Abstract
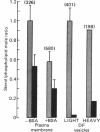
Plasma membrane isolated from rat sperm cells after incubation in vitro had a significantly lower cholesterol/phospholipid mole ratio when the medium contained serum albumin. Transfer of albumin-bound phospholipids to the membrane can largely account for this effect. The result is broadly consistent with a previously proposed model for albumin-induced destabilization of sperm membrane (capacitation) and its reversal by seminal plasma membrane vesicles. Albumin also decreased sialic acid and, more specifically, ganglioside levels, presumably by promoting release of sperm neuraminidase. Cholesteryl ester comprised up to 0.5 mol/mol of cholesterol in these plasma membrane preparations.
Full text
PDF
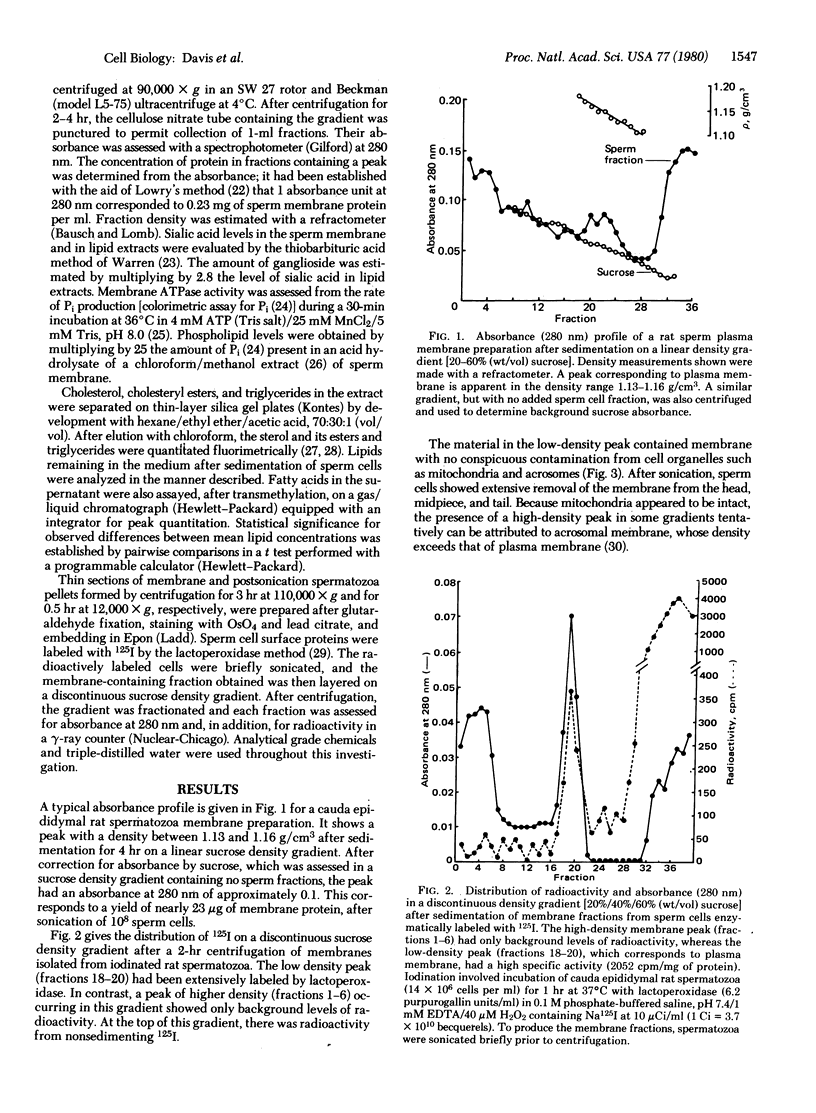

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Barros C., Bedford J. M., Franklin L. E., Austin C. R. Membrane vesiculation as a feature of the mammalian acrosome reaction. J Cell Biol. 1967 Sep;34(3):C1–C5. doi: 10.1083/jcb.34.3.c1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavister B. D. Environmental factors important for in vitro fertilization in the hamster. J Reprod Fertil. 1969 Apr;18(3):544–545. doi: 10.1530/jrf.0.0180544. [DOI] [PubMed] [Google Scholar]
- Beier H. M. Oviducal and uterine fluids. J Reprod Fertil. 1974 Mar;37(1):221–237. doi: 10.1530/jrf.0.0370221. [DOI] [PubMed] [Google Scholar]
- Blank M., Soo L., Britten J. S. Adsorption of albumin on rabbit sperm membranes. J Membr Biol. 1976 Nov 29;29(4):401–409. doi: 10.1007/BF01868973. [DOI] [PubMed] [Google Scholar]
- Chapman D., Penkett S. A. Nuclear magnetic resonance spectroscopic studies of the interaction of phospholipids with cholesterol. Nature. 1966 Sep 17;211(5055):1304–1305. doi: 10.1038/2111304a0. [DOI] [PubMed] [Google Scholar]
- Curtain C. C., Looney F. D., Marchalonis J. J., Raison J. K. Changes in lipid ordering and state of aggregation in lymphocyte plasma membranes after exposure to mitogens. J Membr Biol. 1978 Dec 29;44(3-4):211–232. doi: 10.1007/BF01944222. [DOI] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Nuclear magnetic resonance study of lecithin-cholesterol interactions. J Mol Biol. 1972 Jan 28;63(2):265–279. doi: 10.1016/0022-2836(72)90374-9. [DOI] [PubMed] [Google Scholar]
- Davis B. K., Byrne R., Hungund B. Studies on the mechanism of capacitation. II. Evidence for lipid transfer between plasma membrane of rat sperm and serum albumin during capacitation in vitro. Biochim Biophys Acta. 1979 Dec 12;558(3):257–266. doi: 10.1016/0005-2736(79)90260-8. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Decapacitation and recapacitation of rabbit spermatozoa treated with membrane vesicles from seminal plasma. J Reprod Fertil. 1974 Nov;41(1):241–244. doi: 10.1530/jrf.0.0410241. [DOI] [PubMed] [Google Scholar]
- Davis B. K., Gergely A. F. Studies on the mechanism of capacitation: changes in plasma membrane proteins of rat spermatozoa during incubation in vitro. Biochem Biophys Res Commun. 1979 May 28;88(2):613–618. doi: 10.1016/0006-291x(79)92092-8. [DOI] [PubMed] [Google Scholar]
- Davis B. K., Hunt D. M., Chang M. C. Influence of calcium ions on fertilization in the rabbit. Proc Soc Exp Biol Med. 1974 Nov;147(2):479–481. doi: 10.3181/00379727-147-38369. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Influence of serum albumin on the fertilizing ability in vitro of rat spermatozoa. Proc Soc Exp Biol Med. 1976 Feb;151(2):240–243. doi: 10.3181/00379727-151-39182. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Inhibitory effect of synthetic phospholipid vesicles containing cholesterol on the fertilizing ability of rabbit spermatozoa. Proc Soc Exp Biol Med. 1976 Jun;152(2):257–261. doi: 10.3181/00379727-152-39374. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Macromolecular inhibitor of fertilization in rabbit seminal plasma. Proc Natl Acad Sci U S A. 1971 May;68(5):951–955. doi: 10.1073/pnas.68.5.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis B. K., Niwa K. Inhibition of mammalian fertilization in vitro by membrane vesicles from seminal plasma. Proc Soc Exp Biol Med. 1974 May;146(1):11–16. doi: 10.3181/00379727-146-38033. [DOI] [PubMed] [Google Scholar]
- Davis B. K. Occurrence of vesicles in rabbit seminal plasma. Experientia. 1973 Dec;29(12):1484–1487. doi: 10.1007/BF01943870. [DOI] [PubMed] [Google Scholar]
- Edwards R. G. Follicular fluid. J Reprod Fertil. 1974 Mar;37(1):189–219. doi: 10.1530/jrf.0.0370189. [DOI] [PubMed] [Google Scholar]
- Edwards R. G., Steptoe P. C., Purdy J. M. Fertilization and cleavage in vitro of preovulator human oocytes. Nature. 1970 Sep 26;227(5265):1307–1309. doi: 10.1038/2271307a0. [DOI] [PubMed] [Google Scholar]
- Green D. P. Induction of the acrosome reaction in guinea-pig spermatozoa in vitro by the Ca ionophore A23187 [proceedings]. J Physiol. 1976 Sep;260(2):18P–19P. [PMC free article] [PubMed] [Google Scholar]
- Hope M. J., Bruckdorfer K. R., Hart C. A., Lucy J. A. Membrane cholesterol and cell fusion of hen and guinea-pig erythrocytes. Biochem J. 1977 Aug 15;166(2):255–263. doi: 10.1042/bj1660255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iritani A., Niwa K. Capacitation of bull spermatozoa and fertilization in vitro of cattle follicular oocytes matured in culture. J Reprod Fertil. 1977 May;50(1):119–121. doi: 10.1530/jrf.0.0500119. [DOI] [PubMed] [Google Scholar]
- Katz S. S., Shipley G. G., Small D. M. Physical chemistry of the lipids of human atherosclerotic lesions. Demonstration of a lesion intermediate between fatty streaks and advanced plaques. J Clin Invest. 1976 Jul;58(1):200–211. doi: 10.1172/JCI108450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaulen H. D., Henning R., Stoffel W. Biochemical analsis of the inocytotic process. II. Comparison of some enzymes of the lysosomal nd the plasma membrane of the rat liver cel. Hoppe Seylers Z Physiol Chem. 1970 Dec;351(12):1555–1563. doi: 10.1515/bchm2.1970.351.2.1555. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lui C. W., Cornett L. E., Meizel S. Identification of the bovine follicular fluid protein involved in the in vitro induction of the hamster sperm acrosome reaction. Biol Reprod. 1977 Aug;17(1):34–41. doi: 10.1095/biolreprod17.1.34. [DOI] [PubMed] [Google Scholar]
- Lui C. W., Meizel S. Biochemical studies of the in vitro acrosome reaction inducing activity of bovine serum albumin. Differentiation. 1977 Oct 20;9(1-2):59–66. doi: 10.1111/j.1432-0436.1977.tb01519.x. [DOI] [PubMed] [Google Scholar]
- Niwa K., Imai H., Kim C. I., Iritani A. Fertilization in vitro of hamster and mouse eggs in a chemically defined medium. J Reprod Fertil. 1980 Jan;58(1):109–114. doi: 10.1530/jrf.0.0580109. [DOI] [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Effects of cholesterol and cholesterol derivatives on hydrocarbon chain mobility in lipids. Biochem Biophys Res Commun. 1971 May 7;43(3):610–616. doi: 10.1016/0006-291x(71)90658-9. [DOI] [PubMed] [Google Scholar]
- Papahadjopoulos D., Poste G., Schaeffer B. E., Vail W. J. Membrane fusion and molecular segregation in phospholipid vesicles. Biochim Biophys Acta. 1974 May 30;352(1):10–28. doi: 10.1016/0005-2736(74)90175-8. [DOI] [PubMed] [Google Scholar]
- Poste G., Reeve P., Alexander D. J., Terry G. Effect of plasma membrane lipid composition on cellular susceptibility to virus-induced cell fusion. J Gen Virol. 1972 Oct;17(1):133–136. doi: 10.1099/0022-1317-17-1-133. [DOI] [PubMed] [Google Scholar]
- Setchell B. P. Secretions of the testis and epididymis. J Reprod Fertil. 1974 Mar;37(1):165–177. doi: 10.1530/jrf.0.0370165. [DOI] [PubMed] [Google Scholar]
- Soloni F. G. Simplified manual micromethod for determination of serum triglycerides. Clin Chem. 1971 Jun;17(6):529–534. [PubMed] [Google Scholar]
- Sweet C., Zull J. E. Activation of glucose diffusion from egg lecithin liquid crystals by serum albumin. Biochim Biophys Acta. 1969 Jan 28;173(1):94–103. doi: 10.1016/0005-2736(69)90040-6. [DOI] [PubMed] [Google Scholar]
- Tsai C. M., Chen K. Y., Canellakis E. S. Isolation and characterization of the plasma membrane of L-1210 cells. Iodination as a marker for the plasma membrane. Biochim Biophys Acta. 1975 Aug 20;401(2):196–212. doi: 10.1016/0005-2736(75)90304-1. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]
- Yanagimachi R., Usui N. Calcium dependence of the acrosome reaction and activation of guinea pig spermatozoa. Exp Cell Res. 1974 Nov;89(1):161–174. doi: 10.1016/0014-4827(74)90199-2. [DOI] [PubMed] [Google Scholar]
- Zahler W. L., Doak G. A. Isolation of the outer acrosomal membrane from bull sperm. Biochim Biophys Acta. 1975 Nov 3;406(4):479–488. doi: 10.1016/0005-2736(75)90026-7. [DOI] [PubMed] [Google Scholar]
- van der Bosch J., Schudt C., Pette D. Influence of temperature, cholesterol, dipalmitoyllecithin and Ca2+ on the rate of muscle cell fusion. Exp Cell Res. 1973 Dec;82(2):433–438. doi: 10.1016/0014-4827(73)90362-5. [DOI] [PubMed] [Google Scholar]