Abstract
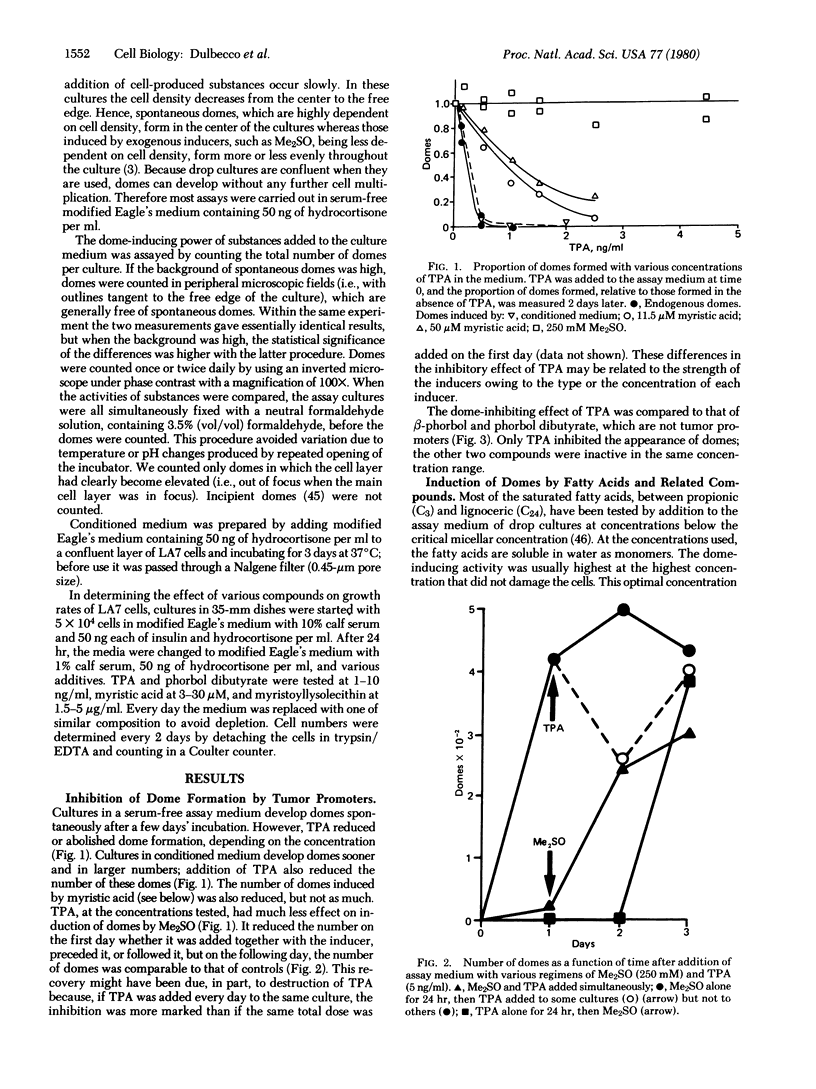
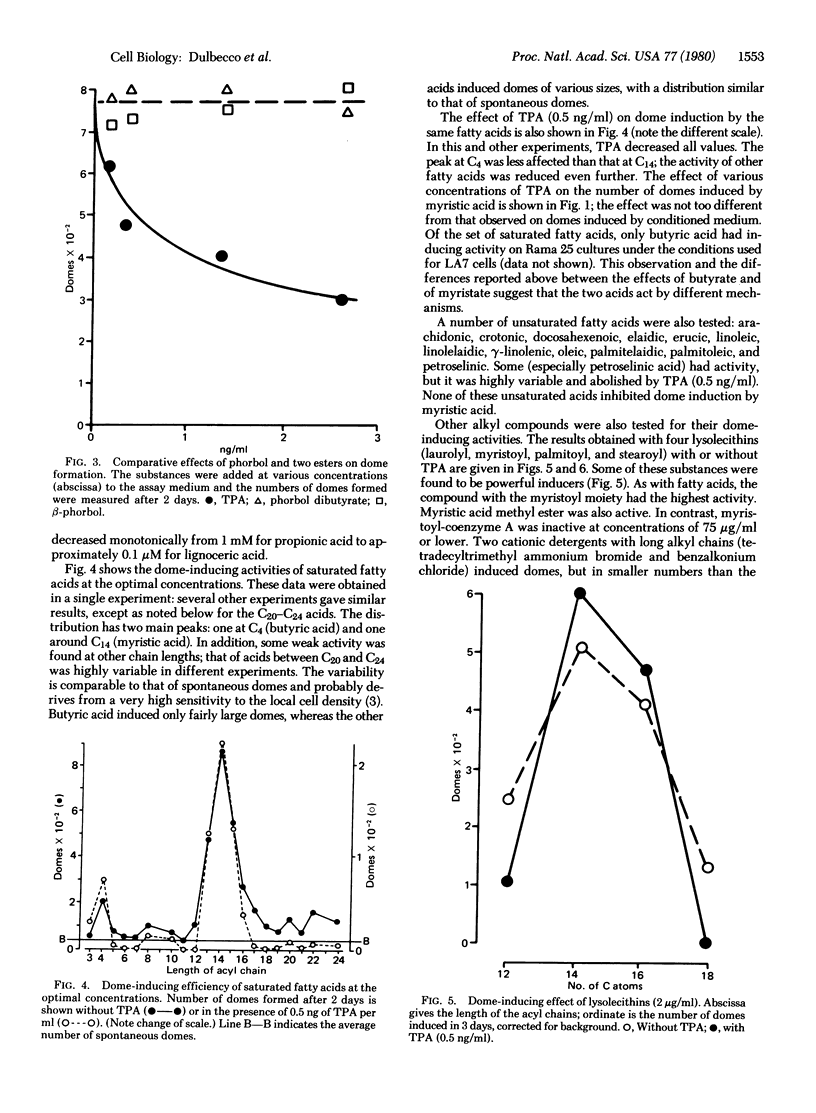
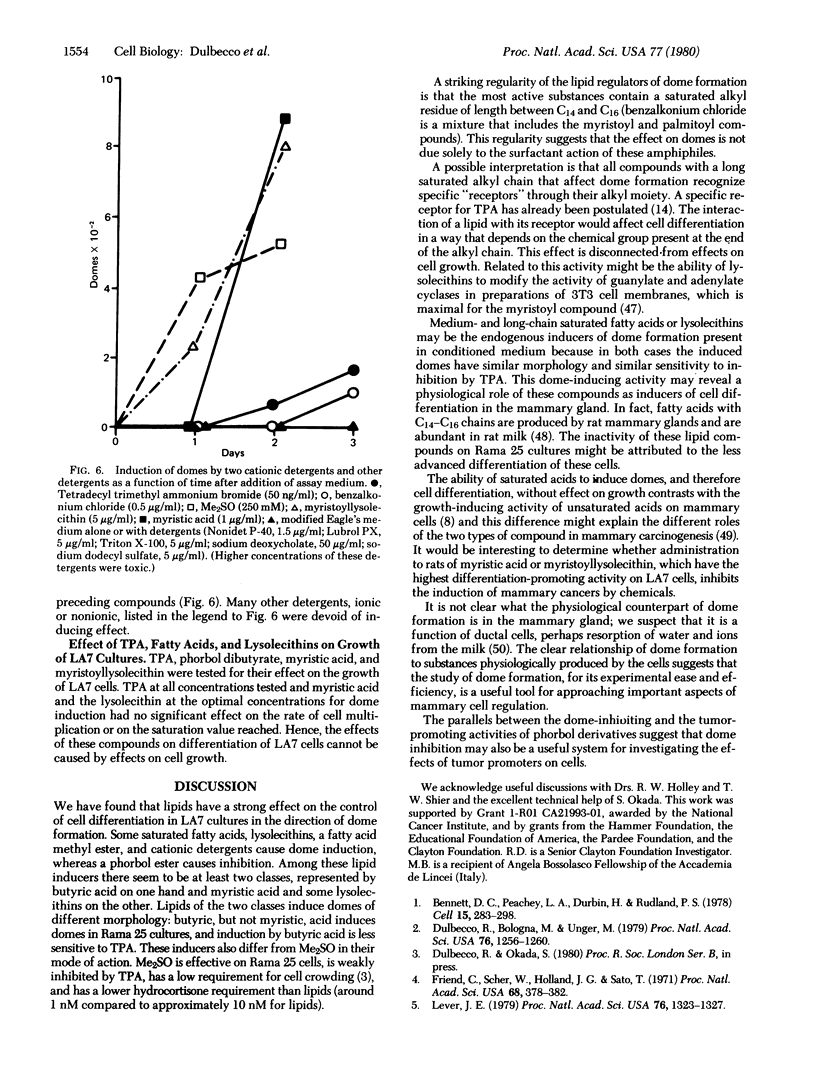
A rat mammary cell line (LA7) undergoes spontaneous differentiation into domes due to production of specific inducers by the cells. Some of these inducers may be lipids, and we show that lipids regulate this differentiation as both inducers and inhibitors. One inhibitor is the tumor promoter tetradecanoyl-13 phorbol 12-acetate. The inducers are saturated fatty acids of two groups: butyric acid and acids with chain lengths from C13 to C16, especially myristic acid (C14). Other inducers are myristoyl and palmitoyl lysolecithins, myristic acid methyl ester, and two cationic detergents with a tetradecenyl chain. We propose that the lipids with a C14-C16 alkyl chain affect differentiation by recognition specific receptors through their alkyl chains and that the effects obtained depend on the head groups. These lipids may be physiological regulators in the mammary gland.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett D. C., Peachey L. A., Durbin H., Rudland P. S. A possible mammary stem cell line. Cell. 1978 Sep;15(1):283–298. doi: 10.1016/0092-8674(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Blumberg P. M., Driedger P. E., Rossow P. W. Effect of a phorbol ester on a transformation-sensitive surface protein of chick fibroblasts. Nature. 1976 Dec 2;264(5585):446–447. doi: 10.1038/264446a0. [DOI] [PubMed] [Google Scholar]
- Boutwell R. K. The function and mechanism of promoters of carcinogenesis. CRC Crit Rev Toxicol. 1974 Jan;2(4):419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- Carroll K. K., Hopkins G. J. Dietary polyunsaturated fat versus saturated fat in relation to mammary carcinogenesis. Lipids. 1979 Feb;14(2):155–158. doi: 10.1007/BF02533866. [DOI] [PubMed] [Google Scholar]
- Christman J. K., Copp R. P., Pedrinan L., Whalen C. E. Specificity of response in hamster cells induced to produce plasminogen activator by the tumor promoter, 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1978 Nov;38(11 Pt 1):3854–3860. [PubMed] [Google Scholar]
- Cohen R., Pacifici M., Rubinstein N., Biehl J., Holtzer H. Effect of a tumour promoter on myogenesis. Nature. 1977 Apr 7;266(5602):538–540. doi: 10.1038/266538a0. [DOI] [PubMed] [Google Scholar]
- Delclos K. B., Blumberg P. M. Decrease in collagen production in normal and Rous sarcoma virus-transformed chick embryo fibroblasts induced by phorbol myristate acetate. Cancer Res. 1979 May;39(5):1667–1672. [PubMed] [Google Scholar]
- Diamond L., O'Brien S., Donaldson C., Shimizu Y. Growth stimulation of human diploid fibro-blasts by the tumor promoter, 12-0-tetradecanoly-phorbol-13-acetate. Int J Cancer. 1974 May 15;13(5):721–730. doi: 10.1002/ijc.2910130516. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Inhibition of adipose conversion of 3T3 fibroblasts by tumour promoters. Nature. 1977 Sep 15;269(5625):247–249. doi: 10.1038/269247a0. [DOI] [PubMed] [Google Scholar]
- Diamond L., O'Brien T. G., Rovera G. Tumor promoters: effects on proliferation and differentiation of cells in culture. Life Sci. 1978 Nov 13;23(20):1979–1988. doi: 10.1016/0024-3205(78)90229-1. [DOI] [PubMed] [Google Scholar]
- Dulbecco R., Bologna M., Unger M. Differentiation of a rat mammary cell line in vitro. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1256–1260. doi: 10.1073/pnas.76.3.1256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Bologna M., Unger M. Role of Thy-1 antigen in the in vitro differentiation of a rat mammary cell line. Proc Natl Acad Sci U S A. 1979 Apr;76(4):1848–1852. doi: 10.1073/pnas.76.4.1848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fibach E., Yamasaki H., Weinstein I. B., Marks P. A., Rifkind R. A. Heterogeneity of murine erythroleukemia cells with respect to tumor promoter-mediated inhibition of cell differentiation. Cancer Res. 1978 Nov;38(11 Pt 1):3685–3688. [PubMed] [Google Scholar]
- Friend C., Scher W., Holland J. G., Sato T. Hemoglobin synthesis in murine virus-induced leukemic cells in vitro: stimulation of erythroid differentiation by dimethyl sulfoxide. Proc Natl Acad Sci U S A. 1971 Feb;68(2):378–382. doi: 10.1073/pnas.68.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Heckman C., Langenbach R. Stimulation of differentiated functions in human melanoma cells by tumor-promoting agents and dimethyl sulfoxide. Cancer Res. 1979 Jul;39(7 Pt 1):2618–2624. [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Epidermal growth factor, like phorbol esters, induces plasminogen activator in HeLa cells. Nature. 1978 Aug 17;274(5672):696–697. doi: 10.1038/274696a0. [DOI] [PubMed] [Google Scholar]
- Lee L. S., Weinstein I. B. Tumor-promoting phorbol esters inhibit binding of epidermal growth factor to cellular receptors. Science. 1978 Oct 20;202(4365):313–315. doi: 10.1126/science.308698. [DOI] [PubMed] [Google Scholar]
- Lever J. E. Inducers of mammalian cell differentiation stimulate dome formation in a differentiated kidney epithelial cell line (MDCK). Proc Natl Acad Sci U S A. 1979 Mar;76(3):1323–1327. doi: 10.1073/pnas.76.3.1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzell J. L., Peaker M. Mechanism of milk secretion. Physiol Rev. 1971 Jul;51(3):564–597. doi: 10.1152/physrev.1971.51.3.564. [DOI] [PubMed] [Google Scholar]
- Lowe M. E., Pacifici M., Holtzer H. Effects of phorbol-12-myristate-13-acetate on the phenotypic program of cultured chondroblasts and fibroblasts. Cancer Res. 1978 Aug;38(8):2350–2356. [PubMed] [Google Scholar]
- Miao R. M., Filedsteel A. H., Fodge D. W. Opposing effects of tumor promoters on erythroid differentiation. Nature. 1978 Jul 20;274(5668):271–272. doi: 10.1038/274271a0. [DOI] [PubMed] [Google Scholar]
- Mondal S., Brankow D. W., Heidelberger C. Two-stage chemical oncogenesis in cultures of C3H/10T1/2 cells. Cancer Res. 1976 Jul;36(7 Pt 1):2254–2260. [PubMed] [Google Scholar]
- O'Brien T. G., Diamond L. A cell culture bioassay to analyze metabolism of phorbol diester tumor promoters. Cancer Res. 1978 Aug;38(8):2567–2572. [PubMed] [Google Scholar]
- O'Brien T. G., Simsiman R. C., Boutwell R. K. Induction of the polyamine-biosynthetic enzymes in mouse epidermis by tumor-promoting agents. Cancer Res. 1975 Jul;35(7):1662–1670. [PubMed] [Google Scholar]
- Rohrschneider L. R., Boutwell R. K. The early stimulation of phospholipid metabolism by 12-0-tetradecanoyl-phorbol-13-acetate and its specificity for tumor promotion. Cancer Res. 1973 Aug;33(8):1945–1952. [PubMed] [Google Scholar]
- Rohrschneider L. R., O'Brien D. H., Boutwell R. K. The stimulation of phospholipid metabolism in mouse skin following phorbol ester treatment. Biochim Biophys Acta. 1972 Sep 7;280(1):57–70. doi: 10.1016/0005-2760(72)90212-3. [DOI] [PubMed] [Google Scholar]
- Rovera G., O'Brien T. G., Diamond L. Tumor promoters inhibit spontaneous differentiation of Friend erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2894–2898. doi: 10.1073/pnas.74.7.2894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shier W. T., Baldwin J. H., Nilsen-Hamilton M., Hamilton R. T., Thanassi N. M. Regulation of guanylate and adenylate cyclase activities by lysolecithin. Proc Natl Acad Sci U S A. 1976 May;73(5):1586–1590. doi: 10.1073/pnas.73.5.1586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoyab M., De Larco J. E., Todaro G. J. Biologically active phorbol esters specifically alter affinity of epidermal growth factor membrane receptors. Nature. 1979 May 31;279(5712):387–391. doi: 10.1038/279387a0. [DOI] [PubMed] [Google Scholar]
- Sivak A. Induction of cell division: role of cell membrane sites. J Cell Physiol. 1972 Oct;80(2):167–173. doi: 10.1002/jcp.1040800203. [DOI] [PubMed] [Google Scholar]
- Sivak A., Van Duuren B. L. Phenotypic expression of transformation: induction in cell culture by a phorbol ester. Science. 1967 Sep 22;157(3795):1443–1444. doi: 10.1126/science.157.3795.1443. [DOI] [PubMed] [Google Scholar]
- Van Duuren B. L., Tseng S. S., Segal A., Smith A. C., Melchionne S., Seidman I. Effects of structural changes on the tumor-promoting activity of phorbol myristate acetate on mouse skin. Cancer Res. 1979 Jul;39(7 Pt 1):2644–2646. [PubMed] [Google Scholar]
- Van Duuren B. L. Tumor-promoting agents in two-stage carcinogenesis. Prog Exp Tumor Res. 1969;11:31–68. doi: 10.1159/000391388. [DOI] [PubMed] [Google Scholar]
- Wertz P. W., Mueller G. C. Rapid stimulation of phospholipid metabolism in bovine lymphocytes by tumor-promoting phorbol esters. Cancer Res. 1978 Sep;38(9):2900–2904. [PubMed] [Google Scholar]
- Wicha M. S., Liotta L. A., Kidwell W. R. Effects of free fatty acids on the growth of normal and neoplastic rat mammary epithelial cells. Cancer Res. 1979 Feb;39(2 Pt 1):426–435. [PubMed] [Google Scholar]
- Wigler M., DeFeo D., Weinstein I. B. Induction of plasminogen activator in cultured cells by macrocyclic plant diterpene esters and other agents related to tumor promotion. Cancer Res. 1978 May;38(5):1434–1437. [PubMed] [Google Scholar]
- Wigler M., Weinstein I. B. Tumour promotor induces plasminogen activator. Nature. 1976 Jan 22;259(5540):232–233. doi: 10.1038/259232a0. [DOI] [PubMed] [Google Scholar]
- Yamasaki H., Fibach E., Nudel U., Weinstein I. B., Rifkind R. A., Marks P. A. Tumor promoters inhibit spontaneous and induced differentiation of murine erythroleukemia cells in culture. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3451–3455. doi: 10.1073/pnas.74.8.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]



