Abstract
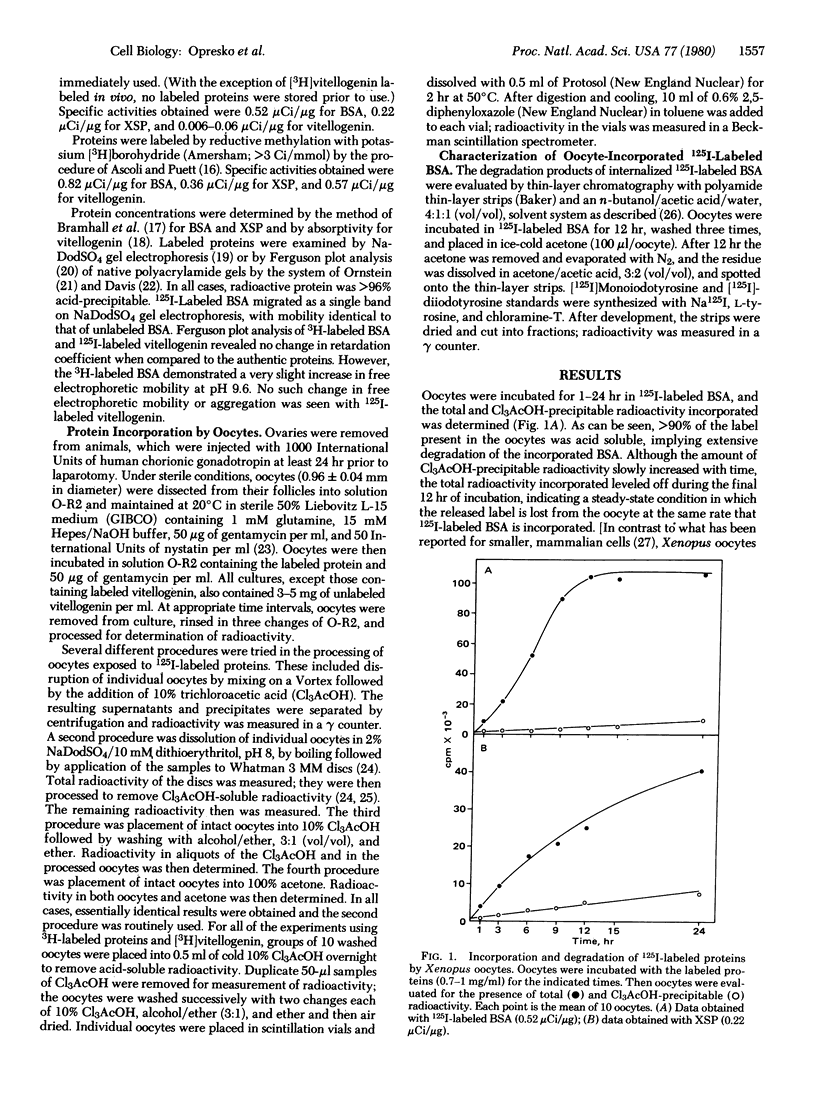
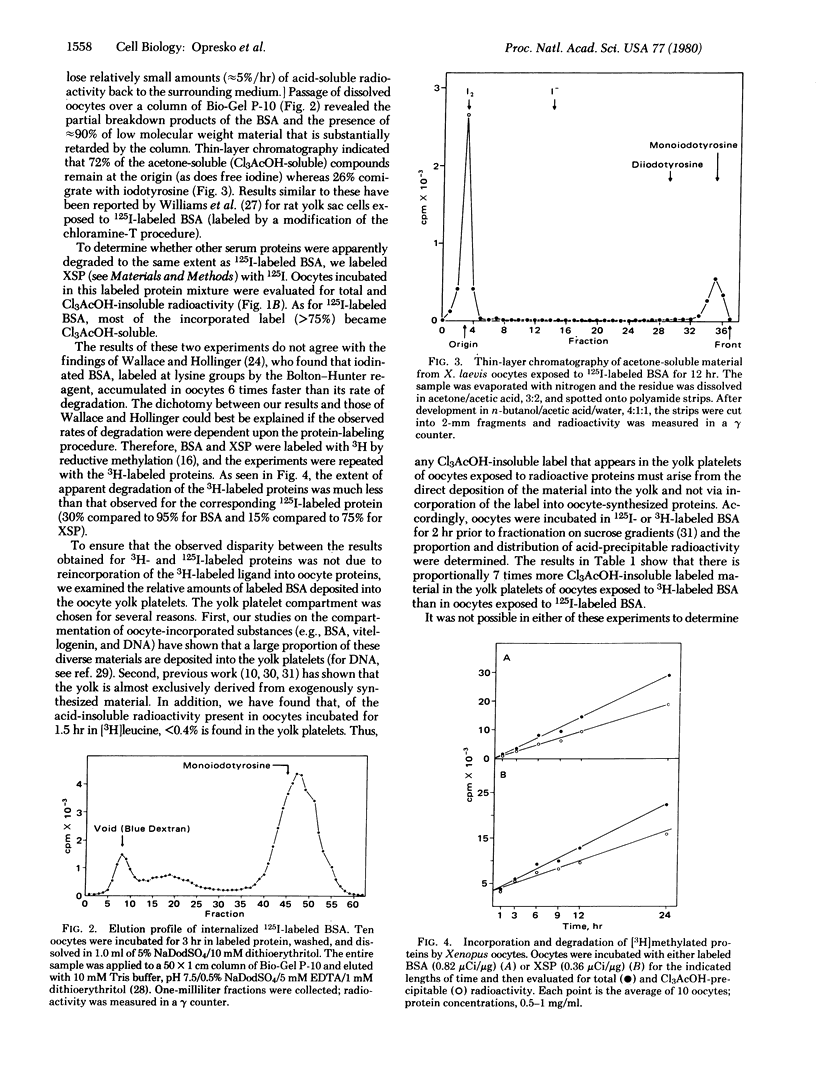
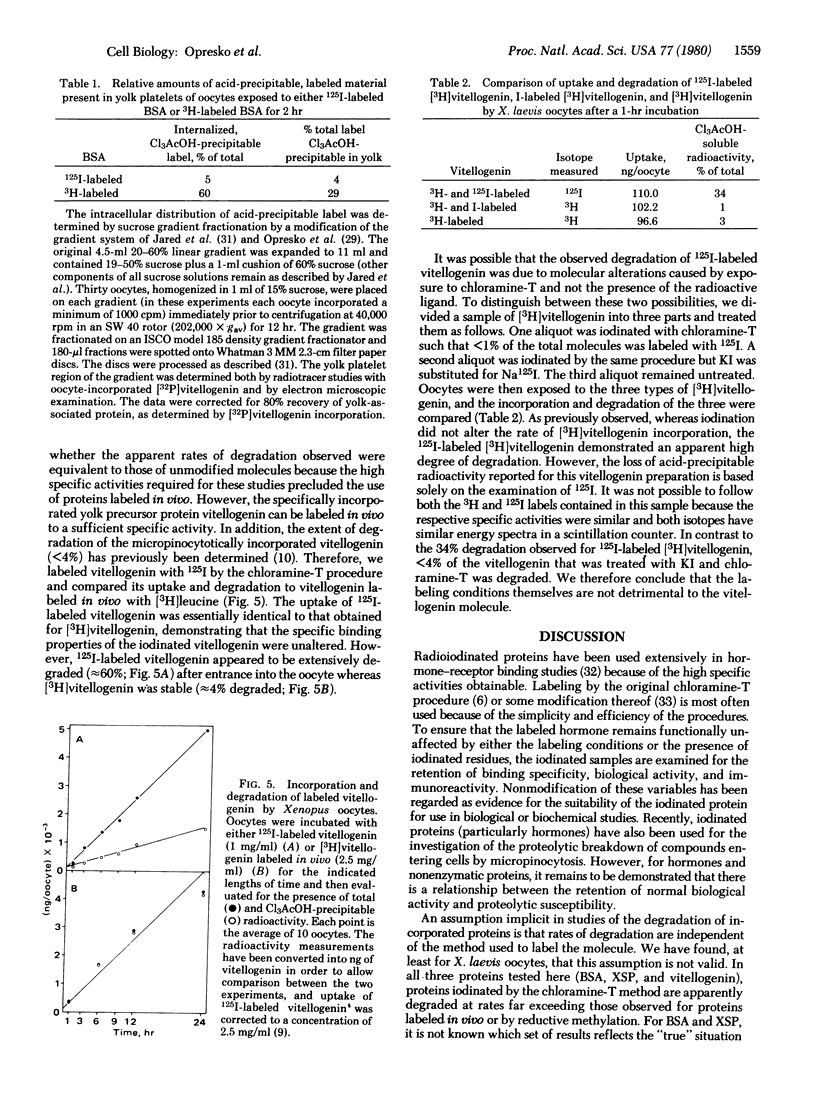
Proteins labeled with either 3H by reductive methylation or 125I by the chloramine-T method were incubated with Xenopus laevis oocytes; the incorporation and acid precipitability of the proteins were then studied. The uptake rates of both specifically incorporated (vitellogenin) and nonspecifically incorporated proteins (bovine serum albumin and X. laevis serum proteins lacking albumin) were not influenced by the method of labeling. However, 125I-labeled proteins were apparently degraded at rates far exceeding their 3H-labeled counterparts, based on the generation of acid-soluble radioactivity. Thus, after a 3-hr incubation, 3-5 times more 125I-labeled bovine serum albumin and X. laevis serum proteins lacking albumin were degraded than the corresponding 3H-labeled proteins (95% compared to 30% and 75% compared to 15%, respectively), whereas after a 24-hr incubation, the degradation of 125I-labeled vitellogenin was 15 times greater than that of [3H]vitellogenin labeled in vivo (60% compared to 4%). Moreover, examination of the relative amounts of 3H- compared to 125I-labeled bovine serum albumin deposited into the exogenously derived yolk platelet compartment of the oocyte revealed 7 times more acid-precipitable 3H-labeled protein, indicating that the observed discrepancies were not due to reincorporation of the 3H-labeled ligands. Passage of dissolved oocytes previously exposed to 125I-labeled bovine serum albumin (chloramine-T method) over a column of Bio-Gel P-10 revealed some breakdown of bovine serum albumin to intermediate molecular weight components and the presence of a large amount (≈90%) of labeled low molecular weight compounds, which analysis showed to be 72% free iodine. The evolution of either iodotyrosine or free iodine would nevertheless be perceived as protein degradation by most analytical procedures (e.g., acid precipitation or autoradiography). We conclude, therefore, that apparent degradation rates observed for endocytotically incorporated proteins may vary depending on the method used to label the protein and caution should be exercised when interpreting results obtained with labeled, particularly chloramine-T labeled, proteins.
Keywords: oocyte, fluid endocytosis, absorptive endocytosis, protein turnover
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ascoli M., Puett D. Tritium labeling of luteinizing hormone by reductive methylation. Biochim Biophys Acta. 1974 Nov 5;371(1):203–210. doi: 10.1016/0005-2795(74)90169-x. [DOI] [PubMed] [Google Scholar]
- Bramhall S., Noack N., Wu M., Loewenberg J. R. A simple colorimetric method for determination of protein. Anal Biochem. 1969 Oct 1;31(1):146–148. doi: 10.1016/0003-2697(69)90251-6. [DOI] [PubMed] [Google Scholar]
- Carpenter G., Cohen S. 125I-labeled human epidermal growth factor. Binding, internalization, and degradation in human fibroblasts. J Cell Biol. 1976 Oct;71(1):159–171. doi: 10.1083/jcb.71.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- GREENWOOD F. C., HUNTER W. M., GLOVER J. S. THE PREPARATION OF I-131-LABELLED HUMAN GROWTH HORMONE OF HIGH SPECIFIC RADIOACTIVITY. Biochem J. 1963 Oct;89:114–123. doi: 10.1042/bj0890114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein J. L., Brown M. S. Binding and degradation of low density lipoproteins by cultured human fibroblasts. Comparison of cells from a normal subject and from a patient with homozygous familial hypercholesterolemia. J Biol Chem. 1974 Aug 25;249(16):5153–5162. [PubMed] [Google Scholar]
- Gorden P., Carpentier J. L., Freychet P., LeCam A., Orci L. Intracellular translocation of iodine-125-labeled insulin: direct demonstration in isolated hepatocytes. Science. 1978 May 19;200(4343):782–785. doi: 10.1126/science.644321. [DOI] [PubMed] [Google Scholar]
- Haigler H., Ash J. F., Singer S. J., Cohen S. Visualization by fluorescence of the binding and internalization of epidermal growth factor in human carcinoma cells A-431. Proc Natl Acad Sci U S A. 1978 Jul;75(7):3317–3321. doi: 10.1073/pnas.75.7.3317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jared D. W., Dumont J. N., Wallace R. A. Distribution of incorporated and synthesized protein among cell fractions of Xenopus oocytes. Dev Biol. 1973 Nov;35(1):19–28. doi: 10.1016/0012-1606(73)90003-1. [DOI] [PubMed] [Google Scholar]
- Lee C. Y., Ryan R. J. Interaction of ovarian receptors with human luteinizing hormone and human chorionic gonadotropin. Biochemistry. 1973 Nov 6;12(23):4609–4615. doi: 10.1021/bi00747a011. [DOI] [PubMed] [Google Scholar]
- ORNSTEIN L. DISC ELECTROPHORESIS. I. BACKGROUND AND THEORY. Ann N Y Acad Sci. 1964 Dec 28;121:321–349. doi: 10.1111/j.1749-6632.1964.tb14207.x. [DOI] [PubMed] [Google Scholar]
- Ohlendorf D. H., Barbarash G. R., Trout A., Kent C., Banaszak L. J. Lipid and polypeptide components of the crystalline yolk system from Xenopus laevis. J Biol Chem. 1977 Nov 25;252(22):7992–8001. [PubMed] [Google Scholar]
- Opresko L., Wiley H. S., Wallace R. A. The origin of yolk-DNA in Xenopus laevis. J Exp Zool. 1979 Sep;209(3):367–376. doi: 10.1002/jez.1402090303. [DOI] [PubMed] [Google Scholar]
- Rodbard D., Chrambach A. Estimation of molecular radius, free mobility, and valence using polyacylamide gel electrophoresis. Anal Biochem. 1971 Mar;40(1):95–134. doi: 10.1016/0003-2697(71)90086-8. [DOI] [PubMed] [Google Scholar]
- Roth J. Peptide hormone binding to receptors: a review of direct studies in vitro. Metabolism. 1973 Aug;22(8):1059–1073. doi: 10.1016/0026-0495(73)90225-4. [DOI] [PubMed] [Google Scholar]
- Sapira J. D. Chromatography of aromatic amino acid derivatives on polyamide thin layers. J Chromatogr. 1969 Jun 3;42(1):134–136. doi: 10.1016/s0021-9673(01)80603-8. [DOI] [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Binding and degradation of 125I-insulin by rat hepatocytes. J Biol Chem. 1975 Nov 10;250(21):8389–8398. [PubMed] [Google Scholar]
- Terris S., Steiner D. F. Retention and degradation of 125I-insulin by perfused livers from diabetic rats. J Clin Invest. 1976 Apr;57(4):885–896. doi: 10.1172/JCI108365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Hollinger T. G. Turnover of endogenous, microinjected, and sequestered protein in Xenopus oocytes. Exp Cell Res. 1979 Mar 15;119(2):277–287. doi: 10.1016/0014-4827(79)90355-0. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Dumont J. N., Sega M. W. Protein incorporation by isolated amphibian oocytes. 3. Optimum incubation conditions. J Exp Zool. 1973 Jun;184(3):321–333. doi: 10.1002/jez.1401840305. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W., Nelson B. L. Protein incorporation by isolated amphibian oocytes. I. Preliminary studies. J Exp Zool. 1970 Nov;175(3):259–269. doi: 10.1002/jez.1401750302. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Protein incorporation by isolated amphibian oocytes. V. Specificity for vitellogenin incorporation. J Cell Biol. 1976 May;69(2):345–351. doi: 10.1083/jcb.69.2.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. 8. The estrogen-induced hepatic synthesis of a serum lipophosphoprotein and its selective uptake by the ovary and trasformation into yolk platelet proteins in Xenopus laevis. Dev Biol. 1969 May;19(5):498–526. doi: 10.1016/0012-1606(69)90085-2. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Jared D. W. Studies on amphibian yolk. VII. Serum phosphoprotein synthesis by vitellogenic females and estrogen-treated males of Xenopus laevis. Can J Biochem. 1968 Aug;46(8):953–959. doi: 10.1139/o68-142. [DOI] [PubMed] [Google Scholar]
- Wallace R. A., Misulovin Z. Long-term growth and differentiation of Xenopus oocytes in a defined medium. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5534–5538. doi: 10.1073/pnas.75.11.5534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace R. A., Nickol J. M., Ho T., Jared D. W. Studies on amphibian yolk. X. The relative roles of autosynthetic and heterosynthetic processes during yolk protein assembly by isolated oocytes. Dev Biol. 1972 Nov;29(3):255–272. doi: 10.1016/0012-1606(72)90066-8. [DOI] [PubMed] [Google Scholar]
- Wallace R. A. Studies on amphibian yolk. IX. Xenopus vitellogenin. Biochim Biophys Acta. 1970 Jul 21;215(1):176–183. doi: 10.1016/0304-4165(70)90400-9. [DOI] [PubMed] [Google Scholar]
- Wiley H. S., Opresko L., Wallace R. A. New methods for the purification of vertebrate vitellogenin. Anal Biochem. 1979 Aug;97(1):145–152. doi: 10.1016/0003-2697(79)90338-5. [DOI] [PubMed] [Google Scholar]
- Wille L. E. A simple and rapid method for isolation of serum lipoproteins from the other serum protein constituents. Clin Chim Acta. 1976 Sep 6;71(2):355–357. doi: 10.1016/0009-8981(76)90554-4. [DOI] [PubMed] [Google Scholar]
- Williams K. E., Kidston E. M., Beck F., Lloyd J. B. Quantitative studies of pinocytosis. II. Kinetics of protein uptake and digestion by rat yolk sac cultured in vitro. J Cell Biol. 1975 Jan;64(1):123–134. doi: 10.1083/jcb.64.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willinger M., Gonatas N., Frankel F. R. Fate of surface proteins of rabbit polymorphonuclear leukocytes during phagocytosis. II. Internalization of proteins. J Cell Biol. 1979 Jul;82(1):45–56. doi: 10.1083/jcb.82.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyckoff M., Rodbard D., Chrambach A. Polyacrylamide gel electrophoresis in sodium dodecyl sulfate-containing buffers using multiphasic buffer systems: properties of the stack, valid Rf- measurement, and optimized procedure. Anal Biochem. 1977 Apr;78(2):459–482. doi: 10.1016/0003-2697(77)90107-5. [DOI] [PubMed] [Google Scholar]



