Abstract
Retrotransposons with long terminal repeats (LTRs) more than 3 kb are not frequent in most eukaryotic genomes. Rice LTR retrotransposon, Retrosat2, has LTRs greater than 3.2 kb and two open reading frames (ORF): ORF1 encodes enzymes for retrotransposition whereas no function can be assigned to ORF0 as it is not found in any other organism. A variety of experimental and in silico approaches were used to determine the origin of Retrosat2 and putative function of ORF0. Our data show that not only is Retrosat2 highly abundant in the Oryza genus, it may yet be active in rice. Homologs of Retrosat2 were identified in maize, sorghum, Arabidopsis and other plant genomes suggesting that the Retrosat2 family is of ancient origin. Several putatively cis-acting elements, some multicopy, that regulate retrotransposon replication or responsiveness to environmental factors were found in the LTRs of Retrosat2. Unlike the ORF1, the ORF0 sequences from Retrosat2 and homologs are divergent at the sequence level, 3D-structures and predicted biological functions. In contrast to other retrotransposon families, Retrosat2 and its homologs are dispersed throughout genomes and not concentrated in the specific chromosomal regions, such as centromeres. The genomic distribution of Retrosat2 homologs varies across species which likely reflects the differing evolutionary trajectories of this retrotransposon family across diverse species.
Introduction
Long terminal repeat (LTR) retrotransposons are the most prominent mobile sequence in many plants. They replicate via an RNA intermediate and can rapidly increase in copy number resulting in large differences in genome sizes between related species (C-value enigma) [1]. For instance, the genome size of maize (Zea mays, 2n = 2x = 20) is six times larger than rice (Oryza sativa, 2n = 2x = 24) due primarily to massive amplifications of LTR retrotransposons in maize after the split of the two species [2], [3]. LTR retrotransposons contain direct LTRs that flank the internal sequences and are similar to retroviruses in structure and replication cycle, although they are unable to move from cell to cell [4]. The internal regions of retrotransposons encode gag and pol polyproteins that catalyze transpositon. Based on sequence divergence and the order of encoded gene products, LTR retrotransposons have been subdivided into Ty1-copia and Ty3-gypsy superfamilies [5]. Some retrotransposons from both Ty1-copia and Ty3-gypsy superfamilies have an additional open reading frame (ORF) located between the ORF encoded gag-pol polyproteins and 3′LTR, which encodes a retroviral envelope-like protein (env) [6], [7]. However, the function of the env gene in plants is not clear.
Unlike the internal regions, the LTRs do not code for enzymes for the replication of retrotransposons they do, however, play a critical role in controlling the activity of LTR retrotransposons. LTRs are composed of three distinct regions (U3, R and U5) that harbor regulatory factors including enhancers, promoters, and termination signals [8], [9]. For instance, a 10-bp sequence divergence in U3 region can result in significant differences in promoter activity [10]. In addition, some LTRs harbor cis-regulatory signals that confer responsiveness to various external stimuli and play a role in reactivation of transposition [11]. Moreover, LTRs from several retrotransposons in yeast have been found to contain sequences complementary to the primer binding sequence (PBS) allowing self primed reverse transcription [12]. Intriguingly, LTRs can serve as alternative promoters or enhancers to regulate genes as far away as 40–70 kb [13]–[15]. Despite their functional conservation, LTRs evolve rapidly and are highly divergent. In addition, the LTR sizes are extremely variable and can range from 80 bp [16], [17] to more than 5 kb [18]. The evolutionary origins and biological functions of larger LTRs are poorly understood.
Massive proliferation of LTR retrotransposons not only results in genome expansion but can also result in deleterious or lethal mutations. Thus, several mechanisms have evolved to suppress retrotransposon activity. This includes an epigenetic silencing mechanism by which transposons are either methylated thereby suppressing transcriptional activity or targeting of transposon transcripts by small RNAs (sRNAs) for post-transcriptional silencing [19]. Retrotransposons may, however, have insertion biases for genomic locations neutral to the host organism which allow the retrotransposon to persist [20]. For example, the Ty3 LTR retrotransposon in Saccharomyces cerevisiae usually targets regions upstream of RNA polymerase III genes and may be harmless for the genome [20], [21]. In rice, the low copy but still active copia-like retrotransposon, Tos17, appears to prefer to insert (or be retained) into genic regions [22]. However, many high copy retrotransposons are concentrated in heterochromatic regions (centromeres, pericentromeres, telomeres) [23]. The distribution patterns of transposon families can be conserved across related genomes. A prominent example is the centromeric retrotransposons (CRs) family in grass species that is highly conserved over long evolutionary periods among a majority of the grass species and concentrated in centromeric regions of rice, wild rice, wheat, barley and maize [24]–[28].
We recently demonstrated that canonical CRs were absent in the wild rice species, O. brachyantha, and replaced by a novel retrotransposon, FRetro3 [29]. FRetro3 has no sequence similarity with the CRs in the grass genomes it does, however, has sequence similarity with a retroelement in rice, Retrosat2. Both FRetro3 and Retrosat2 are large elements (more than 12 kb) and have LTRs longer than 3.2 kb. Intriguingly, they both contain an extra ORF named ORF0 located upstream of the ORF encoding the gag-pol proteins (ORF1). The ORF0 sequence appears to be unique in rice as no other LTR retrotransposon from any organism contains a similar ORF0. The origin and function of the ORF0 sequence is not known.
We used a combination of comparative genomics, protein structure predictions and experimental approaches to gain insight into the function and evolution of this unusual family of retrotransposons, Retrosat2. Our results indicate that the Retrosat2 family is abundant in the Oryza genus and may still be active. Homologs of Retrosat2 were found in maize, sorghum, Arabidopsis and other plant genomes suggesting an ancient evolutionary origin. In contrast to FRetro3, Retrosat2 and its homologous elements were dispersed throughout the host genomes. Sequence analysis and 3D protein structural study of ORF0 sequences from Retrosat2 and other homologs reveal that the ORF0 sequences have evolved more quickly than the retrotransposases. Finally, we show that the genomic distribution and evolutionary functions of this transposon family have likely experienced divergent selection pressures among these various plant genomes.
Results
Retrosat2 is abundant and dispersed throughout the rice genome
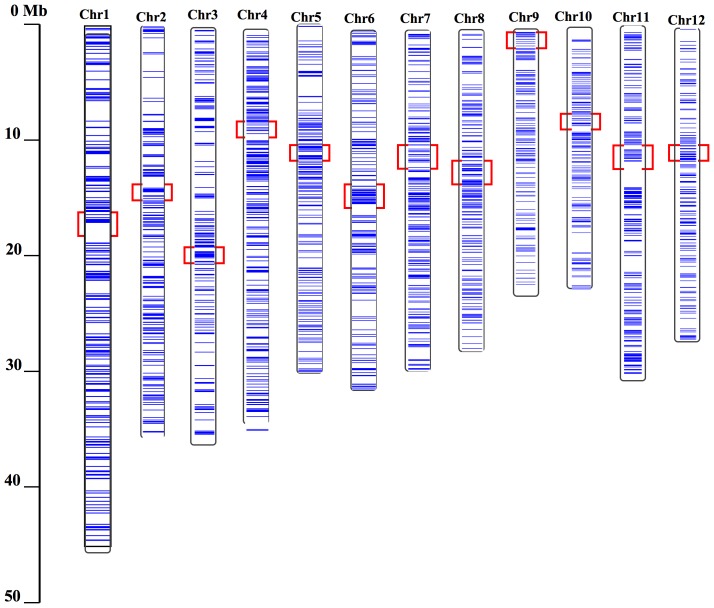
Retrosat2 was originally identified as a nested LTR retrotransposon (Accession number: AF111709) with little other genomic description. We annotated the Nipponbare (Oryza sativa L. ssp. japonica) genome and identified more than 1000 complete or partial copies of Retrosat2, including 162 complete elements and 429 solo-LTRs. Together, these elements comprise about 1.4% of the Nipponbare genome (Table S1). The genome-wide ratio of solo-LTR to complete element is about 2.6∶1 (429/162), although the ratio varies among the 12 chromosomes. Chromosome 3 has the highest ratio of solo-LTR to complete element (42/4 = 10.5∶1), whereas, chromosome 11 has the lowest (29/18 = 1.6∶1). Further investigation indicated that Retrosat2 elements are dispersed unevenly throughout the rice genome (Figure 1).
Figure 1. Distributions of Retrosat2 elements on 12 chromosomes of Nipponbare.
Blue horizontal lines indicate Retrosat2 elements. Red rectangles mark the centromeric positions of 12 rice chromosomes.
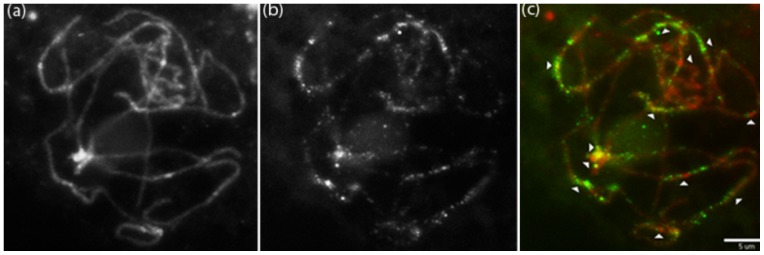
To provide more insight into chromosomal distribution of Retrosat2, fluorescence in situ hybridization (FISH) using the LTR of Retrosat2 as a probe was performed. Some FISH signals derived from Retrosat2 flanked or overlapped the centromeres (Figure 2), but the majority of the signals were dispersed on chromosomal arms which confirm that Retrosat2 is not concentrated at specific chromosomal regions but rather is distributed across the genome. It is interesting that the chromosomal distribution of Retrosat2 differs from its homolog, FRetro3, in O. brachyantha which is enriched in peri- and centromeric regions [29].
Figure 2. FISH image of 1.5 kb LTR sequence of Retrosat2 on pachytene chromosomes.
(a) 4′,6-diamidino-2-phenylindole (DAPI) counterstain, (b) Retrosat2, (c) Merged image. Chromosomes were psuedocolored red and Retrosat2 signals are shown green. Arrows indicate locations of 12 centromeres and the scale bar represents 5 µm.
Retrosat2 elements are present throughout the Oryza genus
The Retrosat2 sequence was used to query GenBank to determine if Retrosat2-like elements are present in other Oryza species. Complete Retrosat2 homologs were found in O. rufipogon (Sat2-ruf), O. punctata (Sat2-pun), O. minuta (Sat2-min), O. officinalis (Sat2-off) and O. australiensis (dingo, [30]) and ranged in size from 11,375 to 13,324 bp (Table S2). No complete element was found in O. glaberrima or O. granulata; however, solo-LTR or fragments (>3 kb) were identified in both genomes with more than 80% sequence similarity to Retrosat2. These results suggest that Retrosat2 and its homologs are present throughout the Oryza genus.
We use the BAC-end sequences (BESs) from 12 Oryza genomes generated by the Oryza Map Alignment Project (OMAP) (http://www.omap.org) to detect the distribution and abundance of Retrosat2 family in the genus Oryza. This survey may not reflect the real situation of Retrosat2 in the genus as BESs only cover the regions having a restriction site for the enzyme that was used to build the BAC libraries However, whole genome sequences are not available for all Oryza species, only available for Oryza sativa. Elements from the Retrosat2 family were used to search against the BESs. Retrosat2 elements were identified in all 12 genomes, but the abundance varies dramatically among the 12 genomes. For example, Retrosat2 elements account for 3.72% of the O. australiensis BESs but only 0.03% of O. coarctata (Table S3).
In order to confirm the distribution of Retrosat2 family in the Oryza genus, Southern blot analysis was performed. Strong signals were detected in all tested genomes except O. coarctata (Figure 3) confirming our results from BES analysis. No hybridization signal was found in O. coarctata, suggesting that Retrosat2 elements are either present in very few copies, highly diverged or in the process of extinction.
Figure 3. Southern blot of Retrosat2.
(1) Oryza brachyantha, (2) Oryza sativa (Nipponbare), (3) Oryza glaberrima, (4) Oryza nivara, (5) Oryza longistaminata, (6) Oryza rufipogon, (7) Oryza minuta, (8) Oryza officinalis, (9) Oryza punctata, (10) Oryza alta, (11) Oryza australiensis, (12) Oryza granulata, (13) Oryza ridleyi, (14) Oryza coarctata and (15) Oryza brachyantha.
Integration times of Retrosat2 in rice
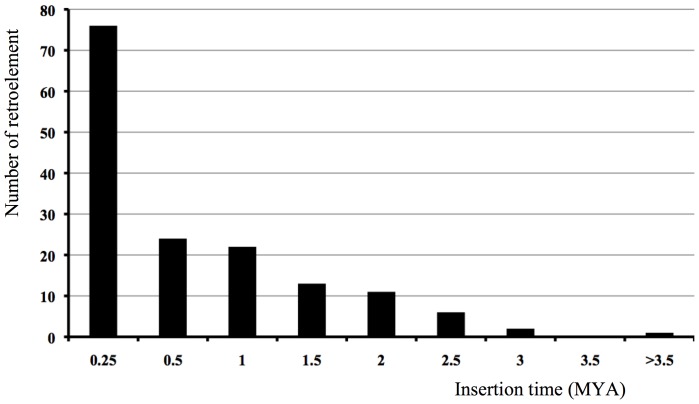
Because LTR retrotransposons replicate via RNA intermediates and has a similar life cycle to the retrovirus, the LTRs should be identical at the time of insertion and then diverge independently through the accumulation of mutations. Thus, the sequence divergence of LTRs of a retrotransposon can be used to estimate the insertion time of the retroelement [31]. The insertion times of 155 complete LTR retrotransposons were calculated for the Nipponbare genome following published methodologies [32] and range from 0 to 3.73 million year ago (MYA). Most elements integrated in the last 0.5 MY, 76 and 24 elements within 0.25 and 0.5 MYA, respectively (Figure 4). This suggests that the majority of Restrosat2 elements were recently inserted into the rice genome and that the element may still be active.
Figure 4. The insertion times of full-length Retrosat2 elements in O. sativa cultivar Nipponbare.
The O. sativa indica and japonica subspecies diverged from a common ancestor ∼0.2–0.4 MYA [32]. Since the insertion times of 49% (76/155) of complete elements of Retrosat2 were less than 0.25 million year (MY), many of the elements may have inserted into the rice genome after the split of two subspecies. To test this hypothesis, we compared the insertion patterns of Retrosat2 between 93–11 (indica) and Nipponbare (japonica). To do this, the presence/absence of orthologous insertions were confirmed by using each element and ∼100bp flanking sequence from 93–11 to search against the Nipponbare genome and the reciprocal using Nipponbare sequences to search against the 93–11 genome. We found 95 and 40 new insertions in Nipponbare and 93–11, respectively. To detect the transcriptional activity of Retrosat2, we conducted RT-PCR analysis and found that Retrosat2 was expressed in leaf, sheath and flower (Figure S1).
Multiple putative regulatory signals are found in the LTRs of Retrosat2
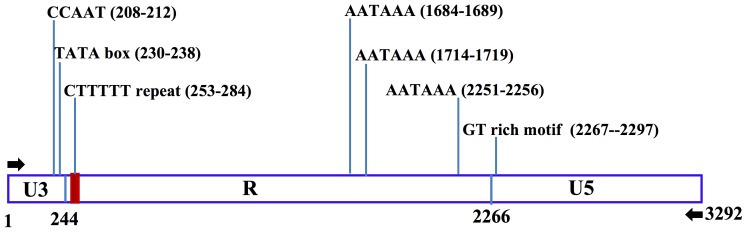
LTRs contain regulatory sequences and are important for the replication and integration of retrotransposons. The LTRs of Retrosat2 are more than 3.2 kb and can be divided into the three canonical regions, U3, R and U5 (Figure 5). The Retrosat2 U3 region contains the 5-bp enhancer motif (CCAAT) [8] found from nucleotides 208–212 and, in an 18-bp region after the enhancer signal, a TATA box for initiation of transcription. The R domain of Retrosat2 is 2023 bp and starts with a G (nucleotide 244) and ends with CA (nucleotides 2064-65). The Retrosat2 R region has unusual sequence features including: 1) Three polyadenylation (poly(A)) sites (AATAAA), located in nucleotides 1684–1689, 1714–1719 and 2251–2256; and 2) Seven tandem repeats of a CTTTTT motif. The U5 region contains GT or T-rich sequences usually located the first 40-bp region of the U5 domain that are thought to be important for retrotransposition [33], [34]. The G/T content of the first 31-bp region of the R domain is 90%, much higher than the average content (51.4%) of the rest of the Retrosat2 LTR. In contrast to the U3 and R regions, the U5 region has sequence conservation between Retrosat2 and homologs within the Oryza genus (Figure S2).
Figure 5. The sequence structure of Retrosat2 LTR.
The LTR is divided into three regions, U3 (1–244), R (245–2266) and U5 (2267–3292). Red rectangle denotes tandem repeats and black arrows indicate the 10-bp inverted terminal repeats of the LTR.
Previous studies have shown that LTRs of the tobacco retrotransposon Tto1 harbor signal sequences for responsiveness to various stresses [11]. Since the LTRs of Retrosat2 are much larger than that of Tto1 (574 bp), we suspected that the LTRs may contain regulatory sequences. To test this hypothesis, the LTRs of Retrosat2 were used to search against PlantCARE (database of plant cis-acting regulatory elements [35]). Interestingly, the LTR sequence matched exactly 22 regulatory elements reported in rice and other plants (Table S4), including the C-repeat/DRE from Arabidopsis, Sp1 from rice and a TGACG-motif from barley. It should be noted that many of these elements are involved in stress responsiveness and that some cis-regulatory elements completely match multiple regions of the Retrosat2 LTR. For instance, we found five CCGTCC sequences that matched the CCGTCC-box from Arabidopsis.
Origin and phylogenetic analysis of the Retrosat2 family
Complete elements or fragments of Retrosat2 homologs were found across the Oryza genus (Tables S2 & S3) which indicate that the ancestor of Retrosat2 likely existed before the divergence of the Oryza genus. To determine if Retrosat2 homologs present outside the genus Oryza, the LTR, ORF0 and ORF1 of Retrosat2 were used individually to search against GenBank and whole genome sequences from maize, sorghum, Brachypodium distachyon (Brachypodium), Arabidopsis, soybean, poplar, grape and Selaginella moellendorffii. No significant hits (E value <10−5) were found in these genomes using either the LTR or ORF0 as queries for BLAST searches. However, complete homologous elements, dispersed across the genomes, were identified in all eight genomes using the ORF1 sequence as a query. Interestingly, the homologs of Retrosat2 in maize (ZMsat2), sorghum (Sorsat2) and Brachypodium (Brasat2) also contain two ORFs, ORF0 and ORF1, whereas homologs in Arabidopsis (Arasat2), soybean (Soysat2), poplar (Popsat2) grape (Grasat2) and Selaginella moellendorffii (Selsat2) have only the ORF1. These results indicate that the ancestor of Retrosat2 and its homologs predates the divergence of dicotyledonous and monocotyledonous plants.
The ORF1 sequences of Retrosat2 and its homologs are more conserved than the ORF0 sequences. For example, the ORF1 proteins of Retrosat2 and Sorsat2 share more than 60% sequence similarity, whereas the ORF0 proteins from the two elements show ∼10% sequence similarity. To gain insight into the sequence divergence of ORF0 and ORF1 of Retrosat2 and its homologs, we calculated the nonsynonymous (Ka) to synonymous (Ks) nucleotide substitution rates using the ORF0 and ORF1 sequences of Retrosa2, Sa2-ruf, Sat2-min and Sat2-off. Dingo, FRetro3, ZMsat2, Sorsat2 and Brasat2 were excluded as their ORF0 sequences were either too divergent or either ORF0 or ORF1 was truncated. The average Ka values of the ORF0 and ORF1 sequences are significantly different, p>0.005, at 0.2574 and 0.0783, respectively. The mean Ks values of the ORF0 and ORF1 sequences are 1.6471 and 1.3298, respectively, and not significantly different. Ka values usually reflect functional divergence whereas Ks values can be used to estimate divergence dates [36]. Thus, these results suggest the ORF0 and ORF1 likely diverged at the same time but have been subjected to different selection pressures.
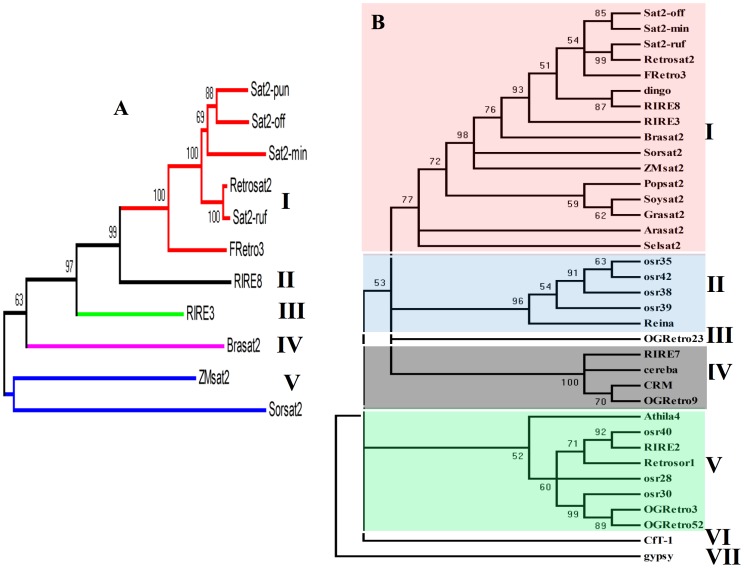
To illuminate the evolutionary relationship between Retrosat2 and its homologs, two phylogenetic trees were constructed using the ORF0 proteins and the conserved reverse transcriptase (RT) domains of ORF1. The phylogenetic tree of ORF0 shows that the sequences fall into five subfamilies where Retrosat2 and the Oryza genus homologs constitute a unique group (Figure 6A). The phylogenetic tree with the conserved RT domains indicates that these elements can be grouped into seven subfamilies. Retrosat2 and all the homologs fall into the subfamily I, other plant retroelements were grouped into subfamilies II-V whereas gypsy from Drosophila and CfT1 from Passalora fulva were grouped into subfamilies VI and VII, respectively (Figure 6B). Notably, RIRE3 and RIRE8 were grouped together with Retrosat2 and its homologs suggesting that they likely diverged from a common ancestral family.
Figure 6. Phylogenetic trees based on the ORF0 sequences (A) and the RT conserved domains (B). Bootstrap values (>50%) are shown.
Structural characterization and functional annotation of ORF0
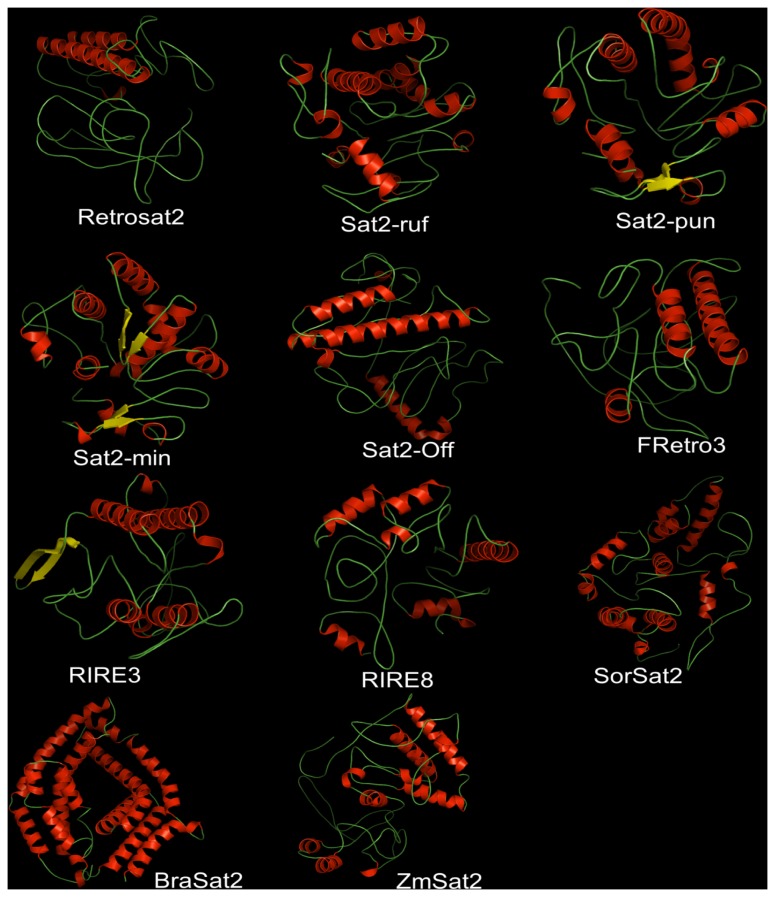
Using computational modeling, we determined the structural features of the 3D general folds and compared the electrostatic surfaces, structural and key functional residues of different ORF0 proteins (Figures 7–8, Figures S3, S4, S5, S6). Because the native structures have not been crystallized, the structural similarity and accuracy of the models were further assessed using structural parameters: root mean square deviation (RMSD), sequence identity and E-value compared with the template, and Z-Score, (model reliability between 0–1). Their pseudo-energies of the contributing terms (C-beta interaction energy, all-atom pairwise energy, solvation energy and torsion angle energy), together with their Z-scores are the indicators of a well built model [37].
Figure 7. Three-dimensional structure analysis of the 11 ORF0 proteins.
All structures are depicted as a cartoon diagram. Within the represented family, the secondary elements are colored in red (α-helix), yellow (β-sheet) and green (coils)
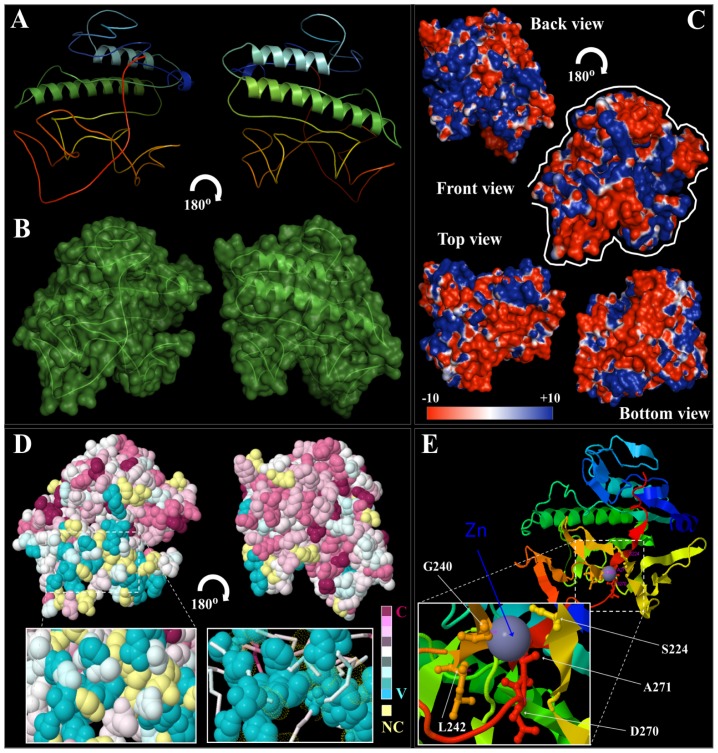
Figure 8. Detailed structural conformation and conservation analysis of Retrosat2, a rice ORF0 protein member.
(A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the rice RetroSat2, where N- and C-terminal are colored blue and red, respectively. Represented structures were rotated at 180u. (B) The surface conformation of RetroSat2 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of RetroSat2 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted RetroSat2 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of RetroSat2 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification highlights the residues implicated in cavity formation and interaction with the ligand zinc (Zn), which are S224, G240, L242, D270, and A271.
General structural comparisons (Figure 7) and phylogenetic analysis (Figure 6A) provide clear insights into the structural divergence of the ORF0 proteins. Following the minimum divergence criterion, RSMD comparisons between every pair of structures were measured (Table S5) indicating that deviations in structural comparisons analyzed were low for Sat2-min, Sat2-pun, Sat2-ruf and Sat2-off (Figure 7–8, Figures S4, S5, S6, Table S5).
Structural comparisons between the most closed structures (Figures 7, 8A, Figures S3A, S4, S5, S6A) are in line with the results obtained in the phylogenetic analyses (Figure 6A). Superimposition between the most similar members of the ORF0 family showed a close structural relationship. However, the special disposition for different 2D elements is the major structural divergence between Sat2-min, Sat2-ruf, Sat2-pun and Sat2-off. The greatest structural differences were found between SorSat2 and ZmSat2 (Table S5, Figure S3A, S4, S5, S6A). The Table S5 showed a strong structural correlation between the three structures Sat2-min, Sat2-pun, Sat2-ruf, as well as between Retrosat2 and Sat2-off, based on the small RMSD values (3.99, 6.21, 6.60, and 5.693Å). Further comparisons between every pair of structures shown major similarities between RIRE3 to FRetro3 (RMSD = 8.508Å), Sat2-min to BraSat2 (RMSD = 9.216 Å), and RIRE8 to SorSat2 (RMSD = 9.970Å).
Proteins folding and structural domains revealed that SorSat2 and ZmSat2 were the most divergent form of the current the ORF0 protein members (Figure 7, Table S5). Differences are also present in other many members of the ORF0 proteins when comparing the general surface domains, cavities and clefts modeled as consequence of the structural parameters variation, and spatial distribution of α-helices, β-sheets, coils, turns and other structural elements of the proteins (depicted in Figure 7, Figures 8B, Figures S3B, S4, S5, S6B).
The Adaptive Poisson-Boltzmann Solver (APBS) package [38] was used to generate the electrostatic surface potentials for all the ORF0 proteins (Figures 8C, Figures S3C, S4, S5, S6C). Although the overall topologies of these proteins are similar between some members of the ORF0 genes, such as Sat2-min, Sat2-pun and Sat2-ruf, several differences can still be observed. A specific electrostatic potential distribution pattern in the surface was observed for Sat2-min and Sat2-pun, as well as for Retrosat2 and Sat2-off, with a abundance of negative and positive charge in the protein surfaceshowing numerous more differences ORF0 protein members, as were depicted in the general view (Figure 7), and isocontour representation data (Figures 8C, Figures S3C, S4, S5, S6C). The surfaces of the ligand-binding domains and the cofactor-binding domains contained the most profound differences in charge distributions inside the ORF0 protein members. However, charge distribution patterns (isocontour ranging from +10 kT to −10 kT) might correlate with differences and/or similarities in their activity, ligand-binding or protein-protein interaction [39], which also denotes differences in the mechanism of action and/or interaction with other proteins and intracellular targets [40].
The conservational analysis of residue implicated in the structural maintenance revealed similar residue patterns in all ORF0 protein members (Figures 8D, Figures S3D, S4, S5, S6D), where Retrosat2 and BraSat2 were slightly more conserved than the rest of the ORF0 members, with the most variable surface residues (depicted in blue) located on the periphery and the conserved residues (depicted in purple) located in the core of the protein structures. The most conserved residues were confined to the catalytic cleft of some ORF0 proteins structures. The most conserved environment around the ligand-binding cleft corresponded to Sat2-min and Sat2-off, whereas Retrosat2 and RIRE8 show the most variable residue composition in its ligand-binding cavity/cleft (Figure 8D, Figure S6D).
The variability of the ligand-binding pockets reflects differential functional features of proteins. We have shown examples of possible ligands and/or cofactors that different ORF0 protein members can hold, which interactions drive the course of reactions, since different enzymatic activities has been exhibited by these ORF0 protein members, i.e. Sat2-ruf, Sat2-pun, and FRetro3 can bind 2-(acetylamino)-2-deoxy-α-D-Glucopyranose (NDG); Retrosat2, Sat2-off, RIRE8, and BraSat2 as example of interaction with cofactors as Co, Zn, SO4, and Fe2/S2 respectively (Figure 8E, Figures S3, S4, S5, S6E). The residue conservation of the ligand/cofactor binding sites and structural comparisons of the ligand-dependent regions inside of the ORF0 protein members are crucial for predicting the cofactor/ligand specificity and the enzymatic mechanism and possible functional characteristics.
We further conducted functional analysis of ORF0 by gene ontology annotations based on ORF0 protein structural information (Table S6). The structure-based functional inferences indicate that ORF0 protein members fall into three groups: Group 1 consists in the class 3 hydrolases (EC 3.1) acting on ester bonds, and includes Sat2-min, Sat2-pun, Sat2-ruf, Retrosat2, Sat2-off, FRetro3, RIRE3, RIRE8, and ZmSat2. Group 2 corresponding to the acyltransferases (EC 2.3) includes SorSat2 (EC 2.3.1 Transferring groups other than amino-acyl groups are implicated in the biosynthesis of lipids. Group 3 of the small molecules transporters (EC 2.A), which includes BraSat2, are implicated in the antiport of glycerol-P (Table S6). The Group 1 can be further differentiated into four functional subgroups. Subgroup 1 includes Sat2-min, Sat2-pun, Sat2-ruf for which the molecular function is Carboxylic (Triacylglycerol) ester hydrolase (EC 3.1.1) implicated in lipid catabolic processes. Subgroup 2 includes ZmSat2 and is implicated in removing 5′-nucleotides from 3′-hydroxyterminated oligonucleotides (EC 3.1.4 or Phosphoric-diester hydrolases). Subgroup 3 which includes RIRE8 is implicated in DNA nicking (endodeoxyribonuclease) activity (EC 3.1.21). Subgroup 4, including Sat2-off, RIRE3, Retrosat2 and FRetro3 shares the same molecular function such as arylsulfatase activity (EC 3.1.6), which could be implicated in metabolic processes of polysaccharides.
Discussion
The evolutionary impacts of the longer LTR of Retrosat2
LTRs are critical for retrotransposon replication because the regions harbor regulatory signals necessary for gene expression. Unlike the coding regions of retrotransposons, LTRs are extremely variable in size ranging in size from 80 bp [16], [17] to more than 5 kb [18]. In many organisms, the LTRs are relative short (<1.5–2.0 kb). For instance, LTRs over 1.5 kb have not been found in Drosophila, yeast or Anopheles gambiae [41]–[43]. In plants, the LTRs of a majority of retrotransposon families are smaller than 2.0 kb [44]–[45]. Retrotransposons with LTRs greater than 3-kb tend to be uncommon.
It is not evident why elements with short LTRs would predominate genomes. Previous results indicated that increased length of LTRs dramatically reduces transcription efficiency and inhibits transposition [46]. Thus, elements with short LTRs may have a higher transposition frequency than those with longer LTRs. The LTRs of Retrosat2 are over 3.2 kb, one of the longest LTRs in rice [45], and yet we show that Retrosat2 is abundant and may still be active in rice.
We identified regulatory putative signals in Retrosat2 LTRs, including cis-acting regulatory elements involved in stress responsiveness (Table S4). Some of the regulatory signals in the LTR are multicopy, such as the three polyadenylation motifs. Multiple regulatory motifs have been found in other retroelements. In barley, for instance, the LTR of the BARE retrotransposon contains two TATA-box motifs, TATA1 and TATA2, that control tissue-specific expression. Both promoters are active in callus but TATA2 is active in embryos, whereas, TATA1 is virtually inactive in embryos [47]. Although RT-PCR indicates that Retrosat2 is expressed in leaf, sheath and flower tissues of rice, we do not know whether the retroelement is expressed in other tissues or how the mulitple regulatory signals may affect expression patterns.
Divergence or mutation of LTR sequences may affect the activity of a retrotransposon. It has been reported that even a 10-bp sequence divergence in the U3 region can result in significant differences in promoter activities [10]. The LTRs of Retrosat2 are more than 3.2 kb and contain multi-copy regulatory signals. Thus, it is possible that the element can accommodate mutations within LTRs due to redundancy of regulatory sequences without losing activity. We hypothesize that elements with long LTRs and multicopy regulatory sequences would be more tolerant of mutations than shorter LTRs with single copy signals.
The genomic distributions of Retrosat2 and the homologs
LTR retrotransposon families in plants have distinct chromosomal distribution patterns. Some LTR retrotransposons are predominantly found in intergenic regions, whereas, others are concentrated in heterochromatic regions [23]. Elements within a family tend to have similar distribution patterns. For example, the rice LTR retroelement Dasheng is related to Gran3 from wild rice, O. granulata, and both elements are concentrated in centromeric and pericentromeric regions [28], [48]–[49]. The CRs are also concentrated in the centromeric regions across the grass genomes including rice, wild rice, wheat, barley and maize [24]–[28]. However, our previous results showed that the canonical CRR (CR of rice) identified in centromeres of all other Oryza species is absent in O. brachyantha and that a new retrotransposon, FRetro3, colonized the centromeres of this species [29].
Sequence comparisons, phylogenetic analysis and structural and functional annotation of proteins indicate that FRetro3 and Retrosat2 share a common ancestor. Despite the evolutionary and structural relationship to Fretro3, however, Retrosat2 is not concentrated in rice centromeric regions but is dispersed across the genome. In addition, homologs of both FRetro3 and Retrosat2 are dispersed throughout the genomes of maize, sorghum and others (data not shown). These observations suggest that homologous elements derived from a common ancestor can have distinct distribution patterns and therefore different roles in shaping host genomes.
Since maize and sorghum are more distantly related than O. brachyantha and rice, the distinct distribution and function of FRetro3 likely emerged after the split of the Oryza genus. It is not clear why or even how FRetro3 became the functional centromeric retroelement in O. brachyantha, although, we assume that selection on FRetro3 in O. brachyantha differed from that on Restrosat2 in Nipponbare that allowed FRetro3 to be domesticated to take over the role of the cannonical CRs.
The origin and functional divergence of the ORF0 sequences
Retrosat2 contains an extra and unique ORF, ORF0, whose origin and function has not been previously described. We identified homologous elements of Retrosat2 in wild rice species, maize and sorghum as well as Arabidopsis, soybean and Selaginella moellendorffii. These results suggest an ancient origin of Retrosat2 where an ancestral element was present before the divergence of S. moellendorffii, dicotyledonous and monocotyledonous plants. We found that homologs in wild rice species, maize and sorghum all contain the ORF0 domain but it was not found outside the grass family. One explanation is that the ORF0 was present the ancestral element but was deleted in Arabidopsis, soybean and other species after the divergence of the grass family from the other lineages. It is more likely, however, that the ORF0 evolved after the radiation of the grass from the other organisms.
In rice, two other LTR retroelements, RIRE3 and RIRE8, also contain ORF0 and ORF1 sequences [50] and are present in other Oryza species (Table S3). Retrosat2 likely shared a common ancestor with RIRE3 and RIRE8 based on the following observations: 1) Retrosat2 is structurally similar to RIRE3 and RIRE8, all three contain longer LTRs (∼3 kb), ORF0 and ORF1 sequences; 2) LTRs of both RIRE3 and RIRE8 show sequence identity with Retrosat2, and the 10-bp inverted terminal repeats of LTRs from RIRE8 and Retrosat2 are identical (5′TGTCACACCC–GGGCGTGACA3′); 3) ORF0 and ORF1 proteins of RIRE3 and RIRE8 share 30–34% and 69–70% similarity with Retrosat2, respectively; and 4) Phylogenetic trees based on both ORF0 and RTs of ORF1 show an evolutionary relationship between RIRE3, RIRE8 and Retrosat2 (Figure 6A,B).
A possible evolutionary scenario would be that the ancestral Retrosat2 element and homologs contained one ORF that encoded a retrotransposase. Sometime between the separation of dicotyledonous and monocotyledonous plants (about 200 MYA) and the formation of the grasses (∼50–80 MYA), ORF0 evolved via a frameshift mutation or through capture of some unrelated DNA sequence. The divergence of Retrosat2, RIRE3 and RIRE8 likely occurred before the divergence of the Oryza genus (10–20 MYA) as the three families are detected in all 12 genomes comprising the Oryza genus (Table S3).
The ORF1 sequences of Retrosat2 and homologs encode all the enzymes necessary for retrotransposition, thus, there is no obvious function for the ORF0. An amino acid sequence alignment of the ORF0 members reveals a wide range of sequence identities (7.6 to 90.2%) which is also reflected in the overall folding pattern of the ORF0 protein members. In a few cases, some of the predicted structures do share a common fold (Sat2-min, Sat2-pun, and Sat2-ruf) with discernible domains in each monomeric subunit, showing differences in the 2D structural elements. Observed differences in organization of these domains among the various ORF0 proteins reflects intrinsic features of structural and functional protein divergences. Comparative analyses of protein electrostatic potentials and structural modeling are key tools for enzyme classification and functional characterization. Electrostatic potentials of ORF0 enzymes allowed us to organize them and compare possible functional differences. The 3D structural and the functional analysis of ORF0 by gene ontology annotations suggest that the ORF0s encode enzymes implicated in metabolism (catabolic processes) with hydrolyses activities, but it is not clear how the ORF0 may affect transposition of Retrosat2. Moreover, we identified specific protein surface interaction properties (protein-protein, protein-cofactor and/or protein-substrate interactions) in different domains of the ORF0 protein members.
We observed differential abundance and distribution of positive and negative charges in the protein surfaces, which might correlate with differences or similarities in their activity, ligand-binding or protein-protein interactions [51], as well as differences in the mechanism of action and/or interaction with other proteins and intracellular targets [52]. This differential distribution could directly affect the interaction of the protein with other partners and target it to different sub-cellular localizations. Functional differences are also reflected in the surfaces of the ligand-binding domains and the cofactor-binding domains, which are more different in electrostatic charge distributions inside the ORF0 protein members.
Multiple regulatory signals were found in LTRs of Retrosat2 and homologs were found in dicotyledons, monocotyledons and S. moellendorffii which suggest ancient origin of this retroelement family. Unlike CRs and other retrotransposons, members of Retrosat2 family had distinct chromosomal/genomic distribution patterns that varied by species. Structural and functional divergence of ORF0s was seen via 3D predictions of ORF0 proteins and comparisons of their electrostatic surfaces, structural and key functional residues. In addition, the ORF0s likely encode enzymes implicated in metabolism (catabolic processes). Our data provide insight into the structural and functional features of ORF0 proteins and indicates that the genomic distribution and evolutionary functions of Retrosat2 homologs, derived from an ancient retrotransposon family, have been subjected to unique selection pressures in their host genomes that have resulted in varied evolutionary trajectories.
Materials and Methods
Genome Database
The genome sequence of Nipponbare (Oryza sativa ssp. japonica) was downloaded from the International Rice Genome Sequencing Project (IRGSP) website (http://rgp.dna.affrc.go.jp/E/IRGSP/index.html). The draft genome sequence of 93–11 was downloaded from the BGI website (http://rice.genomics.org.cn/rice/link/download.jsp). Other genome sequences, including maize, sorghum, Brachypodium, Arabidopsis, papaya, soybean, wine grape poplar and Selaginella moellendorffii, were obtained from the PlantGDB website (http://www.plantgdb.org/prj/GenomeBrowser).
Southern blot analysis
Young leaves from Nipponbare and other 13 rice species, including O. glaberrima (AA), O. nivara (AA), O. longistaminata (AA), O. rufipogon (AA), O. punctata (BB), O. minuta (BBCC), O. officinalis (CC), O. alta (CCDD), O. australiensis (EE), O. brachyantha (FF), O. granulata (GG), O. ridleyi (HHJJ) and O. coarctata (HHKK), were used to extract the genomic DNAs. 6 µg genomic DNAs of all 14 rice species were treated by EcoRI enzyme (Invitrogen, Carlsbad, CA) at 37 C for 10 h. Digested DNAs were separated by electrophoresis on a 1.0% (w/v) agarose gel at 55 V for 11 h, and blotted onto Hybond N+ membrane (Amersham Biosciences, now part of GE Life Sciences). The LTR sequence of Retrosat2 was used as a probe to hybrid the genomic DNAs from 14 rice species. The PCR product was amplified using Nipponbare DNA as the template with the primers (forward: 5′ TGTGGAATTTTCCTTGAGTT3′; reverse: 5′ GAGTGGGGAGGAGAGAGA-3′), and then was labeled with [32P] dCTP using the rediprime II random prime labeling system (Amersham Biosciences). Hybridizations were performed at 56°C for overnight and washed in 1.5×SSC/0.1% SDS solution for 35 min and in 1×SSC/0.1% SDS solution for 35 min. The membrane was exposed on a Fuji-image plate for 36 h, and the hybridization signals were captured using a Fujifilm FLA scanner.
RT-PCR
Total RNA was isolated from leaves, sheaths of 4-week old plants and young spikes (3–5 cm) of Nipponbare using the TRIZOL Reagent (Invitrogen, Carlsbad, CA). Five microgram RNA from each sample was converted into single-strand cDNA with reverse transcriptase (Invitrogen, Carlsbad, CA) according to the manufacture’s recommendations. Reactions were diluted 4- to 5-fold, and 2 µl of the diluted cDNAs were used as templates for PCR amplifications with the forward primer (5′-TGCCCTGGAAGAACTTATCG-3′) and reverse primer (5′ ACCACACCTCAGGTTTCACC-3′) which target the U5 region of Retrosat2 LTRs. The rice actin gene (Os03g0718100) was amplified in parallel with Retrosat2 as the control for quantitative comparison of mRNA levels, using actinF (5′-CAAGGCCAATCGTGAGAA-3′) and actinR (5′-AGCAATGCCAGGGAACATAGT-3′) primers. All plants of Nipponbare were grown in a greenhouse at University of Georgia.
Fluorescence in situ hybridization (FISH) analysis
Slide preparation and FISH were conducted following published protocols [53]. Briefly, young panicles of Oryza sativa L. ssp. japonica cv. Nipponbare were harvested and fixed in 3∶1 ethanol and glacial acetic acid for 24 hrs at room temperature, and then stored at 4°C. Pachytene chromosomes were prepared by squashing anthers in acetic acid. Slides were stored at −80°C until use. The plasmid clone containing the rice specific satellite repeat CentO (GenBank accession: AF058902) was provided by Drs. Jiming Jiang and Jason Walling at University of Wisconsin and used to determine the locations of centromeres. A pair of PCR primers (Forward: 5′-GCTCCGTTTAATCCCATTCA-3′, Reverse: 5′-TGTATTAAAACCCCCGTCCA-3′) were used to amplify the LTR sequence of Retrosat2 from Nipponbare that was then used as a probe for FISH. CentO and Retrosat2 were nick translated with biotin dUTP or digoxigenin dUTP (Roche), and visualized with Streptavidin Alexa Fluor 488 (Invitrogen) or Anti-digoxigenin-rhodamine (Roche), respectively. The chromosome images were captured with a Nikon Eclipse 80i microscope (http://www.nikon.com), equipped with a Photometrics CoolSnap HQ CCD camera (http://www.photometrics.com), controlled with MetaVue imaging software (http://www.moleculardevices.com/). Adobe Photoshop CS3 (Adobe Systems Incorporated) was used to produce publication images.
Phylogenetic analysis and calculation of Ka/Ks values of ORF0 and ORF1 sequences
The ORF0 and ORF1 sequences of Retrosat2 and other LTR retrotransposons were used to generate multiple alignments using CLUSTALW (http://www.ebi.ac.uk/clustalw) with default options. Phylogenetic trees were generated using the neighbor-joining method with the MEGA4 program (http://www.megasoftware.net). A total of 37 LTR retrotransposons were used to construct the phylogenetic trees, including Retrosat2 (AF111709), RIRE2 (AB030283), RIRE3 (AB014738), RIRE7 (AB033235), RIRE8 (AB014740) and other seven rice LTR retroelements (Osr28, Osr30, Osr35, Osr38, Osr39, Osr40 and Osr42 [54]), four elemements (OGRetro3, OGRetro9, OGRetro23 and OGRetro52) in O. glanulata [28], Sat2-ruf in O. rufipogon (FJ581045), Sat2-pun in O. punctata (AC215214), Sat2-min in O. minuta (AC232156), Sat2-off in O. officinalis (AC240793), dingo in O. australiensis [30], FRetro3 in O. brachyantha [29], Reina (U69258) and CRM (AY129008) in maize, Retrosor1 (AF098806) in sorghum, cereba (AY040832) in barley, Athena in Arabidopsis (AC007209), Cft1 in Cladosporium fulvum (Z11866) and gypsy in Drosophila (M12927). The phylogenetic analysis was conducted based on 1000 bootstrap replicates. The synonymous (Ks) and non-synonymous (Ka) substitution rates of the ORF0 and ORF1 sequences were calculated using the PAL2NAL program [55].
Homology modeling
To understand and compare the structural and molecular conformation between proteins, all sequences were modeled using the top ten PDB closed template structures by SWISS-MODEL, a protein structure homology-modeling server, via the ExPASy web server [56]–[58]. Several models were generated and evaluated. The different protein models were subjected to energy minimization with GROMOS96 force field energy [59] implemented in DeepView/Swiss-PDBViewer v3.7 [60] to improve the van der Waals contacts and correct the stereochemistry of the model. Stereochemical quality of the selected model was evaluated using PROCHECK [61], PROSA [62] and WHATCHECK [63] programs, as well as the protein energy with ANOLEA [64]. The Ramachandran plot statistics for the models were calculated to show the number of protein residues in the favored regions. Every protein model was superimposed on the best template crystal structure, as well as structural comparisons between them to calculate average distance between their Cα backbones. Protein superimpositions and surface protein contours analysis were performed and visualized in PyMol software [65].
The theoretical model was submitted to ConSurf server [66] in order to generate evolutionary related conservation scores aimed to the identification of functional region in proteins. Functional and structural key residues in the ORF0 sequences were confirmed by ConSeq server [67].
Ligand-binding domains and function prediction based on the protein structures
Prediction of the best identified ligand-binding site or domain in the built structure was made by sequence and structure-based approaches to protein function inference and ligand screening. This approach use an algorithm for ligand binding site prediction, ligand screening and molecular function prediction, which is based on binding site conservation across evolutionary distant proteins identified by threading [68].
The identification of functional analogs of the query protein based on the built 3D models predicted in Gene Ontology (GO) terms, showing the molecular function and biological processes in which proteins are implicated, as well as Enzyme Classification (EC) numbers are good indicator of the functional similarity between the query and the identified enzyme analogs. This process was developed by using The Gene Ontology project describing fundamental characteristics of genes and their products [69].
Electrostatic potentials
Electrostatic Poisson-Boltzmann (PB) potentials were obtained by using the APBS 1.0.0 plugging [70] for the molecular modeling software PyMol software (DeLano Scientific LLC), with AMBER99 [71] to assign the charges and radii to all of the atoms (including hydrogens), which were added and optimized with the Python software package PDB2PQR [72].
Fine grid spaces of 0.35 A° were used to solve the linearized PB equation in sequential focusing multigrid calculations in a mesh of 130 points per dimension at 310.00 K. The dielectric constants were two for the protein and 80.00 for water. The output mesh was processed in the scalar OpenDX format to render isocontours and maps onto the surfaces with PyMOL software. Potential values are given in units of kT per unit charge (k Boltzmann's constant; T temperature).
Supporting Information
RT-PCR of Retrosat2 . L, S, F means leaf, sheath and flower, respectively.
(TIF)
Dot-plot of LTRs of Retrosat2 and the homologs.
(TIF)
Detailed structural conformation and conservation analysis of ORF0 of Sat2-off. (A) General structure (cartoon diagram) shows the superimposition of ORF0 of Sat2-off (white) and rice RetroSat2 (blue), with a RMSD = 5.693Å calculated for the superimposition of structural carbon α. Represented structures were rotated at 180u. (B) The surface conformation of Sat2-off (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of Sat2-off structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted Sat2-off model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of Sat2-off model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand SO4, which are D138, H141, R146, and A160.
(TIF)
Detailed structural conformation and conservation analysis of FRetro3 , a rice ORF0 protein member. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the rice FRetro3, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of FRetro3 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of FRetro3 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted FRetro3 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of FRetro3 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand 2-(acetylamino)-2-deoxy-α-D-Glucopyranose (NDG), which are F13, V37, L38, R39, A53, E54, A67, and A87.
(TIF)
Detailed structural conformation and conservation analysis of ORF0 sequence of BraSat2. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the ORF0 of BraSat2, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of BraSat2 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of BraSat2 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted BraSat2 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of BraSat2 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand FE2/S2 (inorganic) cluster (FES), which are A10, G11, Q12, D13, L15, F17, E18, Q20, and P60.
(TIF)
Detailed structural conformation and conservation analysis of RIRE8 , a rice ORF0 protein member. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the rice RIRE8, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of RIRE8 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of RIRE8 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted RIRE8 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of RIRE8 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand cobalt (Co), which are E121, T124, D143, and D148.
(TIF)
The Retrosat2 element in Nipponbare genome.
(DOC)
Homologous elements of Retrosat2 in Oryza genus.
(DOC)
Distributions of Retrosat2 , RIRE3 and RIRE8 families in BESs of 12 genomes.
(DOC)
Regulatory signals in the LTR region of Retrosat2 .
(DOC)
Structural similarity among selected ORF0 protein members.
(DOC)
Functional characteristics of the ORF0 protein members based on structural features.
(DOC)
Acknowledgments
We thank Drs. Jiming Jiang and Jason Walling for providing the rice CentO probe and Brian Abernathy for computational assistance.
Funding Statement
The National Science Foundation 0424833, Division of Biological Infrastructure (DBI) 0603927, Integrated and Organismal Biology (IOS) 0701382 and Molecular and Cellular Biosciences (MCB) 1026200 provided funding. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Wessler SR (2006) Transposable elements and the evolution of eukaryotic genomes. Proc Natl Acad Sci U S A. 103: 17600–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. International rice genome sequencing project (2005) The map-based sequence of the rice genome. Nature 436: 793–800. [DOI] [PubMed] [Google Scholar]
- 3. Schnable PS, Ware D, Fulton RS, Stein JC, Wei F, et al. (2009) The B73 maize genome: complexity, diversity, and dynamics. Science. 326: 1112–1115. [DOI] [PubMed] [Google Scholar]
- 4. Perlman PS, Boeke JD (2004) Ring around the retroelement. Science. 303: 182–184. [DOI] [PubMed] [Google Scholar]
- 5. Wicker T, Sabot F, Hua-Van A, Bennetzen JL, Capy P, et al. (2007) A unified classification system for eukaryotic transposable elements. Nat Rev Genet. 8: 973–982. [DOI] [PubMed] [Google Scholar]
- 6. Laten HM, Majumdar A, Gaucher EA (1998) SIRE-1, a copia/Ty1-like retroelement from soybean, encodes a retroviral envelope-like protein. Proc Natl Acad Sci U S A. 95: 6897–6902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Wright DA, Voytas DF (2002) Athila4 of Arabidopsis and Calypso of soybean define a lineage of endogenous plant retroviruses. Genome Res. 12: 122–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Temin HM (1981) Structure, variation and synthesis of retrovirus long terminal repeat. Cell. 27: l–3. [DOI] [PubMed] [Google Scholar]
- 9. Perlman PS, Boeke JD (2004) Molecular biology. Ring around the retroelement. Science. 303: 182–184. [DOI] [PubMed] [Google Scholar]
- 10. Prudhomme S, Oriol G, Mallet F (2004) A retroviral promoter and a cellular enhancer define a bipartite element which controls env ERVWE1 placental expression. J Virol. 78: 12157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Takeda S, Sugimoto K, Otsuki H, Hirochika H (1999) A 13-bp cis-regulatory element in the LTR promoter of the tobacco retrotransposon Tto1 is involved in responsiveness to tissue culture, wounding, methyl jasmonate and fungal elicitors. Plant J. 18: 383–393. [DOI] [PubMed] [Google Scholar]
- 12. Rhind N, Chen Z, Yassour M, Thompson DA, Haas BJ, et al. (2011) Comparative functional genomics of the fission yeasts. Science. 332: 930–936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ling J, Pi W, Yu X, Bengra C, Long Q, et al. (2003) The ERV-9 LTR enhancer is not blocked by the HS5 insulator and synthesizes through the HS5 site non-coding, long RNAs that regulate LTR enhancer function. Nucleic Acids Res. 31: :4582–4596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pi W, Zhu X, Wu M, Wang Y, Fulzele S, et al. (2007) Long-range function of an intergenic retrotransposon. Proc Natl Acad Sci U S A. 107: 12992–12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Romanish MT, Lock WM, van de Lagemaat LN, Dunn CA, Mager DL (2007) Repeated recruitment of LTR retrotransposons as promoters by the anti-apoptotic locus NAIP during mammalian evolution. PLoS Genet. 3: e10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Michaille JJ, Mathavan S, Gaillard J, Garel A (1990) The complete sequence of mag, a new retrotransposon in Bombyx mori. Nucleic Acids Res. 18(3): 674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gao D, Chen JF, Chen MS, Meyers B, Jackson A (2012) A highly conserved, small LTR retrotransposon that preferentially targets genes in grass genomes. Plos One. 7: e32010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Neumann P, Pozárková D, Macas J (2003) Highly abundant pea LTR retrotransposon Ogre is constitutively transcribed and partially spliced. Plant Mol Biol. 53: 399–410. [DOI] [PubMed] [Google Scholar]
- 19. Feschotte C, Jiang N, Wessler SR (2002) Plant transposable elements: where genetics meets genomics. Nat Rev Genet. 3: 329–341. [DOI] [PubMed] [Google Scholar]
- 20. Bushman FD (2003) Targeting survival: integration site selection by retroviruses and LTR-retrotransposons. Cell. 115: 135–138. [DOI] [PubMed] [Google Scholar]
- 21. Chalker DL, Sandmeyer SB (1992) Ty3 integrates within the region of RNA polymerase III transcription initiation. Genes Dev. 6: 117–128. [DOI] [PubMed] [Google Scholar]
- 22. Miyao A, Tanaka K, Murata K, Sawaki H, Takeda S, et al. (2003) Target site specificity of the Tos17 retrotransposon shows a preference for insertion within genes and against insertion in retrotransposon-rich regions of the genome. Plant Cell. 15: 1771–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kumar A, Bennetzen JL (1999) Plant retrotransposons. Annu Rev Genet. 33: :479–532. [DOI] [PubMed] [Google Scholar]
- 24. Dong F, Miller JT, Jackson SA, Wang GL, Ronald PC, et al. (1998) Rice (Oryza sativa) centromeric regions consist of complex DNA. Proc Natl Acad Sci U S A. 95: 8135–8140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Miller JT, Dong F, Jackson SA, Song J, Jiang J (1998) Retrotransposon-related DNA sequences in the centromeres of grass chromosomes. Genetics. 150: :1615–1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Presting GG, Malysheva L, Fuchs J, Schubert I (1998) A Ty3/gypsy retrotransposon-like sequence localizes to the centromeric regions of cereal chromosomes. Plant J. 16: 721–728. [DOI] [PubMed] [Google Scholar]
- 27. Liu Z, Yue W, Li D, Wang RR, Kong X (2008) et (2008) Structure and dynamics of retrotransposons at wheat centromeres and pericentromeres. Chromosoma. 117: 445–456. [DOI] [PubMed] [Google Scholar]
- 28. Gao D, Gong Z, Wing RA, Jiang J, Jackson SA (2011) Molecular and cytological characterization of centromeric retrotransposons in a wild relative of rice, Oryza granulata . Tropical Plant Biol. 4: 217–227. [Google Scholar]
- 29. Gao D, Gill N, Kim HR, Walling JG, Zhang W, et al. (2009) A lineage-specific centromere retrotransposon in Oryza brachyantha . Plant J. 60: 820–831. [DOI] [PubMed] [Google Scholar]
- 30.Piegu B, Guyot R, Picault N, Roulin A, Sanyal A, et al. (2006) Doubling genome size without polyploidization: dynamics of retrotransposition-driven genomic expansions in Oryza australiensis, a wild relative of rice. Genome Res. 16: :1262–1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. SanMiguel P, Gaut BS, Tikhonov A, Nakajima Y, Bennetzen JL (1998) The paleontology of intergene retrotransposons of maize. Nat Genet. 20: 43–45. [DOI] [PubMed] [Google Scholar]
- 32. Ma J, Bennetzen JL (2004) Rapid recent growth and divergence of rice nuclear genomes. Proc. Natl. Acad. Sci. USA 101: 12404–12410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Guntaka RV (1993) Transcription termination and polyadenylation in retroviruses. Microbiol Rev (57): 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Benachenhou F, Jern P, Oja M, Sperber G, Blikstad V, et al. (2009) Evolutionary conservation of orthoretroviral long terminal repeats (LTRs) and ab initio detection of single LTRs in genomic data. PLoS One. 4: e5179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lescot M, Déhais P, Thijs G, Marchal K, Moreau Y, et al. (2002) PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 30: 325–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Matthews CM, Vandenberg CJ, Trotman CN (1998) Variable substitution rates of the 18 domain sequences in Artemia hemoglobin. J Mol Evol. 46: 729–733. [DOI] [PubMed] [Google Scholar]
- 37. Benkert P, Biasini M, Schwede T (2011) Toward the estimation of the absolute quality of individual protein structure models. Bioinformatics 27: 343–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA 98: 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Mandell JG, Roberts VA, Pique ME, Kotlovyi V, Mitchell JC, et al. (2001) Protein docking using continuum electrostatics and geometric fit. Peds 14: 105–113. [DOI] [PubMed] [Google Scholar]
- 40. Goldenberg NM, Steinberg BE (2010) Surface charge: a key determinant of protein localization and function. Cancer Research 70: 1277–1280. [DOI] [PubMed] [Google Scholar]
- 41. Goodwin TJ, Poulter RT (2000) Multiple LTR-retrotransposon families in the asexual yeast Candida albicans. Genome Res. 10: 174–191. [DOI] [PubMed] [Google Scholar]
- 42. Bowen NJ, McDonald JF (2001) Drosophila euchromatic LTR retrotransposons are much younger than the host species in which they reside. Genome Res. 11: 1527–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marsano RM (2005) Caizzi R.A genome-wide screening of BEL-Pao like retrotransposons in Anopheles gambiae by the LTR_STRUC program (2005) Gene. 357: 115–121. [DOI] [PubMed] [Google Scholar]
- 44. Wang H, Liu JS (2008) LTR retrotransposon landscape in Medicago truncatula: more rapid removal than in rice. BMC Genomics. 9: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Vitte C, Panaud O, Quesneville H (2007) LTR retrotransposons in rice (Oryza sativa, L.): recent burst amplifications followed by rapid DNA loss. BMC Genomics. 8: 218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lauermann V, Hermankova M, Boeke JD (1997) Increased length of long terminal repeats inhibits Ty1 transposition and leads to the formation of tandem multimers. Genetics. 145: 911–922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Chang W, Schulman AH (2008) BARE retrotransposons produce multiple groups of rarely polyadenylated transcripts from two differentially regulated promoters. Plant J. 56: 40–50. [DOI] [PubMed] [Google Scholar]
- 48.Jiang N, Bao Z, Temnykh S, Cheng Z, Jiang J, et al. (2002) Dasheng: a recently amplified nonautonomous long terminal repeat element that is a major component of pericentromeric regions in rice. Genetics 161: , 1293–1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ammiraju JS, Zuccolo A, Yu Y, Song X, Piegu B, et al. (2007) Evolutionary dynamics of an ancient retrotransposon family provides insights into evolution of genome size in the genus Oryza. . Plant J. 52: 342–351. [DOI] [PubMed] [Google Scholar]
- 50. Kumekawa N, Ohtsubo H, Horiuchi T, Ohtsubo E (1999) Identification and characterization of novel retrotransposons of the gypsy type in rice. Mol Gen Genet. 260: 593–602. [DOI] [PubMed] [Google Scholar]
- 51. Mandell JG, Roberts VA, Pique ME, Kotlovyi V, Mitchell JC, et al. (2001) Protein docking using continuum electrostatics and geometric fit. Peds. 14: 105–113. [DOI] [PubMed] [Google Scholar]
- 52. Goldenberg NM, Steinberg BE (2010) Surface charge: a key determinant of protein localization and function. Cancer Research. 70: 1277–1280. [DOI] [PubMed] [Google Scholar]
- 53.Cheng Z, Buell CR, Wing RA, Gu M, Jiang J (2001) Toward a cytological characterization of the rice genome. Genome Res. 11: , 2133–2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Gao L, McCarthy EM, Ganko EW, McDonald JF (2004) Evolutionary history of Evolutionary history of Oryza sativa LTR retrotransposons: a preliminary survey of the rice genome sequences. BMC Genomics. 5: 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Suyama M, Torrents D, Bork P (2006) PAL2NAL: robust conversion of protein sequence alignments into the corresponding codon alignments. Nucleic Acids Res. 34: , W609–W612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Peitsch MC (1995) Protein modelling by e-mail. Nat Biotechnol 13: 658–660. [Google Scholar]
- 57. Arnold K, Bordoli L, Kopp J, Schwede T (2006) The SWISSMODEL workspace: a web-based environment for protein structure homology modeling. Bioinformatics. 22: 195–201. [DOI] [PubMed] [Google Scholar]
- 58. Kiefer F, Arnold K, Kunzli M, Bordoli L, Schwede T (2009) The SWISS-MODEL repository and associated resources. Nucl Acids Res. 37: D387–D392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Christen M, Hunenberger PH, Bakowies D, Baron R, Burgi R, et al. (2005) The GROMOS software for biomolecular simulation: GROMOS05. J Comput Chem. 26: 1719–1751. [DOI] [PubMed] [Google Scholar]
- 60. Guex N, Peitsch MC (1997) SWISS-MODEL and the Swiss-PdbViewer: an environment for comparative protein modeling. Electrophoresis. 18: 2714–2723. [DOI] [PubMed] [Google Scholar]
- 61. Laskowski RA, MacArthur MW, Moss DS, Thornton JM (1993) PROCHECK: a program to check the stereo-chemical quality of protein structures. J Appl Cryst 26: 283–291. [Google Scholar]
- 62.Wiederstein M, Sippl MJ. (2007). ProSA-web: interactive web service for the recognition of errors in three-dimensional structures of proteins. Nucl Acids Res. 35: W407–W410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hooft RWW, Vriend G, Sander C, Abola EE (1996). WHATCHECK. This is the “official” WHAT_CHECK reference Errors in protein structures. Nature. 381: , 272–272. [DOI] [PubMed] [Google Scholar]
- 64. Melo F, Feytmans E (1998) Assessing protein structures with a non-local atomic interaction energy. J Mol Biol. 277: 1141–1152. [DOI] [PubMed] [Google Scholar]
- 65.DeLano W.L. (2002). The PyMOL molecular graphics system. DeLano Scientific, San Carlos
- 66. Landau M, Mayrose I, Rosenberg Y, Glaser F, Martz E, et al. (2005) ConSurf: the projection of evolutionary conservation scores of residues on protein structures. Nucl Acids Res. 33: 299–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Berezin C, Glaser F, Rosenberg Y, Paz I, Pupko T, et al. (2004) ConSeq: The Identification of Functionally and Structurally Important Residues in Protein Sequences. Bioinformatics 20: 1322–1324. [DOI] [PubMed] [Google Scholar]
- 68. Skolnick J, Brylinski M (2009) FINDSITE: a combined evolution/structure-based approach to protein function prediction. Briefings in bioinformatics. 10: 378–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Carbon S, Ireland A, Mungall CJ, Shu S, Marshall B, et al. (2009) AmiGO: online access to ontology and annotation data. Bioinformatics. 25: 288–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Baker NA, Sept D, Joseph S, Holst MJ, McCammon JA (2001) Electrostatics of nanosystems: application to microtubules and the ribosome. Proc Natl Acad Sci USA. 98: 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Cieplak P, Kollman PA (2000) How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J Comput Chem. 21: 1049–1074. [Google Scholar]
- 72. Dolinsky TJ, Czodrowski P, Li H, Nielsen JE, Jensen JH, et al. (2007) PDB2PQR: expanding and upgrading automated preparation of biomolecular structures for molecular simulations. Nucl Acids Res 35: 522–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
RT-PCR of Retrosat2 . L, S, F means leaf, sheath and flower, respectively.
(TIF)
Dot-plot of LTRs of Retrosat2 and the homologs.
(TIF)
Detailed structural conformation and conservation analysis of ORF0 of Sat2-off. (A) General structure (cartoon diagram) shows the superimposition of ORF0 of Sat2-off (white) and rice RetroSat2 (blue), with a RMSD = 5.693Å calculated for the superimposition of structural carbon α. Represented structures were rotated at 180u. (B) The surface conformation of Sat2-off (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of Sat2-off structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted Sat2-off model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of Sat2-off model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand SO4, which are D138, H141, R146, and A160.
(TIF)
Detailed structural conformation and conservation analysis of FRetro3 , a rice ORF0 protein member. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the rice FRetro3, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of FRetro3 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of FRetro3 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted FRetro3 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of FRetro3 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand 2-(acetylamino)-2-deoxy-α-D-Glucopyranose (NDG), which are F13, V37, L38, R39, A53, E54, A67, and A87.
(TIF)
Detailed structural conformation and conservation analysis of ORF0 sequence of BraSat2. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the ORF0 of BraSat2, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of BraSat2 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of BraSat2 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted BraSat2 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of BraSat2 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand FE2/S2 (inorganic) cluster (FES), which are A10, G11, Q12, D13, L15, F17, E18, Q20, and P60.
(TIF)
Detailed structural conformation and conservation analysis of RIRE8 , a rice ORF0 protein member. (A) General structure (cartoon diagram rainbow colored) shows the 2D structural elements of the rice RIRE8, where N- and C-terminal are colored blue and red respectively. Represented structures were rotated at 180u. (B) The surface conformation of RIRE8 (rotated 180u) showing the secondary structure elements inside is depicted. (C) Electrostatic surface potential showing front, back, top and bottom views of RIRE8 structure. The surface colors are clamped at red (−10) or blue (+10). Top and bottom views are highlighted with a white line coming from front view. (D) Best predicted RIRE8 model (2D-structure) was subject to consurf-conservational analysis searching for close homologous sequences with known structures using PSI-BLAST. The protein was finally visualized using FirstGlance in Jmol with the conservation scores being color-coded. The conserved and variable residues are presented as space-filled models and colored according to the conservation scores. A detailed view of the predicted ligand-binding cavity holding up the cofactor/ligand (van der Walls spheres and/or lines) is shown in high magnification. Represented structures were rotated at 180u. (E) Cartoon structural representation of a general front view of RIRE8 model (C- and N-terminal colored as blue and red respectively), showing the morphology of the predicted cofactor/ligand-binding pocket/cavity. A detailed view at higher magnification is highlighting the residues implicated in this cavity formation and interaction with the ligand cobalt (Co), which are E121, T124, D143, and D148.
(TIF)
The Retrosat2 element in Nipponbare genome.
(DOC)
Homologous elements of Retrosat2 in Oryza genus.
(DOC)
Distributions of Retrosat2 , RIRE3 and RIRE8 families in BESs of 12 genomes.
(DOC)
Regulatory signals in the LTR region of Retrosat2 .
(DOC)
Structural similarity among selected ORF0 protein members.
(DOC)
Functional characteristics of the ORF0 protein members based on structural features.
(DOC)