 30 OsBBXs which can be classified into four groups. Nine cis-elements that are involved in the light responsiveness of rice are: the circadian, G-box, Sp1, Box I, GT1-motif, Box 4, I-box, GAG-motif and TCCC-motif elements. Six of the cis-elements are well-characterized stress-responsive elements: HSE, ARE, W box, GC-motif, Box-W1 and MBS elements. The three cis-acting regulatory elements that are involved in hormone responsiveness are: ABRE, TGACG-motif and CGTCA-motif elements. One cis-acting regulatory element (Skn-1_motif) is required for endosperm expression. The cis-elements that were identified among the 30 OsBBX genes and the putative functions and positions of these elements are listed in Table 2 and Table S5, respectively.
Table 2. The cis-elements that have been identified in more than ten OsBBX genes.
| Cis-elements | Number of genes | Functions of the cis-elements | Cis-elements types |
| CAAT-box | 30 | Common cis-acting element in promoter and enhancer regions | |
| TATA-box | 30 | Core promoter element around −30 of transcription start | |
| Sp1 | 28 | Light responsive element | Light responsive |
| G-box | 27 | Cis-acting regulatory element involved in light responsiveness | Light responsive |
| ABRE | 21 | Cis-acting element involved in the abscisic acid responsiveness | Hormone responsive |
| TGACG-motif | 20 | Cis-acting regulatory element involved in the meja-responsiveness | Hormone responsive |
| ARE | 19 | Cis-acting regulatory element essential for the anaerobic induction | Stress responsive |
| CGTCA-motif | 19 | Cis-acting regulatory element involved in the meja-responsiveness | Hormone responsive |
| Skn-1_motif | 19 | Cis-acting regulatory element required for endosperm expression | Endosperm expression |
| I-box | 18 | Part of a light responsive element | Light responsive |
| circadian | 16 | Cis-acting regulatory element involved in circadian control | Light responsive |
| W box | 14 | Wounding and pathogen responsievness. | Stress responsive |
| GC-motif | 14 | Enhancer-like element involved in anoxic specific inducibility | Stress responsive |
| Box-W1 | 14 | Fungal elicitor responsive element | Stress responsive |
| Box 4 | 14 | Part of a conserved DNA module involved in light responsiveness | Light responsive |
| GAG-motif | 13 | Part of a light responsive element | Light responsive |
| HSE | 12 | Cis-acting element involved in heat stress responsiveness | Stress responsive |
| Box I | 12 | Light responsive element | Light responsive |
| MBS | 12 | MYB binding site involved in drought-inducibility | Stress responsive |
| GT1-motif | 11 | Light responsive element | Light responsive |
| TCCC-motif | 11 | Part of a light responsive element | Light responsive |
Discussion
Evolution of the OsBBX gene family
The rice genome size and gene number are approximately 3.7 times (450 Mb vs. 130 Mb) and 1.5 times (37,000 vs. 25,000) larger, respectively, than those of Arabidopsis [50]. However, we identified fewer BBX genes in rice than in Arabidopsis. The reason for this discrepancy could be the variable status of genome duplications in Arabidopsis and rice [51], [52]. Eighteen OsBBX genes are involved in segmental duplication events of the rice chromosome, fourteen of which contains one CCT domain and either one or two B-box domains, two with two B-box domains, and two genes with only one B-box domain. The rice BBX gene family contains 17 OsBBXs with one or two B-box domains and one CCT domain, which is similar to the genes found in Arabidopsis. Ten OsBBXs contained two B-box domains, and three OsBBXs contain only one B-box domain; in contrast, eight AtBBXs contain two B-box domains, and seven AtBBXs contain only one B-box domain. These results suggest that the rice and Arabidopsis BBX genes probably had a common ancestor and that the expansion occurred independently after the divergence of the monocots and the dicots.
During the course of plant evolution, tandem duplication and large-scale segmental duplication play an important role in maintaining the large number of genes that belong to specific gene families [53]. To elucidate the potential mechanism of evolution of the OsBBX gene family, both the tandem and segmental duplication events were analyzed. Although no tandem duplication was found, 18 OsBBXs participated in segmental duplication. These results suggest that segmental duplication events contributed significantly to the expansion of the OsBBX gene family. The same potential mechanism of gene family evolution was also found in the bZIP transcription factor gene family in rice [54].
A phylogenic tree of the BBXs from rice, Arabidopsis, maize, poplar and sorghum bicolor was constructed. In the above mentioned tree, the phylogenic relationships among the OsBBXs were almost the same as in a phylogenic tree that contained only the BBXs from rice. Moreover, many of the BBXs from the monocot were clustered together, which indicated that these genes were orthologous; this finding is consistent with the results reported by Griffiths et al [24]. The phylogenic tree that contains the OsBBX and AtBBX showed that most of the BBXs are clustered in species-specific clades, indicating that the BBXs evolved separately after the monocot and dicot divergence (Figure S3). Griffiths et al [24] suggested that the monocot plants have a unique subfamily that contains one B-box motif and a CCT motif that is derived through the internal deletion of the second B-box domain. The position of OsBBX25 and OsBBX27 in the phylogenic trees confirmed this conclusion. The sequence alignment showed that the OsBBX7 and OsBBX19 possessed the second B-box domains, which indicated that these proteins diverged from the other BBXs that contain two B-box domains. The aligned B-box 1, B-Box 2, CCT and concatenated B-Box and CCT domain sequences were also used for phylogenetic analysis. Similar overall patterns compared with the full-length sequence tree were observed. These results indicate that the B-Box and CCT domains may be relevant for the conserved functions of the BBX family.
Divergent expression profile of the OsBBX genes
Microarray analysis is a high-throughput method that can be used to analyze the expression pattern of many genes at the transcription level. Our analyses indicated that the expression patterns of the 30 OsBBX genes could be classified into eight groups. From the phylogenetic analysis, we identified several paralogous OsBBXs. Some paralogous genes (OsBBX3/OsBBX20, OsBBX4/OsBBX11, OsBBX7/OsBBX19 and OsBBX8/OsBBX10/OsBBX17) have similar expression patterns. Therefore, we inferred that these genes had obtained redundant expression pattern during the evolution. On the other hand, some paralogous genes (OsBBX1/OsBBX14, OsBBX2/OsBBX21, OsBBX5/OsBBX12, OsBBX6/OsBBX13, OsBBX9/OsBBX23 and OsBBX26/OsBBX28) have divergent expression patterns indicating that these genes had evolutionarily obtained unique expression pattern.
The development of comparative genomics has made possible to analyze the genes of the same gene family among different species. The phylogenetic analysis indicated that most OsBBXs and AtBBXs are clustered in species-specific clades with high bootstrap supporting, except for four sets of orthologous genes: OsBBX16 and AtBBX22; OsBBX29, AtBBX18 and AtBBX19; OsBBX24, AtBBX28 and AtBBX29; OsBBX18, AtBBX1, AtBBX2 and AtBBX3. The AtBBX22, AtBBX18 (DBB1a) and AtBBX19 (DBB1b) are positive or negative regulate genes of light signaling in Arabidopsis [9], [10], [19]. Therefore, we inferred that OsBBX16 and OsBBX29 might participate in the light signaling pathway even though OsBBX16 did not have an obviously diurnal expression pattern. Previous studies showed that the expression of OsBBX29 oscillates in continuous light [10].
Light regulation of the OsBBX genes and cis-elements analysis
Light is one of the major environmental factors that regulate plant growth and development. Plants perceive various light signals and subsequently regulate diverse developmental processes, such as skotomorphogenesis, photomorphogenesis, shade avoidance, circadian growth and flowering time control. Skotomorphogenesis and photomorphogenesis are two developmental processes that occur in plants that grow in the dark and light, respectively. The proper regulation of these two stages is important for the optimization of plant growth and for their success in response to the environment [55]. Seedlings at the plumule and radicle stages were placed under 48 h of continuous light or darkness, and samples were collected for microarray experiments. Analysis of the microarray data showed that eight genes were differentially regulated by the light and dark treatments. All eight genes were up-regulated under light conditions compared to darkness. In addition, OsBBX5 (OsCOL4) also exhibited differential expression in our microarray analysis. Therefore, we inferred that these differentially expressed genes might play a role in the light signaling pathway, especially in the early photomorphogenesis stage in rice. Previous reports have suggested that many BBX genes are implicated in a light signal transduction pathway [9], [10], [15], [16], [18], [19]. The analysis of data obtained from the Diurnal Project and the qPCR data showed that most of the rice BBX genes had diurnal expression patterns, suggesting that the OsBBX family members may be involved in the light signaling pathway in rice.
A total of 90 cis-acting regulatory elements were identified. The frequency of light-responsive cis-regulatory elements in the OsBBX promoter region ranges from three to seven. OsBBX6 had the maximum number of light-responsive cis-elements followed by OsBBX1, 5, 8, 10, 16, 25, 26 and 27, which have six light-responsive cis-elements. The light-responsive element Sp1 was found in the promoter region of 28 OsBBX genes. The cis-element circadian was found in the promoter region of 16 OsBBXs, including OsBBX7, 10, 11, 19, 26 and 27. All of these genes exhibit the diurnal expression patterns. Therefore, we inferred that the genes with the circadian cis-element may have a diurnal expression pattern that is regulated by light. The presence of many light responsive elements in the promoter region of the OsBBX genes strongly suggests the involvement of these BBX genes in the regulation of the photoperiodic control of flowering.
The majority of the OsBBX genes have hormone-responsive cis-elements, except OsBBX1, OsBBX14 and OsBBX19, which indicates that these genes have a putative role in hormone regulation. Twenty-nine OsBBX genes contain at least one of the stress-responsive cis-elements (ARE, W box, GC-motif, Box-W1, HSE and MBS), suggesting that these genes have a putative role in the response to abiotic and biotic stress. The identified cis-elements are mainly related to five important physiological phenomena: light, hormone and stress responsiveness and endosperm- and meristem-specific gene expression. Their presence in the upstream sequence of OsBBX genes indicates that the OsBBX members have additional unknown functions.
Phytohormonal regulation of OsBBX genes
Plant hormones are essential for plant growth and development. Auxin, GA, cytokinin, ABA and ethylene are the ‘classical’ phytohormones. Notably, these hormones can regulate many processes independently. However, their overlapping influence on various cellular processes suggests that there is cooperation and crosstalk between their signaling pathways [56]. Furthermore, an increasing number of studies showed that a tight crosstalk between light and hormones to regulates seed germination and seedling photomorphogenesis, and can lead to a dramatic change in plant morphology [57]. Previous studies have shown that the BBX genes participate in light signaling and hormone responses. DBB1a functions as a negative regulator of blue light-mediated hypocotyl elongation and is involved in gibberellin homeostasis in Arabidopsis [15]. AtBBX22 may play a role in regulating light signaling and hormone responses [20]. Our results showed that eleven OsBBX genes are regulated by hormones. Eight of these genes are regulated by more than one hormone treatment, which suggests that these genes may be involved in the interaction of different hormone signals at the physiological level. In addition, three genes (OsBBX8, 27 and 30) responded to both hormone and light treatments, which indicated that these genes may integrate light and hormone signals. Further characterization of additional OsBBX genes that are involved in different hormone and light responses will greatly expand our understanding of the functions of the OsBBX genes and the crosstalk that occurs between the hormone and light signaling pathways.
In conclusion, this study provided a genomic framework, uniform nomenclature and phylogenetic analysis of the 30 OsBBX genes. In addition, we studied the expression profile of these 30 OsBBX genes during the whole life-cycle of rice and under phytohormone and light/dark treatments. Furthermore, the diurnal expression patterns of the OsBBX genes were also investigated. Our investigation provides insight into the role of OsBBX genes in certain developmental stages and in the phytohormone and light signaling pathways. Our analysis provides a useful reference for more detailed functional analyses of these BBX genes in rice and will be helpful in the selection of appropriate candidate genes for further study.
Supporting Information
Gene structure of each OsBBX gene. The white rectangles represent the exons and the black lines represent the introns. The UTR regions are marked as black rectangles.
(TIF)
Diurnal expression pattern of OsBBX genes from the Diurnal Project. The x-axis represents the time course over two days. The y-axis represents the average expression values obtained from the microarrays. The blue line represents the LDHC condition and the green line represents the LLHH (LDHC) condition.
(TIF)
Phylogenetic analysis of the OsBBX and AtBBX members. The unrooted tree was generated from the OsBBX and AtBBX full length protein sequences. The bootstrap values from 1000 replicates are indicated at each node. The triangles in front of the members indicate the predicted paralogous proteins. The members marked in red contain two B-box domains and one CCT domains. The scale bar represents 0.1 amino acid substitutions per site.
(PDF)
Phylogenetic analysis of the B-box TFs from rice, Arabidopsis , maize, poplar and sorghum bicolor . The sequences of the maize (Zm: Zea Mays), poplar ( Pt: Populus trichocarpa) and sorghum bicolor (Sb: Sorghum bicolor) BBX proteins were identified and downloaded from NCBI. The unrooted tree was generated from the full length protein sequences. The bootstrap values from 1000 replicates are indicated at each node. The scale bar represents 0.1 amino acid substitutions per site.
(PDF)
Detailed information of the rice samples used in the microarray analysis.
(DOC)
Primers used for the qPCR analysis.
(DOC)
Average expression signal values of the 30 OsBBX genes.
(XLS)
Results of the differential expression analysis of the OsBBX genes in the plumule and radicle under NAA, GA3 and KT treatment and light/dark regulation.
(XLS)
The positions of the cis -elements that were identified in more than ten OsBBX genes.
(XLS)
Acknowledgments
We thank our supervisor, Professor Qifa Zhang, for his encouragement and direction. We also thank the editor and reviewers for their critical comments and thoughtful suggestions.
Funding Statement
This work was supported by grants from the National 863 Project (2012AA100103) and the National Natural Science Foundation of China (30921091). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Gong W, Shen YP, Ma LG, Pan Y, Du YL, et al. (2004) Genome-wide ORFeome cloning and analysis of Arabidopsis transcription factor genes. Plant Physiol 135: 773–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Gao G, Zhong YF, Guo AY, Zhu QH, Tang W, et al. (2006) DRTF: a database of rice transcription factors. Bioinformatics 22: 1286–1287. [DOI] [PubMed] [Google Scholar]
- 3. Khanna R, Kronmiller B, Maszle DR, Coupland G, Holm M, et al. (2009) The Arabidopsis B-box zinc finger family. Plant Cell 21: 3416–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Klug A, Schwabe JW (1995) Protein motifs 5. Zinc fingers. FASEB J 9: 597–604. [PubMed] [Google Scholar]
- 5. Putterill J, Robson F, Lee K, Simon R, Coupland G (1995) The CONSTANS gene of Arabidopsis promotes flowering and encodes a protein showing similarities to zinc finger transcription factors. Cell 80: 847–857. [DOI] [PubMed] [Google Scholar]
- 6. Robson F, Costa MM, Hepworth SR, Vizir I, Pineiro M, et al. (2001) Functional importance of conserved domains in the flowering-time gene CONSTANS demonstrated by analysis of mutant alleles and transgenic plants. Plant J 28: 619–631. [DOI] [PubMed] [Google Scholar]
- 7. Borden KL (1998) RING fingers and B-boxes: zinc-binding protein-protein interaction domains. Biochem Cell Biol 76: 351–358. [DOI] [PubMed] [Google Scholar]
- 8. Torok M, Etkin LD (2000) Two B or not two B? Overview of the rapidly expanding B-box family of proteins. Differentiation 67: 63–71. [DOI] [PubMed] [Google Scholar]
- 9. Chang CS, Li YH, Chen LT, Chen WC, Hsieh WP, et al. (2008) LZF1, a HY5-regulated transcriptional factor, functions in Arabidopsis de-etiolation. Plant J 54: 205–219. [DOI] [PubMed] [Google Scholar]
- 10. Kumagai T, Ito S, Nakamichi N, Niwa Y, Murakami M, et al. (2008) The common function of a novel subfamily of B-Box zinc finger proteins with reference to circadian-associated events in Arabidopsis thaliana . Biosci Biotechnol Biochem 72: 1539–1549. [DOI] [PubMed] [Google Scholar]
- 11. Ledger S, Strayer C, Ashton F, Kay SA, Putterill J (2001) Analysis of the function of two circadian-regulated CONSTANS-LIKE genes. Plant J 26: 15–22. [DOI] [PubMed] [Google Scholar]
- 12. Datta S, Hettiarachchi GH, Deng XW, Holm M (2006) Arabidopsis CONSTANS-LIKE3 is a positive regulator of red light signaling and root growth. Plant Cell 18: 70–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hassidim M, Harir Y, Yakir E, Kron I, Green RM (2009) Over-expression of CONSTANS-LIKE 5 can induce flowering in short-day grown Arabidopsis . Planta 230: 481–491. [DOI] [PubMed] [Google Scholar]
- 14. Cheng X-F, Wang Z-Y (2005) Overexpression of COL9, a CONSTANS-LIKE gene, delays flowering by reducing expression of CO and FT in Arabidopsis thaliana . Plant J 43: 758–768. [DOI] [PubMed] [Google Scholar]
- 15. Wang QM, Zeng JX, Deng KQ, Tu XJ, Zhao XY, et al. (2011) DBB1a, involved in gibberellin homeostasis, functions as a negative regulator of blue light-mediated hypocotyl elongation in Arabidopsis . Planta 233: 13–23. [DOI] [PubMed] [Google Scholar]
- 16. Indorf M, Cordero J, Neuhaus G, Rodríguez-Franco M (2007) Salt tolerance (STO), a stress-related protein, has a major role in light signalling. Plant J 51: 563–574. [DOI] [PubMed] [Google Scholar]
- 17. Holm M, Hardtke CS, Gaudet R, Deng X-W (2001) Identification of a structural motif that confers specific interaction with the WD40 repeat domain of Arabidopsis COP1. EMBO J 20: 118–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Datta S, Hettiarachchi C, Johansson H, Holm M (2007) SALT TOLERANCE HOMOLOG2, a B-box protein in Arabidopsis that activates transcription and positively regulates light-mediated development. Plant Cell 19: 3242–3255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Datta S, Johansson H, Hettiarachchi C, Irigoyen ML, Desai M, et al. (2008) LZF1/SALT TOLERANCE HOMOLOG3, an Arabidopsis B-box protein involved in light-dependent development and gene expression, undergoes COP1-mediated ubiquitination. Plant Cell 20: 2324–2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chang C-SJ, Maloof JN, Wu S-H (2011) COP1-mediated degradation of BBX22/LZF1 optimizes seedling development in Arabidopsis . Plant Physiol 156: 228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Holtan HE, Bandong S, Marion CM, Adam L, Tiwari S, et al. (2011) BBX32, an Arabidopsis b-box protein, functions in light signaling by suppressing hy5-regulated gene expression and interacting with STH2/BBX21. Plant Physiol 156: 2109–2123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Yano M, Katayose Y, Ashikari M, Yamanouchi U, Monna L, et al. (2000) Hd1, a major photoperiod sensitivity quantitative trait locus in rice, is closely related to the Arabidopsis flowering time gene CONSTANS . Plant Cell 12: 2473–2484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kim SK, Yun CH, Lee JH, Jang YH, Park HY, et al. (2008) OsCO3, a CONSTANS-LIKE gene, controls flowering by negatively regulating the expression of FT-like genes under SD conditions in rice. Planta 228: 355–365. [DOI] [PubMed] [Google Scholar]
- 24. Griffiths S, Dunford RP, Coupland G, Laurie DA (2003) The evolution of CONSTANS-like gene families in barley, rice, and Arabidopsis. Plant Physiol 131: 1855–1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee YS, Jeong DH, Lee DY, Yi J, Ryu CH, et al. (2010) OsCOL4 is a constitutive flowering repressor upstream of Ehd1 and downstream of OsphyB . Plant J 63: 18–30. [DOI] [PubMed] [Google Scholar]
- 26. Altschul SF, Madden TL, Schäffer AA, Zhang J, Zhang Z, et al. (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25: 3389–3402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ouyang S, Zhu W, Hamilton J, Lin H, Campbell M, et al. (2007) The TIGR Rice Genome Annotation Resource: improvements and new features. Nucleic Acids Res 35: D883–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Tanaka T, Antonio BA, Kikuchi S, Matsumoto T, Nagamura Y, et al. (2008) The Rice Annotation Project Database (RAP-DB): 2008 update. Nucleic Acids Res 36: D1028–D1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Itoh T, Tanaka T, Barrero RA, Yamasaki C, Fujii Y, et al. (2007) Curated genome annotation of Oryza sativa ssp. japonica and comparative genome analysis with Arabidopsis thaliana . Genome Res 17: 175–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kikuchi S, Satoh K, Nagata T, Kawagashira N, Doi K, et al. (2003) Collection, mapping, and annotation of over 28,000 cDNA clones from japonica rice. Science 301: 376–379. [DOI] [PubMed] [Google Scholar]
- 31. Schultz J, Milpetz F, Bork P, Ponting CP (1998) SMART, a simple modular architecture research tool: identification of signaling domains. Proc Natl Acad Sci U S A 95: 5857–5864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Letunic I, Doerks T, Bork P (2012) SMART 7: recent updates to the protein domain annotation resource. Nucleic Acids Res 40: D302–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Punta M, Coggill PC, Eberhardt RY, Mistry J, Tate J, et al. (2012) The Pfam protein families database. Nucleic Acids Res 40: D290–D301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Guo AY, Zhu QH, Chen X, Luo JC (2007) GSDS: a gene structure display server. Yi chuan 29: 1023–1026. [PubMed] [Google Scholar]
- 35. Voorrips RE (2002) MapChart: software for the graphical presentation of linkage maps and QTLs. J Hered 93: 77–78. [DOI] [PubMed] [Google Scholar]
- 36. Tamura K, Peterson D, Peterson N, Stecher G, Nei M, et al. (2011) MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol 28: 2731–2739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14: 1188–1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang L, Xie W, Chen Y, Tang W, Yang J, et al. (2010) A dynamic gene expression atlas covering the entire life cycle of rice. Plant J 61: 752–766. [DOI] [PubMed] [Google Scholar]
- 39. Zhao X, Huang J, Yu H, Wang L, Xie W (2010) Genomic survey, characterization and expression profile analysis of the peptide transporter family in rice (Oryza sativa L.). BMC Plant Biol 10: 92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods 25: 402–408. [DOI] [PubMed] [Google Scholar]
- 41. Mockler TC, Michael TP, Priest HD, Shen R, Sullivan CM, et al. (2007) The DIURNAL project: DIURNAL and circadian expression profiling, model-based pattern matching, and promoter analysis. Cold Spring Harb Symp Quant Biol 72: 353–363. [DOI] [PubMed] [Google Scholar]
- 42. Michael TP, Mockler TC, Breton G, McEntee C, Byer A, et al. (2008) Network discovery pipeline elucidates conserved time-of-day-specific cis-regulatory modules. PLoS Genet 4: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Xue W, Xing Y, Weng X, Zhao Y, Tang W, et al. (2008) Natural variation in Ghd7 is an important regulator of heading date and yield potential in rice. Nat Genet 40: 761–767. [DOI] [PubMed] [Google Scholar]
- 44. Lescot M, Dehais P, Thijs G, Marchal K, Moreau Y, et al. (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res 30: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Zhang H, Jin J, Tang L, Zhao Y, Gu X, et al. (2011) PlantTFDB 2.0: update and improvement of the comprehensive plant transcription factor database. Nucleic Acids Res 39: D1114–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Perez-Rodriguez P, Riano-Pachon DM, Correa LGG, Rensing SA, Kersten B, et al. (2010) PInTFDB: updated content and new features of the plant transcription factor database. Nucleic Acids Res 38: D822–D827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Mochida K, Yoshida T, Sakurai T, Yamaguchi-Shinozaki K, Shinozaki K, et al. (2011) In silico analysis of transcription factor repertoires and prediction of stress-responsive transcription factors from six major gramineae plants. DNA Res 18: 321–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yilmaz A, Nishiyama MY Jr, Fuentes BG, Souza GM, Janies D, et al. (2009) GRASSIUS: a platform for comparative regulatory genomics across the grasses. Plant Physiol 149: 171–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Gray J, Bevan M, Brutnell T, Buell CR, Cone K, et al. (2009) A recommendation for naming transcription factor proteins in the grasses. Plant Physiol 149: 4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Vij S, Gupta V, Kumar D, Vydianathan R, Raghuvanshi S, et al. (2006) Decoding the rice genome. Bioessays 28: 421–432. [DOI] [PubMed] [Google Scholar]
- 51. Paterson AH, Bowers JE, Chapman BA (2004) Ancient polyploidization predating divergence of the cereals, and its consequences for comparative genomics. Proc Natl Acad Sci U S A 101: 9903–9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Yu J, Wang J, Lin W, Li S, Li H, et al. (2005) The Genomes of Oryza sativa: a history of duplications. PLoS Biol 3: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cannon SB, Mitra A, Baumgarten A, Young ND, May G (2004) The roles of segmental and tandem gene duplication in the evolution of large gene families in Arabidopsis thaliana. BMC Plant Biol 4: 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Nijhawan A, Jain M, Tyagi AK, Khurana JP (2008) Genomic survey and gene expression analysis of the basic leucine zipper transcription factor family in rice. Plant Physiol 146: 333–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Casal JJ, Fankhauser C, Coupland G, Blázquez MA (2004) Signalling for developmental plasticity. Trends Plant Sci 9: 309–314. [DOI] [PubMed] [Google Scholar]
- 56. Santner A, Calderon-Villalobos LIA, Estelle M (2009) Plant hormones are versatile chemical regulators of plant growth. Nat Chem Biol 5: 301–307. [DOI] [PubMed] [Google Scholar]
- 57. Lau OS, Deng XW (2010) Plant hormone signaling lightens up: integrators of light and hormones. Curr Opin Plant Biol 13: 571–577. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Gene structure of each OsBBX gene. The white rectangles represent the exons and the black lines represent the introns. The UTR regions are marked as black rectangles.
(TIF)
Diurnal expression pattern of OsBBX genes from the Diurnal Project. The x-axis represents the time course over two days. The y-axis represents the average expression values obtained from the microarrays. The blue line represents the LDHC condition and the green line represents the LLHH (LDHC) condition.
(TIF)
Phylogenetic analysis of the OsBBX and AtBBX members. The unrooted tree was generated from the OsBBX and AtBBX full length protein sequences. The bootstrap values from 1000 replicates are indicated at each node. The triangles in front of the members indicate the predicted paralogous proteins. The members marked in red contain two B-box domains and one CCT domains. The scale bar represents 0.1 amino acid substitutions per site.
(PDF)
Phylogenetic analysis of the B-box TFs from rice, Arabidopsis , maize, poplar and sorghum bicolor . The sequences of the maize (Zm: Zea Mays), poplar ( Pt: Populus trichocarpa) and sorghum bicolor (Sb: Sorghum bicolor) BBX proteins were identified and downloaded from NCBI. The unrooted tree was generated from the full length protein sequences. The bootstrap values from 1000 replicates are indicated at each node. The scale bar represents 0.1 amino acid substitutions per site.
(PDF)
Detailed information of the rice samples used in the microarray analysis.
(DOC)
Primers used for the qPCR analysis.
(DOC)
Average expression signal values of the 30 OsBBX genes.
(XLS)
Results of the differential expression analysis of the OsBBX genes in the plumule and radicle under NAA, GA3 and KT treatment and light/dark regulation.
(XLS)
The positions of the cis -elements that were identified in more than ten OsBBX genes.
(XLS)