Abstract
Human papillomavirus (HPV) is a risk factor in a subset of oropharyngeal cancer; however, the contribution of HPV in the malignancy of oral squamous cell carcinomas (OSCC) is not fully understood in Taiwanese. Herein, 61 patients with no risk factors and 117 patients with one or more risk factors were enrolled in this study. HPV16/18 infection rate in non-smokers, non-drinkers and non-betel quid chewers was higher than their counterparts. The development of HPV-infected cancer has been shown to be associated with interleukin-10 (IL-10) expression. To this end, IL-10 mRNA expression in OSCC tumors was evaluated by real-time RT-PCR. Data showed that HPV-positive patients had higher IL-10 mRNA levels than in HPV-negative patients. Kaplan-Meier and Cox-regression analysis indicated that the prognostic significance of IL-10 mRNA on overall survival and relapse free survival was only observed in HPV-positive OSCC, but not in HPV-negative OSCC. Mechanistically, the elevation of IL-10 by E6 was responsible for increased colony formation and migration capability in OSCC cells. Therefore, we suggest that IL-10 induced by E6 promotes cell growth and migration capability and consequent poor survival and relapse in HPV-positive OSCC.
Introduction
Oral squamous cell carcinomas (OSCC) arise from the mucosa of the oral cavity including border of tongue, buccal cavity, palate, gingival and lip. This disease is the tenth most common cancer in the world and is the fourth most common cancer among men in Taiwan [1]. However, the disease is rare among women in Taiwan, possibly because of etiological factors such as cigarette smoking, alcohol drinking, and betel quid chewing habits that are predominately observed in men and rarely in women [2]–[4]. A subset of oropharyngeal cancer including pharynx, tonsil, and base of tongue of non-smokers and non-drinkers in the Caucasian and Chinese population has been shown to be linked to human papillomavirus (HPV) infection, but the contribution of HPV in the malignancy of oral squamous cell carcinomas (OSCC) is not fully identified in Taiwanese [5]–[15]. Therefore, we hypothesized that HPV infections might play a role in the development of OSCC in members of the Taiwanese population who do not have the habits of alcohol drinking, betel quid chewing, or cigarette smoking [16]. In addition, HPV16-positive OSCC has been shown to have a more favorable outcome than HPV16-negative OSCC [7], [17]–[23]. However, HPV16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival [24].The reason remains unclear, but may involve interactions of cytokines such as interleukin-10 (IL-10) which have been shown to associate with viral-infected cancer development [25]–[28].
IL-10 is an important immunoinhibitory cytokine that is part of a balanced cytokine network [29], [30]. It is produced by several cells including normal and neoplastic B cells, stimulated monocytes/macrophages, a subset of T cells, and some cancer cells [30]–[32]. Brook et al. showed that persistent viral infection in mice results in a significant upregulation of IL-10 by antigen-presenting cells, and leads to impaired T-cell responses [28]. Genetic removal of IL-10 resulted in the rapid elimination of the virus and the development of antiviral memory T-cell responses [28]. Therefore, IL-10 has been documented to determine in vivo virus clearance or persistent infection. Previously, IL-10 expression levels were reported as significantly higher in an HPV16-infected high grade of cervical intraepithelial neoplasia (CIN) II and III when compared to normal cervical epithelium and CIN I [33]–[35]. This suggests that IL-10 production may play a role in the progression of HPV-associated cervical precancer [34].
In the present paper, 61 patients with no risk factors and 117 patients with one or more risk factors (smoking, drinking, and betel quid chewing) were enrolled to explore: (I) whether HPV16/18 infection may be a predominant etiological factor for OSCC of non-smokers, non-drinkers, and non-betel quid chewers when compared with smokers, drinkers, and betel quid chewers; and (II) whether IL-10 expression might play a more important role in the progression of HPV16/18-positive OSCC than in HPV16/18-negative OSCC and consequently would result in poor overall survival (OS) and relapse free survival (RFS) in these patients.
Materials and Methods
Study subjects
OSCC tumor specimens were collected between 2001 and 2010 from 178 patients with primary oral cancers in the Department of Otolaryngology, Chung-Shan Medical University Hospital (Taichung, Taiwan) and the Department of Surgical Pathology, Changhua Christian Hospital (Changhua, Taiwan). This study is approved by the Institutional Review Board, Chung Shan Medical University Hospital (CSMUH No: CS11178; informed consent form: waiver). The tumor type and stage of each collected specimen were histologically determined according to the WHO classification system. Cancer relapse data were obtained by chart review and confirmed.
Cell lines
The SiHa (cervical cancer) [36], SAS, TW2.6, HSC3, GNM, SCC4, and SCC25 oral cancer cell lines were maintained in DMEM. Cell lines were kindly provided by Dr. T. C. Lee, Institute of Biomedical Sciences, Academia Sinica, Taipei, Taiwan [37]. The GNM cell line was kindly provided by Dr. M. Y. Chou, Department of Dentistry, Chung Shan Medical University, Taichung, Taiwan [38]. The medium contained 10% fetal bovine serum supplemented with penicillin (100 U/ml) and streptomycin (100 mg/ml). Cells were grown at 37°C in a humidified incubator at 5% CO2.
Nested polymerase chain reaction (Nested-PCR)
Tumor genomic DNA was extracted from the tumor portion of whole-mount paraffin sections of OSCC specimens. SiHa and HeLa cervical cancer cells were used as positive controls for the detection of HPV16 and HPV18 DNA, and PBS was used as a negative control. HPV viral DNA was first amplified with type consensus primers MY09 and MY11 followed by a second round of amplification with type-specific primers flanking the L1 region to identify the subtype. The detailed procedures were described previously [39].
Plasmid construction and transfection reaction
Different concentrations of HPV16 E6 plasmids were transiently transfected into oral cancer cells (1×106) using the TurboFect reagent (Fermentas) as described previously [40].
Silencing of HPV-18 E6 and IL-10 expression by RNA interference
The RNA interference target sequences for HPV18 E6 shRNA have been previously verified [41]. IL-10 shRNA was purchased from National RNAi Core Facility, Academia Sinica, Taiwan. Detailed procedures were described previously [40].
RNA isolation and real-time RT-PCR
Total RNA was extracted from the tumor portions of whole-mount paraffin sections of OSCC specimens, by TRIzol reagent chloroform extraction and isopropanol precipitation. Total RNA from tumor tissues was transcribed using a High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, ABI) to obtain a cDNA library [40]. Real-time RT-PCR primers were as follows: for IL-10 transcripts, 5′-GGCGCTGTCATCGATTTCTT-3′ (forward) and 5′-TGGAGCTTATTAAAGGCAT -TCTTCAC-3′ (reverse); for 18S gene transcripts, 5′-TCGGAACTGAGGCC -ATGA-3′ (forward) and 5′-CCGGTCGGCATCGTTTA-3′ (reverse), and the reactions were performed as described previously [40], [41]. The PCR product of IL-10 was confirmed by direct sequencing.
Protein extraction and Western blot
Anti-IL10 (E-10; Santa Cruz Biotechnology), anti-HPV16 E6 (N-17; Santa Cruz Biotechnology) and anti- HPV18 E6 (G-7; Santa Cruz Biotechnology) primary antibody were used in western blot. The procedures were described previously [41].
Colony formation and migration assay
Transfected cells were seeded into 6-well plate (100 cell per well) and, then, fixed and stained with 10% Geimsa after a ten-day colony formation period. Cell migration ability was measured by Boyden chamber assay with an 8 µm pore polycarbonate membrane, according to the manufacturer's protocol. Transfected cells were fixed and stained with crystal violet after a 24-hour migration period.
Immunohistochemical staining
Immunohistochemical staining to evaluate p16 expression in tumor tissue was performed on whole-mount paraffin sections of OSCC specimens. Anti-p16 (1/200) polyclonal primary antibody (Santa Cruz Biotechnology, USA) was used. An immunohistochemistry (IHC) detection kit for in vitro diagnostic use (Invitrogen, USA) was used according to the standard protocol. IHC staining scores were defined as previously described [40].
Statistical analysis
The Student's t-test and the Chi-square test were applied for continuous or discrete data analysis, respectively. The associations between IL-10 expression and patient survival were estimated using the Kaplan-Meier method and assessed using the log-rank test. Potential confounders including gender, age and stage were adjusted by Cox regression models, with IL-10 expression fitted as indicator variables. Interaction was further assessed using the likelihood ratio test to calculate χ2 and p values. In the test for interaction, the Cox regression model with only main effects was compared to that with both main effect terms and interaction term. Interaction effect was defined as the difference of their deviance All statistical analyses were conducted using the SPSS statistical software program (version 13.0) (SPSS, Inc., Chicago, IL). All statistical testing was performed using two-sided tests and P values<0.05 were considered to be statistically significant.
Results
HPV 16/18 infections in Taiwanese OSCC patients are more common in females, non-smokers, non-drinkers, and non-betel quid chewers
We explored whether HPV16/18 infections in OSCC are more prevalent in non-smokers, non-drinkers, and non-betel quid chewers in Taiwan. A total of 178 OSCC patients were enrolled to determine the type of HPV16 and HPV18 DNA by nested PCR. As shown in Table 1, HPV16, HPV18, and combined HPV16/18 (HPV16/18) infection rates in females, non-smokers, non-drinkers, and non-betel quid chewers were higher than in males, smokers, drinkers, and betel quid chewers. HPV16 and HPV16/18 infections were more common in patients with OSCC of the tongue than in OSCC of the buccal or other areas (e.g., palate, gingiva, and lip). Surprisingly, a more than 70% HPV16/18 infection was found in females, non-smokers, and non-betel quid chewers. This finding suggests that HPV16/18 infections may play a crucial role in cancer development in this subset of OSCC patients.
Table 1. Relationships between HPV16, HPV18 and HPV16/18 infection and clinical parameters in OSCC patients.
| Parameters | Case No. | HPV 16 | HPV 18 | HPV 16/18 |
| Age | ||||
| <56 | 105 | 30 (28.6) | 30 (28.6) | 50 (47.6) |
| ≥56 | 73 | 24 (32.9) | 24 (32.9) | 44 (60.3) |
| Gender | ||||
| Female | 70 | 34 (48.6)* | 31 (44.3)* | 56 (80.0)* |
| Male | 108 | 20 (18.5) | 23 (21.3) | 38 (35.2) |
| Smoking | ||||
| No | 73 | 33 (45.2)* | 30 (41.1)* | 53 (72.6)* |
| Yes | 105 | 21 (20.0) | 24 (22.9) | 41 (39.0) |
| Drinking | ||||
| No | 99 | 35 (35.4) | 39 (39.4)* | 64 (64.6)* |
| Yes | 79 | 19 (24.1) | 15 (19.0) | 30 (38.0) |
| Betel quid | ||||
| No | 80 | 33 (41.3)* | 36 (45.0)* | 60 (75.0)* |
| Yes | 98 | 21 (21.4) | 18 (18.4) | 34 (34.7) |
| Stage | ||||
| I+II | 103 | 31 (30.1) | 26 (25.2) | 50 (48.5) |
| III+IV | 75 | 23 (30.7) | 28 (37.3) | 44 (58.7) |
| T value | ||||
| T1+T2 | 135 | 40 (29.6) | 40 (29.6) | 69 (51.1) |
| T3+T4 | 43 | 14 (32.6) | 14 (32.6) | 25 (58.1) |
| N value | ||||
| N0 | 128 | 39 (30.5) | 34 (26.6) | 64 (50.0) |
| N1+N2 | 50 | 15 (30.0) | 20 (40.0) | 30 (60.0) |
| Tumor site | ||||
| Tongue | 92 | 35 (38.0)* | 35 (38.0) | 60 (65.2)* |
| Buccal | 68 | 17 (25.0) | 15 (22.1) | 28 (41.2) |
| Other1 | 18 | 2 (11.1) | 4 (22.2) | 6 (33.3) |
| IL10 | ||||
| Low | 89 | 18 (20.2)* | 21 (23.6)# | 34 (38.2)* |
| High | 89 | 36 (40.4) | 33 (37.1) | 60 (67.4) |
Other tumor sites includes plate (n = 1), gingival (n = 8), and lip (n = 9).
p<0.05;
p = 0.05.
P16 expression has been documented as a surrogate marker of HPV infection [5]. In the present study, 147 out of 178 OSCC paraffin sections were available for evaluation of p16 expression by immunohistochemical analysis. A prevalence of OSCC with p16-positive immunostaining was found and positive staining was significantly higher in HPV16, HPV18, and HPV16/18-positive tumors compared with their counterparts (Table S1). This result seems to confirm the detection of HPV16/18 DNA in OSCC by nested PCR in this study.
Higher IL-10 mRNA expression levels are seen in HPV16, HPV18, and HPV16/18-positive OSCCs than in HPV-negative OSCCs
A link has been shown between IL-10 expression and HPV-infected CIN progression [35]. Therefore, we expected to find differential IL-10 mRNA expression levels between HPV-positive and -negative OSCC. The IL-10 mRNA expression levels in OSCC, evaluated by real-time RT-PCR, ranged from 0∼1402.2. A median value of 2.38 was used as a cut-off point to divide OSCC into low and high groups (low: 0.6±0.8, 0.0∼2.38; High: 237.0±413.5, 2.39∼1402.2). The IL-10 mRNA expression levels were not associated with any clinico-pathological parameters except for age: the prevalence of high IL-10 mRNA was greater in older patients (>56 years of age) than in younger patients (58.9% vs. 43.8%, P = 0.048; Table S2). Interestingly, the prevalence of high IL-10 mRNA was also greater in OSCC patients with HPV16, HPV18, and HPV16/18 infections than without HPV16, HPV18, and HPV16/18 infections. The odds ratios of HPV16-, HPV18-, and HPV16/18-positive OSCC were 2.679, 1.908, and 3.347, respectively, when compared with HPV16-, HPV18-, and HPV16/18-negative OSCC (Table 2). These results suggest that HPV16/18 infection could elevate IL-10 transcription in OSCC patients.
Table 2. Comparison of IL-10 mRNA expression levels between HPV-positive and HPV–negative OSCC patients.
| IL-10 mRNA | |||||
| HPV type | Case No. | Low (%) | High (%) | Odds Ratio (95% CI) | P value |
| HPV 16 | |||||
| Negative | 124 | 71 (57.3) | 53 (42.7) | Referent | |
| Positive | 54 | 18 (33.3) | 36 (66.7) | 2.679 (1.373–5.227) | 0.003 |
| HPV 18 | |||||
| Negative | 124 | 68 (54.8) | 56 (45.2) | Referent | |
| Positive | 54 | 21 (38.9) | 33 (61.1) | 1.908 (0.995–3.661) | 0.050 |
| HPV 16/18 | |||||
| Negative | 84 | 55 (65.5) | 29 (34.5) | Referent | |
| Positive | 94 | 34 (36.2) | 60 (63.8) | 3.347 (1.808–6.196) | <0.001 |
Fourteen of 178 patients had both HPV16 and 18 infections.
IL-10 low: 0.6±0.8 (0.0–2.38); IL-10 high: 237.0±413.5 (2.39–1402.2).
IL-10 mRNA expression predicts OS and RFS in HPV-positive OSCC, but not in HPV-negative OSCC
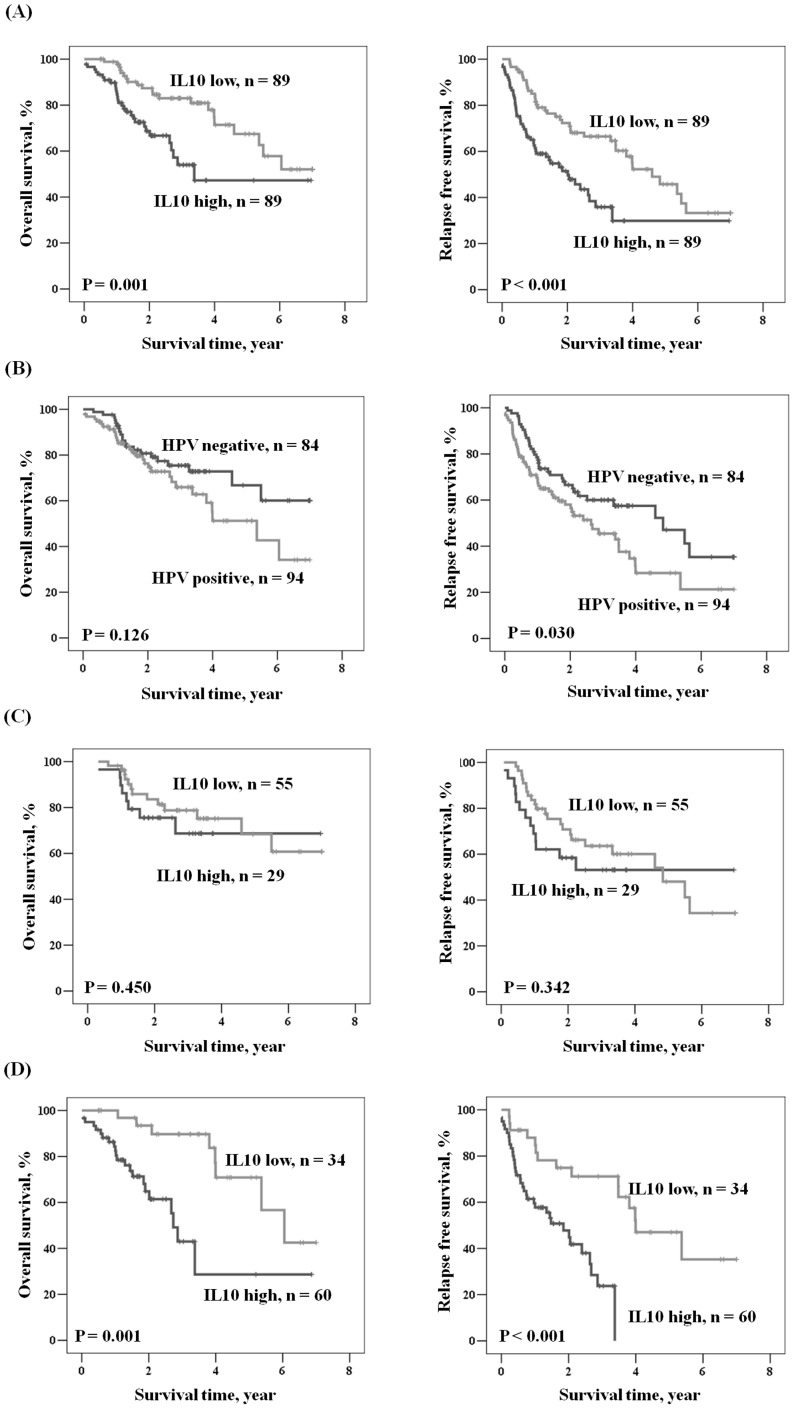
IL-10 has been shown to promote tumor progression via suppressed tumor immune surveillance [25], [32]. We therefore expected that IL-10 might be associated with the clinical outcome in HPV-positive OSCC. Kaplan-Meier analysis showed that patients with high IL-10 mRNA had shorter OS and RFS periods than did those with low IL-10 mRNA (P = 0.001 for OS; P<0.001 for RFS; Fig. 1A). Patients with HPV-positive tumors had shorter RFS when compared with those with HPV-negative tumors (P = 0.030), but a prognostic significance of HPV was not observed for OS (P = 0.126; Fig. 1B). More interestingly, the prognostic significance of IL-10 mRNA for OS and RFS was only shown in HPV-positive OSCC patients (Fig. 1D; P = 0.001 for OS; P<0.001 for RFS), but not in HPV-negative OSCC patients (Fig. 1C). Multivariate logistic regression analysis further indicated that patients with high IL-10 mRNA had hazard ratios (HR) of 2.438 and 2.098 for OS and RFS, respectively, when compared with patients with low IL-10 mRNA (95% CI, 1.345–4.419, P = 0.003 for OS; 95% CI, 1.329–3.311, P = 0.001 for RFS; Table 3). The five-year survival rate in patients with high IL-10 mRNA was lower than for patients with low IL-10 mRNA (47.2% vs. 67.4% for OS; 29.9% vs. 45.7% for RFS). Among HPV-positive OSCC patients, those with high IL-10 mRNA had HR of 4.810 and 3.807 for OS and RFS, respectively, when compared with patients with low IL-10 mRNA (95% CI, 1.946–11.892 for OS; 95% CI, 1.771–8.184 for RFS; Table 3). The five-year survival rate was significantly lower in patients with high IL-10 mRNA than with low IL-10 mRNA (28.7% vs. 70.8% for OS, P = 0.001; 0.0% vs. 47.0% for RFS, P = 0.001). As expected, we did not observe any prognostic significance of IL-10 mRNA in the entire study population or in HPV-negative patients (Table 3).
Figure 1. Kaplan-Meier analysis was used to assess the influence of IL-10 on OS and RFS.
(A) in all study population, (B) in patients with HPV 16/18 infection compared to those without HPV 16/18 infection, (C) in patients with HPV 16/18 non-infection, and (D) in patients with HPV16/18 infection.
Table 3. Multivariate analysis of the influence of HPV 16/18 expression and IL-10 mRNA expression on overall survival (OS) and relapse free survival (RFS) in oral cancer patients.
| Parameter | Case No. | OS | RFS | ||||||
| 5-year survival (%) | HR | 95% CI | P | 5-year survival (%) | HR | 95%CI | P | ||
| IL-10 | |||||||||
| Low | 89 | 67.4 | 1.000 | Referent | 45.7 | 1.000 | Referent | ||
| High | 89 | 47.2 | 2.438 | 1.345–4.419 | 0.003 | 29.9 | 2.098 | 1.329–3.311 | 0.001 |
| HPV | |||||||||
| Negative | 84 | 72.6 | 1.000 | Referent | 49.2 | 1.000 | Referent | ||
| Positive | 94 | 46.3 | 1.765 | 0.790–3.945 | 0.166 | 27.2 | 1.604 | 0.847–3.038 | 0.147 |
| HPV negative | |||||||||
| IL-10 Low | 55 | 68.4 | 1.000 | Referent | 48.0 | 1.000 | Referent | ||
| IL-10 High | 29 | 68.7 | 1.323 | 0.521–3.361 | 0.556 | 53.1 | 1.237 | 0.610–2.507 | 0.555 |
| HPV positive | |||||||||
| IL-10 Low | 34 | 70.8 | 1.000 | Referent | 47.0 | 1.000 | Referent | ||
| IL-10 High | 60 | 28.7 | 4.810 | 1.946–11.892 | 0.001 | 0.0 | 3.807 | 1.771–8.184 | 0.001 |
The interaction between HPV and IL-10: χ2 = 2.675, df = 1, P = 0.102 for OS; χ2 = 2.781, df = 1, P = 0.095 for RFS.
A prognostic significance of IL-10 mRNA on OS and RFS was shown in female OSCC patients and OSCC patients who were non-smokers, non-drinkers, and non-betel quid chewers
As mentioned above, HPV infection and high IL-10 mRNA levels were more common in females, non-smokers, non-drinkers, and non-betel quid chewers. In addition, patients with HPV infection had higher levels of IL-10 mRNA expression than did patients without HPV infection. We therefore expected that the impact of IL-10 mRNA on OS and RFS would be predominantly observed in females, non-smokers, non-drinkers, and non-betel quid chewers. Multivariate logistic regression analysis indicated that the impact of IL-10 mRNA on both OS and RFS was indeed revealed in females, non-smokers, non-drinkers, and non-betel quid chewers, and not in males, smokers, drinkers, and betel-quid chewers (Table 4). The prognostic value of IL-10 mRNA on OS was also seen in males and smokers (HR, 2.14, 95% CI, 1.002–4.571, P = 0.049 for male; HR, 2.497, 95% CI, 1.160–5.373, P = 0.019 for smokers; Table 4). However, the HR value for OS and RFS in female and nonsmokers was higher than in male and smokers (Table 4). Therefore, these results suggest that the level of IL-10 mRNA expression is more feasible predictor of survival and relapse in female OSCC patients and in OSCC patients who are non-smokers, non-drinkers, and non-betel quid chewers.
Table 4. Multivariate analysis of the influence of various parameters on overall survival (OS) and relapse free survival (RFS) in oral cancer patients according to gender.
| Parameter | Category | Case No. | OS | RFS | ||||||
| 5-year survival (%) | HR | 95% CI | P | 5-year survival (%) | HR | 95%CI | P | |||
| Female | ||||||||||
| Stage | III+IV/I+II | 25/45 | 36.3/72.0 | 3.393 | 1.389–8.289 | 0.007 | 10.6/58.3 | 2.844 | 1.441–5.614 | 0.003 |
| HPV | Positive/Negative | 56/14 | 51.9/83.9 | 1.715 | 0.484–6.081 | 0.404 | 26.9/84.6 | 3.764 | 1.111–12.749 | 0.033 |
| IL-10 | High/Low | 38/32 | 24.5/76.3 | 3.610 | 1.345–9.689 | 0.011 | 0.0/65.0 | 9.258 | 3.272–26.196 | <0.001 |
| Male | ||||||||||
| Stage | III+IV/I+II | 50/58 | 22.7/81.9 | 3.707 | 1.622–8.471 | 0.002 | 0.0/60.3 | 4.017 | 2.112–7.641 | <0.001 |
| HPV | Positive/Negative | 38/70 | 52.3/62.2 | 1.170 | 0.553–2.475 | 0.682 | 32.3/37.5 | 1.020 | 0.563–1.847 | 0.948 |
| IL-10 | High/Low | 51/57 | 60.5/60.4 | 2.140 | 1.002–4.571 | 0.049 | 51.5/30.7 | 1.309 | 0.741–2.314 | 0.354 |
| Non-smoker | ||||||||||
| Stage | III+IV/I+II | 24/49 | 35.8/74.0 | 2.791 | 1.108–7.031 | 0.029 | 5.9/58.5 | 2.459 | 1.200–5.036 | 0.014 |
| HPV | Positive/Negative | 53/20 | 53.8/77.9 | 0.732 | 0.213–2.517 | 0.620 | 27.9/63.8 | 0.988 | 0.376–2.596 | 0.981 |
| IL-10 | High/Low | 39/34 | 32.1/75.9 | 3.121 | 1.011–9.635 | 0.048 | 0.0/60.6 | 5.894 | 2.081–16.695 | 0.001 |
| Smoker | ||||||||||
| Stage | III+IV/I+II | 51/54 | 30.2/81.8 | 3.953 | 1.671–9.351 | 0.002 | 10.9/69.2 | 3.460 | 1.816–6.592 | <0.001 |
| HPV | Positive/Negative | 64/41 | 50.0/71.1 | 1.279 | 0.595–2.748 | 0.528 | 31.1/51.9 | 1.121 | 0.612–2.052 | 0.712 |
| IL-10 | High/Low | 50/55 | 56.1/55.1 | 2.497 | 1.160–5.373 | 0.019 | 46.8/31.9 | 1.428 | 0.803–2.539 | 0.225 |
| Non-drinker | ||||||||||
| Stage | III+IV/I+II | 38/61 | 39.8/78.9 | 4.206 | 1.828–9.679 | 0.001 | 13.8/66.9 | 3.692 | 1.994–6.841 | <0.001 |
| HPV | Positive/Negative | 64/35 | 55.9/77.1 | 1.335 | 0.480–3.711 | 0.579 | 33.6/61.5 | 1.290 | 0.614–2.710 | 0.502 |
| IL-10 | High/Low | 47/52 | 45.5/75.9 | 2.995 | 1.292–6.939 | 0.011 | 23.5/59.0 | 2.643 | 1.389–5.029 | 0.003 |
| Drinker | ||||||||||
| Stage | III+IV/I+II | 37/42 | 21.9/76.4 | 2.902 | 1.178–7.151 | 0.021 | 0.0/33.1 | 2.952 | 1.434–6.075 | 0.003 |
| HPV | Positive/Negative | 30/49 | 57.4/53.1 | 1.083 | 0.437–2.686 | 0.864 | 12.8/18.1 | 1.252 | 0.608–2.578 | 0.542 |
| IL-10 | High/Low | 42/37 | 52.7/48.7 | 1.760 | 0.721–4.297 | 0.215 | 41.4/13.5 | 1.397 | 0.696–2.806 | 0.347 |
| Non-betel quid chewer | ||||||||||
| Stage | III+IV/I+II | 34/46 | 34.3/64.2 | 3.523 | 1.446–8.583 | 0.006 | 11.0/64.7 | 2.911 | 1.456–5.818 | 0.003 |
| HPV | Positive/Negative | 60/20 | 51.3/68.0 | 0.598 | 0.192–1.864 | 0.376 | 27.3/70.9 | 1.238 | 0.459–3.341 | 0.673 |
| IL-10 | High/Low | 40/40 | 25.9/70.2 | 5.395 | 1.886–15.431 | 0.002 | 0.0/58.1 | 7.002 | 2.640–18.571 | <0.001 |
| Betel quid chewer | ||||||||||
| Stage | III+IV/I+II | 41/57 | 27.3/81.6 | 3.118 | 1.322–7.356 | 0.009 | 0.0/54.6 | 3.459 | 1.819–6.579 | <0.001 |
| HPV | Positive/Negative | 34/64 | 60.4/63.8 | 1.274 | 0.552–2.942 | 0.570 | 41.2/35.7 | 1.160 | 0.609–2.212 | 0.650 |
| IL-10 | High/Low | 49/49 | 61.6/64.9 | 1.871 | 0.815–5.296 | 0.140 | 48.2/30.8 | 1.075 | 0.582–1.986 | 0.816 |
Elevation of IL-10 expression by HPV16/18 E6 may be responsible for colony formation and migration capability in oral cancer cells
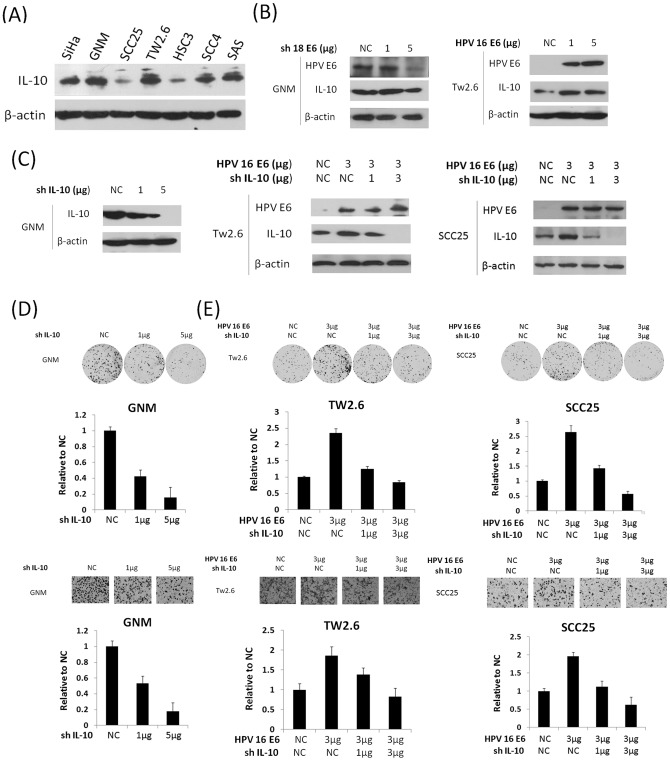
As mentioned above, the prognostic significance of IL-10 mRNA expression was only observed in HPV-positive OSCC, not in HPV-negative OSCC. Therefore, we hypothesized that elevation of IL-10 by HPV infection could promote cell growth rate and migration capability. Western blotting analysis showed that HPV18-infected OSCC (GNM) had the highest IL-10 expression among all OSCC cells tested (Fig. 2A). The relatively low IL-10 expression in HPV16-infected SiHa cervical cancer cells was used as a positive control (Fig. 2A). To verify whether IL-10 could be elevated by HPV16/18 E6 oncoprotein, HPV18 E6 of GNM cells were knocked down by a small hairpin RNA (shRNA) (Fig. 2B). We also overexpressed an HPV16 E6 cDNA plasmid in HPV-negative TW2.6 and SCC25 cells (Fig. 2B). Western blotting analysis showed that IL-10 expression was decreased by E6-knockdown in GNM cells and increased by E6-overexpression in TW2.6 and SCC25 cells (Fig. 2B). Additionally, IL-10 expression was decreased by HPV16 or18 E6-knockdown in SiHa or HeLa cervical cancer cells and increased by HPV16 E6 overexpression C33A cervical cancer cells (Figure S1). These results suggest that HPV16/18 E6 infection may not only elevate IL-10 expression in OSCC cells but also observed in cervical cancer cells.
Figure 2. IL-10 expression increases associated with HPV16/18 E6 is responsible for the migration capability of OSCC cell lines.
(A) IL-10 expression in a panel of OSCC cell lines was evaluated by Western blotting; (B) GNM cells were knocked down by transfection of shHPV18 E6 and Tw2.6 cells were transiently transfected with HPV16 E6 cDNA plasmid for 48 hrs. β-actin was used as a protein loading control; (C) GNM cells were knocked down by transfection of shIL-10. Tw2.6 and SCC25 cells were co-transfected with HPV16 E6 cDNA plasmid, and then E6 and IL-10 expression were determined by Western blotting. β-actin was used as a protein loading control; (D) The migration and colony formation capability of GNM cells with or without transfection of shIL-10 was evaluated by transwell migration assay; and (E) E6 transfected-Tw2.6 and -SCC25 cells were co-transfected with two doses of shIL-10, and then the migration and colony formation capability of both cells was evaluated by transwell migration assay.
We next questioned whether IL-10 might be responsible for OSCC cell growth and migration capability. The colon formation and Boyden chamber assays indicated that the colony formation and migration capability decreased markedly in IL-10 knockdown GNM cells (upper panel for colony formation; lower panel for migration; Fig. 2C and 2D). In addition, the colony formation and migration capability was elevated by E6-overexpression in TW2.6 and SCC25 cells were eliminated by IL-10 knockdown (upper panel for colony formation; lower panel for migration; Fig. 2C and 2E). The representative colony formation and migration cell numbers with different treatments were shown in Figure 2D and 2E. The results from the cell model experiment may partially explain the observation that OSCC patients with high IL-10 expression had poorer survival and greater relapse with HPV-positive OSCC.
Discussion
Our present study supports the finding that HPV-positive OSCC is a cancer that is distinct from HPV-negative OSCC; this distinction arises due to the association of HPV-negative OSCC with different etiological factors including cigarette smoking, alcohol drinking, and betel quid chewing. HPV16 has been considered as the predominant HPV subtype involved in oropharyngeal cancer [9], [10], [12]–[16], [27]. However, the infection rates for HPV18 were the same as for HPV16 (30.3%) in this study population (Table 1). More interestingly, HPV16 or HPV18 infection in these patients was exclusive because fewer than 10% patients (14 out of 178) had both types of HPV infection. Therefore, we speculated that HPV16 or HPV18 had an equal involvement in OSCC in the subset of Taiwanese patients.
HPV-infected oropharyngeal cancer has been shown to be more prevalent at the base of the tongue, in the pharynx, and in the tonsils, and less prevalent at the mobile or border of the tongue and buccal area [12], [16], [27], [33]. This phenomenon was explained by sexual behavior or marijuana use [42], [43]. However, the oral sexual behavior and marijuana use were seldom observed in the Taiwanese population [44], [45]. The infection rate of HPV16/18 was 65.2% and 41.2% for OSCC of tongue and buccal cavity, respectively (Table 1). This finding suggests that HPV16/18 infection may play an important role in patients with OSCC of tongue and buccal cavity who are non-smokers, non-drinkers, and non-betel quid chewers.
A high prevalence of OSCC patients who were females, non-smokers, non-drinkers, and non-betel quid chewers was noted in this study when compared with previous Taiwanese OSCC reports. This study population was similar to those reported previously that showed that the majority of Taiwanese male OSCC patients had one or more habit of cigarette smoking, alcohol drinking, and/or betel quid chewing (∼90%; Table S3). Interestingly, HPV-positive OSCC was more common in females than in males, which revealed that the existence of a different etiological profile between genders. The majority of female OSCC patients were non-smokers (84.9%), non-drinkers (62.6%), and non-betel quid chewers (82.5%); conversely, most male OSCC patients were smokers (92.4%), drinkers (89.9%), and betel quid chewers (95.9%).
Previous reports from the US and Sweden have also shown that HPV-positive OSCC in males was more common than in females [46]. However, in the present study, HPV-positive OSCC was more prevalent in females than in males. Therefore, our finding strongly supports a previous report that indicated a distinct risk factor profile for patients with HPV-positive OSCC when compared with HPV-negative OSCC patients.
A large body of evidence now reveals that HPV-positive OSCC has a more favorable outcome when compared with HPV-negative OSCC [7], [17]–[23]. However, HPV infection was not associated with patient outcomes in the present study population. Moreover, there is no difference in clinical outcome between HPV16- and HPV18-positive OSCC patients (p = 0.461 for OS; p = 0.403 for RFS). In current study, p16 expression used as a surrogate marker of HPV infection had a consistent prognostic value of previous reports [6], [16], [47], [48] showing that patients with p16 negative tumors had poorer outcome than those with p16-positive tumors (Table S4). This could be due to the bias of the study population, as our patients had one or more risk factors of cigarette smoking, alcohol drinking, and betel quid chewing. In the present study, 61 out of 178 patients (57 female and 4 male) had no risk factors for OSCC. To the best of our knowledge, this is the first study to use a similar number of risk and non-risk patients in a study population to investigate whether HPV-positive OSCC could be a distinct disease from the HPV-negative OSCC that is more common in smokers and drinkers.
HPV infection has been considered to be a favorable prognostic factor for OSCC, but no molecular evidence has yet supported this observation [7], [17]–[23]. In the present study, we provide in vitro cellular and in vivo human tumor evidence to indicate that IL-10 mRNA expression levels may independently predict the survival and relapse rates in patients with HPV-positive OSCC, but not in those with HPV-negative OSCC. This finding not only supports HPV-positive OSCC as a different disease from HPV-negative OSCC, but it also reveals that IL-10 may play a crucial role in the progression of HPV-positive OSCC.
It is conceivable that IL-10 may suppress T cell immunity, thereby resulting in a persistent HPV infection [2], [3], [32]. Consequently, the E6 oncoprotein will be expressed when HPV DNA becomes integrated into the host chromosome. The cell model data further showed that IL-10 expression elevated by E6 was responsible for colony formation and migration of OSCC cells. Promotion of lung cancer cell invasion by IL-10 was also reported previously [49]. Therefore, a positive feedback loop of IL-10 expression may contribute to HPV-positive OSCC progression.
Supporting Information
IL-10 expression was decreased by HPV16/18 E6-knockdown in SiHa or HeLa cells and increased by HPV16 E6 overexpression C33A cells. SiHa cells were knocked down by transfection of shHPV16 E6 and HeLa cells were knocked down by transfection of shHPV18 E6. C33A cells were transfected with HPV16 E6 cDNA plasmid. HPV16/18 E6 and IL-10 expression was determined by Western blotting. β-actin was used as a protein loading control.
(TIFF)
Relationships between HPV infection and p16 expression in oral cancer patients.
(DOC)
Relationships between IL-10 mRNA and clinical parameters in oral cancer patients.
(DOC)
Relationships between gender and parameters in oral cancer patients.
(DOC)
Multivariate analysis of the influence of HPV 16/18 expression and p16 expression on overall survival (OS) and relapse free survival (RFS) in oral cancer patients.
(DOC)
Funding Statement
This work was jointly supported by grants from the Chung Shan Medical University Hospital (CSH-2010-C-023). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. No additional external funding sources for this study.
References
- 1. Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69–90. [DOI] [PubMed] [Google Scholar]
- 2. Chung CH, Yang YH, Wang TY, Shieh TY, Warnakulasuriya S (2005) Oral precancerous disorders associated with areca quid chewing, smoking, and alcohol drinking in southern Taiwan. J Oral Pathol Med 34: 460–466. [DOI] [PubMed] [Google Scholar]
- 3. Lee CH, Ko YC, Huang HL, Chao YY, Tsai CC, et al. (2003) The precancer risk of betel quid chewing, tobacco use and alcohol consumption in oral leukoplakia and oral submucous fibrosis in southern Taiwan. Br J Cancer 88: 366–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Chen PC, Kuo C, Pan CC, Chou MY (2002) Risk of oral cancer associated with human papillomavirus infection, betel quid chewing, and cigarette smoking in Taiwan–an integrated molecular and epidemiological study of 58 cases. J Oral Pathol Med 31: 317–322. [DOI] [PubMed] [Google Scholar]
- 5. Kreimer AR, Clifford GM, Boyle P, Franceschi S (2005) Human papillomavirus types in head and neck squamous cell carcinomas worldwide: a systematic review. Cancer Epidemiol Biomarkers Prev 14: 467–475. [DOI] [PubMed] [Google Scholar]
- 6. Marur S, D'Souza G, Westra WH, Forastiere AA (2010) HPV-associated head and neck cancer: a virus-related cancer epidemic. Lancet Oncol 11: 781–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ringstrom E, Peters E, Hasegawa M, Posner M, Liu M, et al. (2002) Human papillomavirus type 16 and squamous cell carcinoma of the head and neck. Clin Cancer Res 8: 3187–3192. [PubMed] [Google Scholar]
- 8. Lopes V, Murray P, Williams H, Woodman C, Watkinson J, et al. (2011) Squamous cell carcinoma of the oral cavity rarely harbours oncogenic human papillomavirus. Oral Oncol 47: 698–701. [DOI] [PubMed] [Google Scholar]
- 9. Gillison ML, Broutian T, Pickard RK, Tong ZY, Xiao W, et al. (2012) Prevalence of oral HPV infection in the United States, 2009–2010. JAMA 307: 693–703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Reddout N, Christensen T, Bunnell A, Jensen D, Johnson D, et al. (2007) High risk HPV types 18 and 16 are potent modulators of oral squamous cell carcinoma phenotypes in vitro. Infect Agent Cancer 2: 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gillison ML, Shah KV (2003) Chapter 9: Role of mucosal human papillomavirus in nongenital cancers. J Natl Cancer Inst Monogr 57–65. [DOI] [PubMed] [Google Scholar]
- 12. D'Souza G, Kreimer AR, Viscidi R, Pawlita M, Fakhry C, et al. (2007) Case-control study of human papillomavirus and oropharyngeal cancer. N Engl J Med 356: 1944–1956. [DOI] [PubMed] [Google Scholar]
- 13. Dai M, Clifford GM, le Calvez F, Castellsague X, Snijders PJ, et al. (2004) Human papillomavirus type 16 and TP53 mutation in oral cancer: matched analysis of the IARC multicenter study. Cancer Res 64: 468–471. [DOI] [PubMed] [Google Scholar]
- 14. Ha PK, Pai SI, Westra WH, Gillison ML, Tong BC, et al. (2002) Real-time quantitative PCR demonstrates low prevalence of human papillomavirus type 16 in premalignant and malignant lesions of the oral cavity. Clin Cancer Res 8: 1203–1209. [PubMed] [Google Scholar]
- 15. Zhu C, Ling Y, Dong C, Zhou X, Wang F (2012) The relationship between oral squamous cell carcinoma and human papillomavirus: a meta-analysis of a Chinese population (1994–2011). PLoS One 7: e36294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen YW, Kao SY, Yang MH (2012) Analysis of p16(INK4A) expression of oral squamous cell carcinomas in Taiwan: Prognostic correlation without relevance to betel quid consumption. J Surg Oncol [DOI] [PubMed] [Google Scholar]
- 17. Dahlgren L, Dahlstrand HM, Lindquist D, Hogmo A, Bjornestal L, et al. (2004) Human papillomavirus is more common in base of tongue than in mobile tongue cancer and is a favorable prognostic factor in base of tongue cancer patients. Int J Cancer 112: 1015–1019. [DOI] [PubMed] [Google Scholar]
- 18. Chien CY, Su CY, Fang FM, Huang HY, Chuang HC, et al. (2008) Lower prevalence but favorable survival for human papillomavirus-related squamous cell carcinoma of tonsil in Taiwan. Oral Oncol 44: 174–179. [DOI] [PubMed] [Google Scholar]
- 19. Licitra L, Perrone F, Bossi P, Suardi S, Mariani L, et al. (2006) High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal squamous cell carcinoma. J Clin Oncol 24: 5630–5636. [DOI] [PubMed] [Google Scholar]
- 20. Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, et al. (2010) Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 363: 24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Gillison ML (2006) Human papillomavirus and prognosis of oropharyngeal squamous cell carcinoma: implications for clinical research in head and neck cancers. J Clin Oncol 24: 5623–5625. [DOI] [PubMed] [Google Scholar]
- 22. Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, et al. (2000) Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst 92: 709–720. [DOI] [PubMed] [Google Scholar]
- 23. Chaturvedi AK, Engels EA, Anderson WF, Gillison ML (2008) Incidence trends for human papillomavirus-related and -unrelated oral squamous cell carcinomas in the United States. J Clin Oncol 26: 612–619. [DOI] [PubMed] [Google Scholar]
- 24. Lee LA, Huang CG, Liao CT, Lee LY, Hsueh C, et al. (2012) Human papillomavirus-16 infection in advanced oral cavity cancer patients is related to an increased risk of distant metastases and poor survival. PLoS One 7: e40767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vicari AP, Trinchieri G (2004) Interleukin-10 in viral diseases and cancer: exiting the labyrinth? Immunol Rev 202: 223–236. [DOI] [PubMed] [Google Scholar]
- 26. Shrestha S, Wang C, Aissani B, Wilson CM, Tang J, et al. (2007) Interleukin-10 gene (IL10) polymorphisms and human papillomavirus clearance among immunosuppressed adolescents. Cancer Epidemiol Biomarkers Prev 16: 1626–1632. [DOI] [PubMed] [Google Scholar]
- 27. Brooks DG, Ha SJ, Elsaesser H, Sharpe AH, Freeman GJ, et al. (2008) IL-10 and PD-L1 operate through distinct pathways to suppress T-cell activity during persistent viral infection. Proc Natl Acad Sci U S A 105: 20428–20433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, et al. (2006) Interleukin-10 determines viral clearance or persistence in vivo. Nat Med 12: 1301–1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Saraiva M, O'Garra A (2010) The regulation of IL-10 production by immune cells. Nat Rev Immunol 10: 170–181. [DOI] [PubMed] [Google Scholar]
- 30. O'Garra A, Vieira P (2007) T(H)1 cells control themselves by producing interleukin-10. Nat Rev Immunol 7: 425–428. [DOI] [PubMed] [Google Scholar]
- 31. Mosser DM, Zhang X (2008) Interleukin-10: new perspectives on an old cytokine. Immunol Rev 226: 205–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kim R, Emi M, Tanabe K, Arihiro K (2006) Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer Res 66: 5527–5536. [DOI] [PubMed] [Google Scholar]
- 33. El-Sherif AM, Seth R, Tighe PJ, Jenkins D (2001) Quantitative analysis of IL-10 and IFN-gamma mRNA levels in normal cervix and human papillomavirus type 16 associated cervical precancer. J Pathol 195: 179–185. [DOI] [PubMed] [Google Scholar]
- 34. Mindiola R, Caulejas D, Nunez-Troconis J, Araujo M, Delgado M, et al. (2008) Increased number of IL-2, IL-2 receptor and IL-10 positive cells in premalignant lesions of the cervix. Invest Clin 49: 533–545. [PubMed] [Google Scholar]
- 35. Syrjanen S, Naud P, Sarian L, Derchain S, Roteli-Martins C, et al. (2009) Immunosuppressive cytokine Interleukin-10 (IL-10) is up-regulated in high-grade CIN but not associated with high-risk human papillomavirus (HPV) at baseline, outcomes of HR-HPV infections or incident CIN in the LAMS cohort. Virchows Arch 455: 505–515. [DOI] [PubMed] [Google Scholar]
- 36. Cheng YW, Wu TC, Chen CY, Chou MC, Ko JL, et al. (2008) Human telomerase reverse transcriptase activated by E6 oncoprotein is required for human papillomavirus-16/18-infected lung tumorigenesis. Clin Cancer Res 14: 7173–7179. [DOI] [PubMed] [Google Scholar]
- 37. Sanjiv K, Su TL, Suman S, Kakadiya R, Lai TC, et al. (2012) The novel DNA alkylating agent BO-1090 suppresses the growth of human oral cavity cancer in xenografted and orthotopic mouse models. Int J Cancer 130: 1440–1450. [DOI] [PubMed] [Google Scholar]
- 38. Lee SS, Yang SF, Tsai CH, Chou MC, Chou MY, et al. (2008) Upregulation of heme oxygenase-1 expression in areca-quid-chewing-associated oral squamous cell carcinoma. J Formos Med Assoc 107: 355–363. [DOI] [PubMed] [Google Scholar]
- 39. Cheng YW, Chiou HL, Sheu GT, Hsieh LL, Chen JT, et al. (2001) The association of human papillomavirus 16/18 infection with lung cancer among nonsmoking Taiwanese women. Cancer Res 61: 2799–2803. [PubMed] [Google Scholar]
- 40. Sung WW, Wang YC, Cheng YW, Lee MC, Yeh KT, et al. (2011) A polymorphic −844T/C in FasL promoter predicts survival and relapse in non-small cell lung cancer. Clin Cancer Res 17: 5991–5999. [DOI] [PubMed] [Google Scholar]
- 41. Wu DW, Liu WS, Wang J, Chen CY, Cheng YW, et al. (2011) Reduced p21(WAF1/CIP1) via alteration of p53-DDX3 pathway is associated with poor relapse-free survival in early-stage human papillomavirus-associated lung cancer. Clin Cancer Res 17: 1895–1905. [DOI] [PubMed] [Google Scholar]
- 42. Smith EM, Ritchie JM, Summersgill KF, Klussmann JP, Lee JH, et al. (2004) Age, sexual behavior and human papillomavirus infection in oral cavity and oropharyngeal cancers. Int J Cancer 108: 766–772. [DOI] [PubMed] [Google Scholar]
- 43. Huang LW, Seow KM (2010) Oral sex is a risk factor for human papillomavirus-associated nasopharyngeal carcinoma in husbands of women with cervical cancer. Gynecol Obstet Invest 70: 73–75. [DOI] [PubMed] [Google Scholar]
- 44. Wang PD, Lin RS (1994) Sexual activity of women in Taiwan. Soc Biol 41: 143–149. [DOI] [PubMed] [Google Scholar]
- 45. Chen WJ, Fu TC, Ting TT, Huang WL, Tang GM, et al. (2009) Use of ecstasy and other psychoactive substances among school-attending adolescents in Taiwan: national surveys 2004–2006. BMC Public Health 9: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Jalouli J, Jalouli MM, Sapkota D, Ibrahim SO, Larsson PA, et al. (2012) Human papilloma virus, herpes simplex virus and epstein barr virus in oral squamous cell carcinoma from eight different countries. Anticancer Res 32: 571–580. [PubMed] [Google Scholar]
- 47.Huber GF, Albinger-Hegyi A, Soltermann A, Roessle M, Graf N, et al.. (2011) Expression patterns of Bmi-1 and p16 significantly correlate with overall, disease-specific, and recurrence-free survival in oropharyngeal squamous cell carcinoma. Cancer. [DOI] [PubMed] [Google Scholar]
- 48. Kuo KT, Hsiao CH, Lin CH, Kuo LT, Huang SH, et al. (2008) The biomarkers of human papillomavirus infection in tonsillar squamous cell carcinoma-molecular basis and predicting favorable outcome. Mod Pathol 21: 376–386. [DOI] [PubMed] [Google Scholar]
- 49. Zeng L, O'Connor C, Zhang J, Kaplan AM, Cohen DA (2010) IL-10 promotes resistance to apoptosis and metastatic potential in lung tumor cell lines. Cytokine 49: 294–302. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
IL-10 expression was decreased by HPV16/18 E6-knockdown in SiHa or HeLa cells and increased by HPV16 E6 overexpression C33A cells. SiHa cells were knocked down by transfection of shHPV16 E6 and HeLa cells were knocked down by transfection of shHPV18 E6. C33A cells were transfected with HPV16 E6 cDNA plasmid. HPV16/18 E6 and IL-10 expression was determined by Western blotting. β-actin was used as a protein loading control.
(TIFF)
Relationships between HPV infection and p16 expression in oral cancer patients.
(DOC)
Relationships between IL-10 mRNA and clinical parameters in oral cancer patients.
(DOC)
Relationships between gender and parameters in oral cancer patients.
(DOC)
Multivariate analysis of the influence of HPV 16/18 expression and p16 expression on overall survival (OS) and relapse free survival (RFS) in oral cancer patients.
(DOC)