Abstract
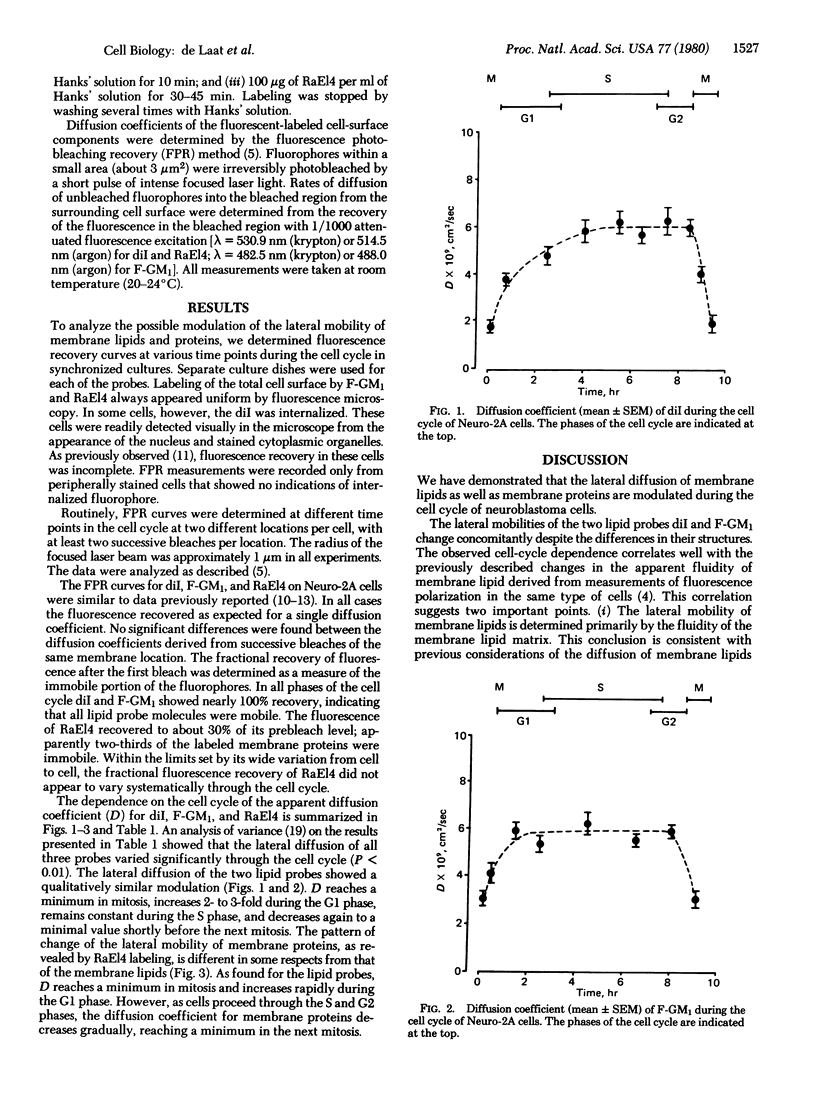
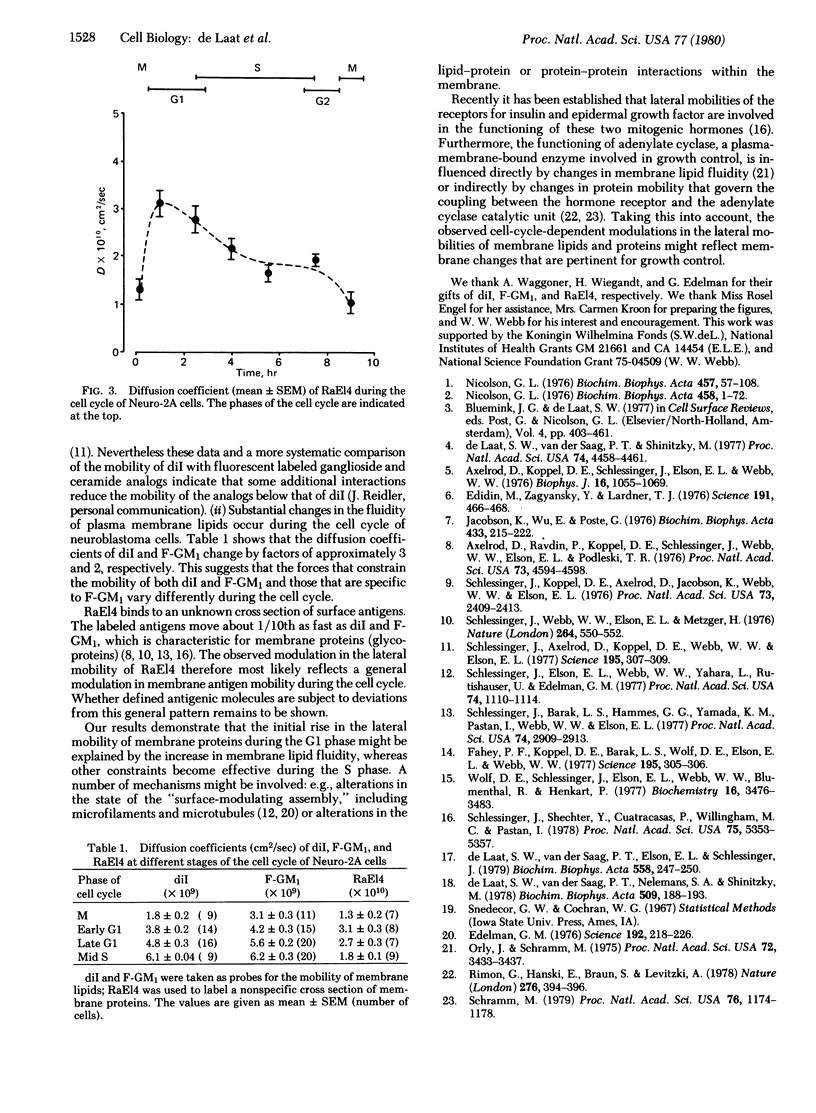
The fluorescence photobleaching recovery method has been used to determine the lateral mobilities of membrane lipids and proteins during the cell cycle of synchronized C1300 mouse neuroblastoma cells (clone Neuro-2A). As probes for lipid mobility, 3,3'-dioctadecylindocarbocyanine iodide and a fluorescein-labeled analog of ganglioside GM1 were used. Membrane proteins were labeled with rhodamine-labeled rabbit antibodies against mouse E14 cells. For both lipid probes the diffusion coefficients reach a minimum in mitosis, increase 2- to 3-fold during G1, remain constant at maximal values during S, and decrease again shortly before mitosis. Membrane proteins also exhibit minimum diffusion coefficients in mitosis, followed by a similar rise in G1. However, as cells proceed through S and G2, the lateral mobility of the membrane proteins gradually decreases. It is argued that lipid mobility is controlled by the fluidity of the membrane lipid matrix whereas protein mobility is governed also by other constraints.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod D., Koppel D. E., Schlessinger J., Elson E., Webb W. W. Mobility measurement by analysis of fluorescence photobleaching recovery kinetics. Biophys J. 1976 Sep;16(9):1055–1069. doi: 10.1016/S0006-3495(76)85755-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Axelrod D., Ravdin P., Koppel D. E., Schlessinger J., Webb W. W., Elson E. L., Podleski T. R. Lateral motion of fluorescently labeled acetylcholine receptors in membranes of developing muscle fibers. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4594–4598. doi: 10.1073/pnas.73.12.4594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman G. M. Surface modulation in cell recognition and cell growth. Science. 1976 Apr 16;192(4236):218–226. doi: 10.1126/science.769162. [DOI] [PubMed] [Google Scholar]
- Edidin M., Zagyansky Y., Lardner T. J. Measurement of membrane protein lateral diffusion in single cells. Science. 1976 Feb 6;191(4226):466–468. doi: 10.1126/science.1246629. [DOI] [PubMed] [Google Scholar]
- Fahey P. F., Koppel D. E., Barak L. S., Wolf D. E., Elson E. L., Webb W. W. Lateral diffusion in planar lipid bilayers. Science. 1977 Jan 21;195(4275):305–306. doi: 10.1126/science.831279. [DOI] [PubMed] [Google Scholar]
- Jacobson K., Wu E., Poste G. Measurement of the translational mobility of concanavalin A in glycerol-saline solutions and on the cell surface by fluorescence recovery after photobleaching. Biochim Biophys Acta. 1976 Apr 16;433(1):215–222. doi: 10.1016/0005-2736(76)90189-9. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Trans-membrane control of the receptors on normal and tumor cells. II. Surface changes associated with transformation and malignancy. Biochim Biophys Acta. 1976 Apr 30;458(1):1–72. doi: 10.1016/0304-419x(76)90014-7. [DOI] [PubMed] [Google Scholar]
- Nicolson G. L. Transmembrane control of the receptors on normal and tumor cells. I. Cytoplasmic influence over surface components. Biochim Biophys Acta. 1976 Apr 13;457(1):57–108. doi: 10.1016/0304-4157(76)90014-9. [DOI] [PubMed] [Google Scholar]
- Orly J., Schramm M. Fatty acids as modulators of membrane functions: catecholamine-activated adenylate cyclase of the turkey erythrocyte. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3433–3437. doi: 10.1073/pnas.72.9.3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimon G., Hanski E., Braun S., Levitzki A. Mode of coupling between hormone receptors and adenylate cyclase elucidated by modulation of membrane fluidity. Nature. 1978 Nov 23;276(5686):394–396. doi: 10.1038/276394a0. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Axelrod D., Koppel D. E., Webb W. W., Elson E. L. Lateral transport of a lipid probe and labeled proteins on a cell membrane. Science. 1977 Jan 21;195(4275):307–309. doi: 10.1126/science.556653. [DOI] [PubMed] [Google Scholar]
- Schlessinger J., Barak L. S., Hammes G. G., Yamada K. M., Pastan I., Webb W. W., Elson E. L. Mobility and distribution of a cell surface glycoprotein and its interaction with other membrane components. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2909–2913. doi: 10.1073/pnas.74.7.2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Elson E. L., Webb W. W., Yahara I., Rutishauser U., Edelman G. M. Receptor diffusion on cell surfaces modulated by locally bound concanavalin A. Proc Natl Acad Sci U S A. 1977 Mar;74(3):1110–1114. doi: 10.1073/pnas.74.3.1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Koppel D. E., Axelrod D., Jacobson K., Webb W. W., Elson E. L. Lateral transport on cell membranes: mobility of concanavalin A receptors on myoblasts. Proc Natl Acad Sci U S A. 1976 Jul;73(7):2409–2413. doi: 10.1073/pnas.73.7.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Shechter Y., Cuatrecasas P., Willingham M. C., Pastan I. Quantitative determination of the lateral diffusion coefficients of the hormone-receptor complexes of insulin and epidermal growth factor on the plasma membrane of cultured fibroblasts. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5353–5357. doi: 10.1073/pnas.75.11.5353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlessinger J., Webb W. W., Elson E. L., Metzger H. Lateral motion and valence of Fc receptors on rat peritoneal mast cells. Nature. 1976 Dec 9;264(5586):550–552. doi: 10.1038/264550a0. [DOI] [PubMed] [Google Scholar]
- Schramm M. Transfer of glucagon receptor from liver membranes to a foreign adenylate cyclase by a membrane fusion procedure. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1174–1178. doi: 10.1073/pnas.76.3.1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf D. E., Schlessinger J., Elson E. L., Webb W. W., Blumenthal R., Henkart P. Diffusion and patching of macromolecules on planar lipid bilayer membranes. Biochemistry. 1977 Jul 26;16(15):3476–3483. doi: 10.1021/bi00634a029. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Elson E. L., Schlessinger J. Lateral diffusion of membrane lipids and proteins is increased specifically in neurites of differentiating neuroblastoma cells. Biochim Biophys Acta. 1979 Dec 4;558(2):247–250. doi: 10.1016/0005-2736(79)90064-6. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Nelemans S. A., Shinitzky M. Microviscosity changes during differentiation of neuroblastoma cells. Biochim Biophys Acta. 1978 May 4;509(1):188–193. doi: 10.1016/0005-2736(78)90019-6. [DOI] [PubMed] [Google Scholar]
- de Laat S. W., van der Saag P. T., Shinitzky M. Microviscosity modulation during the cell cycle of neuroblastoma cells. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4458–4461. doi: 10.1073/pnas.74.10.4458. [DOI] [PMC free article] [PubMed] [Google Scholar]



