Abstract
This study was to determine the effect of exercise on the recovery of dopaminergic neuron loss and muscle atrophy in 6-OHDA-induced hemi Parkinson's disease model. Exercise was loaded twice per day for 30 minutes each time, at 5 days after 6-OHDA lesioning and continued for 16 days using a treadmill. Exercise significantly increased the number of tyrosine hydroxylase positive neuron in the lesioned substantia nigra and the expression level of tyrosine hydroxylase in the striatum compared with the control group. To examine which signaling pathways may be involved in the exercise, the phosphorylation of GSK3β and ERK were observed in the striatum. In the control group, basal level of GSK3β phosphorylation was less than in both striatum, but exercise increased it. ERK phosphorylation decreased in the lesioned striatum, but exercise recovered it. These findings suggest that exercise inactivates GSK3β by phosphorylation which may be involved in the neuroprotective effect of exercise on the 6-OHDA-induced cell death. In the exercise group, weight, and Type I and II fiber cross-sectional area of the contralateral soleus significantly recovered and expression of myosin heavy chain and Akt and ERK phosphorylation significantly increased by exercise. These results suggest that exercise recovers Parkinson's disease induced dopaminergic neuron loss and contralateral soleus muscle atrophy.
Keywords: ERK, Exercise, GSK3β, Muscle atrophy, Parkinson's disease
INTRODUCTION
Parkinson's disease (PD) is a neurodegenerative disease in the central nervous system (CNS), characterized by dopaminergic neuronal loss in the nigrostriatal system with clinical symptoms such as resting tremor, rigidity, akinesia, and disturbances of postural reflex [1]. These deficits might be compensated for by adjustments that are typical for PD, including widened stance, increased ratio of support period to stride period, exaggerated activities of the thigh and leg muscles, and low walking speed [2].
The human pathological features of PD can be mimicked in rats by injection of the neurotoxin 6-hydroxydopamine (6-OHDA) to induce striatal dopamine depletion [3]. The injections are usually made unilaterally and so affect motor performance on the contralateral side of the body, including skilled fore- and hindlimb use [4] and sensorimotor functions [5]. Rats with unilateral 6-OHDA lesions exhibit characteristic gait disturbances during overground locomotion such as compensatory weight support shifts to the unaffected side during propulsion and turning [5]. Recent studies have provided evidence that muscle strength is reduced in patients with PD compared with age-matched controls even at early stages of the disease and in the unaffected side [6] and we also reported that contralateral soleus muscle atrophy occurs at 21 days after establishing the PD rat model with unilateral 6-OHDA lesions [7], supporting that muscle atrophy is present in PD patients. However, there are few studies on the recovery of muscle atrophy induced by PD.
Physical exercise has been shown to exert neuroprotective effects, enhance neurogenesis [8,9], and increase angiogenesis [10,11]. Several trophic factors might be involved in the rationale for mechanisms of these beneficial effects of exercise [12]. A meta-analysis demonstrated that exercise might improve physical functions, health-related quality of life, strength, balance, and gait speed of PD patients [13].
Thus, we examined whether exercise could recover the muscle atrophy in the PD animal model and which signaling pathway is involved in the recovery of atrophied muscle.
METHODS
Animal experiments
Male Sprague-Dawley rats (Daehan Experimental Co., Korea) 260±15 g (8 weeks) were used for the experiment. The animals were housed under laboratory conditions at a controlled temperature (20±2℃) and maintained under light-dark cycles, 12 hours of each (from 07:00 to 19:00 h). Water and pellets (Samyang Co., Cheonan, Korea) were provided ad libitum. The experimental procedures were performed in accordance with the animal care guidelines of the National Institute of Health (NIH) and carried out with a prior approval from the Institutional Animal Ethical Committee of Dongguk University (IRB No. 2009-1116). Rats were assigned randomly to an exercise (n=9) and control group (n=9). All rats had inducing operation of Parkinson's disease performed. Exercise group started treadmill training at 5 days after 6-OHDA lesioning and continued for 16 days twice per day for 30 minutes at 10 m/minute and at a grade of 10, whereas control group received no treatment after operation. The body weights were measured twice a week by a rat digital balance (Daejong instruments, Seoul, Korea). Food pellets were weighed and a consistent amount added to the food tray. Food intake was measured twice a week for 21 days and the total amount across the 21 days was used in the data analysis.
Induction of Parkinson's disease and behavior test
Rats were anesthetized with Rompun (Vial Korea, 10 mg/kg) plus Zoletil 50 (Virbac Korea, 25 mg/kg) intramuscular injection and given a unilateral stereotaxic injection of 20 µg 6-OHDA (4 µg/µl) with 0.2 mg/ml L-ascorbic acid into the left striatum (AP 0.7, ML 2.6, DV 4.5; all coordinates represent millimeter adjustments from bregma) at a rate of 0.5 µl/min using a 26-gauge Hamilton syringe. The day after stereotaxic injection, amphetamine-induced rotation test was performed [7] and rats showing rotation rate more than 5 times/min were selected for experiments (average rate is 7±2). After finishing exercise, amphetamine-induced rotation test was also performed. In the exercise group, rotation rate was significantly reduced compared with that of control group (Table 1).
Table 1.
Amphetamine-induced rotation rate
n, number of animals; C, control with Parkinson's disease; E, exercise with Parkinson's disease.
Muscle weight and cross-sectional area of Type I and II fibers
Rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg, supplemented as required) at 22 days after 6-OHDA lesioning. The soleus and plantaris muscles were excised bilaterally, the wet mass of individual muscles was obtained, and the tissue was rinsed with normal saline. Following muscle dissection, sodium pentobarbital was supplemented intraperitoneally to euthanize the rats. The weights of dissected individual muscles were measured using a microbalance (Mettler PE 160, Columbus, OH, USA) after fat and connective tissues were trimmed.
The method that we used for measuring the cross-sectional areas of Types I and II muscle fibers was described in Kim and Choe [7]. From each muscle, a portion was cut transversely from the midsection, mounted in wooden pieces and quick frozen by immersion in isopentane cooled with liquid nitrogen. Transverse sections 10 µm thick were sectioned in a cryostat at -20℃, adhered to cover glasses, thawed, and air-dried at room temperature for 30 min. Acid-stable myosin ATPase reactions were performed on serial sections and fibers were classified as Type I (slow-twitch) or Type II (fast-twitch) based on this reaction.
Pre-incubation was carried out for 5 min in a medium of both pH 4.3 and pH 4.6 acetate buffer. The medium contained 200 mM acetate, and pH was adjusted by 2 M HCl. The sections were then rinsed with normal saline. Incubation was carried out for 30 min at 37℃ in a medium that contained 1.1 M sodium barbiturate 20 ml, 180 mM CaCl2 10 ml and 152 mg ATP. The pH was adjusted to 9.4. Sections were rinsed with normal saline following the incubation then rinsed with 1% CoCl2 once every 2 min for 6 min (i.e., a total of three times). Sections were reacted with 2% CoCl2 for 3 min and then rinsed with normal saline five times. Sections were allowed to react with 2% ammonium sulfate for 2 min and then rinsed with normal saline five times.
Following histochemical staining and incubations, sections were dehydrated through a series of ethanol concentrations from 70% to 100%, cleared in xylene and mounted. Type I fibers were identified as those staining dark, while Type II fibers were identified as those staining light in ATPase reactions after pre-incubation. Fiber cross-sectional area was calculated from tracings of 50~100 muscle fibers at 100× magnifications by microscopic image analyzer (Leity, ASM 68k, Netzler).
Tyrosine hydroxylase (TH) expression by immunohistochemistry
Rats were anesthetized intraperitoneally with sodium pentobarbital (50 mg/kg, supplemented as required) at 22 days after 6-OHDA lesioning. After dissecting muscles, rats were perfused transcardially with 4% paraformaldehyde in 0.05 M phosphate buffer (PB) for brain isolation. The brains were removed, postfixed, and cryoprotected. Immunohistochemistry was performed by using free-floating cryomicrotome-cut sections (40 mm thickness) that encompassed the entire substantia nigra (SN). After incubation with 3% H2O2 in 0.05 M PBS, the sections were stained overnight at room temperature (RT) using an anti-TH (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA) primary antibody for dopamine (DA) neurons. The Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA, USA) was used as the secondary antibody. For visualization of TH protein, 3,3'-Diaminobenzidine (DAB) staining method was used. Diaminobenzidine is oxidized by hydrogen peroxide in the presence of hemoglobin to give a dark-brown color.
Protein expression and phosphorylation by Western blot analysis
For western blotting, the striatum and muscle tissues were homogenized in lysis buffer containing 50 mM Trisbase (pH 7.5), 150 mM NaCl, 2 mM EDTA, 1% glycerol, 10 mM NaF, 10 mM Na-pyrophosphate, 1% NP-40 and protease inhibitors (0.1 mM phenylmethylsulfonylfluoride, 5 µg/ml aprotinin, and 5 µg/ml leupeptin). Thirty µg of cell lysates were electrophoresed in 10% SDS-polyacrylamide gels and transferred to nitrocellulose membranes which were then incubated with anti-TH (1:1,000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-phospho-MAPK, anti-MAPK, anti-phospho-Akt, anti-Akt, anti-phosph-GSK3β (Ser 9), anti-GSK3β (Cell signaling technology, Beverly, MA, USA), anti-MHC (Abcam, Cambridge, UK) and anti-β actin (Sigma, St. Louis, MO, USA) for 16 h at 4℃. After washing with TBS-T (0.05%), the blots were incubated with horseradish peroxidase-conjugated anti-rabbit or anti-mouse IgG, and the bands were visualized using the ECL system (Thermo Fisher Scienctific, USA). Band images were obtained by using Molecular Imager ChemiDoc XRS+ (Bio-Rad, Hercules, CA, USA) and band intensity was analyzed by Image Lab™ software version 2.0.1 (Bio-Rad).
Data analysis
All statistical analyses were conducted with SPSS (ver. 19, Somers, NY, USA). Values are expressed as means±SEM. Independent t-tests were used to compare the exercise and control groups. Statistical significance was accepted at a p value less than .05.
RESULTS
Body weight and dietary intake
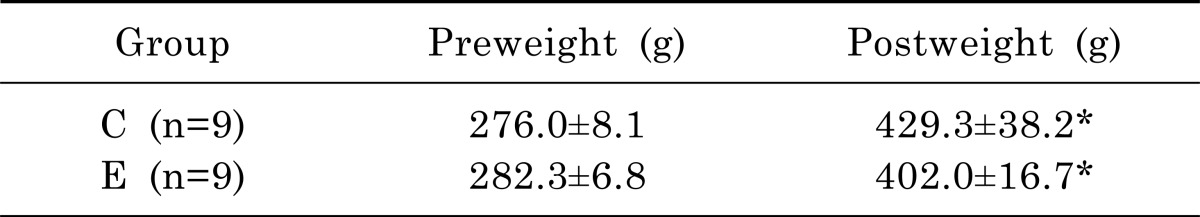
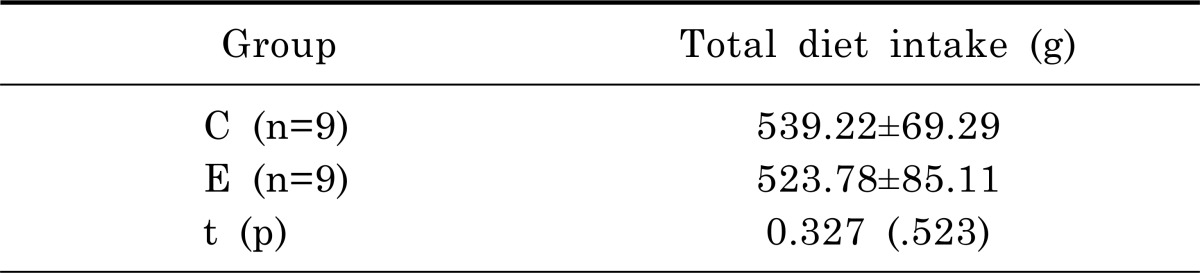
As shown in Table 2, there was no significant difference on body weight between exercise and control groups at the first day of the experiment (t=1.691, p=.113). Two groups demonstrated a significant increase in body weight between pre-experiment and post-experiment: weight of the exercise group increased by 140.0% (p<.01), that of the control group increased by 155.5% (p<.01). There was no significant difference on body weight on 22 days following exercise between the exercise and control groups (t=1.854, p=.095). As presented in Table 3, there was no significant difference on total dietary intake between exercise and control groups (t=0.327, p=.523).
Table 2.
Pre-and post-experimental weight of rats in the control with PD and exercise with PD groups
n, number of animals; C, control with Parkinson's disease; E, exercise with Parkinson's disease.
*Significant difference between preweight & postweight (p<.05).
Table 3.
Total amount of diet intake during the experimental period
n, number of animals; C, control with Parkinson's disease; E, exercise with Parkinson's disease.
Muscle weight and cross-sectional area of type I and II fibers
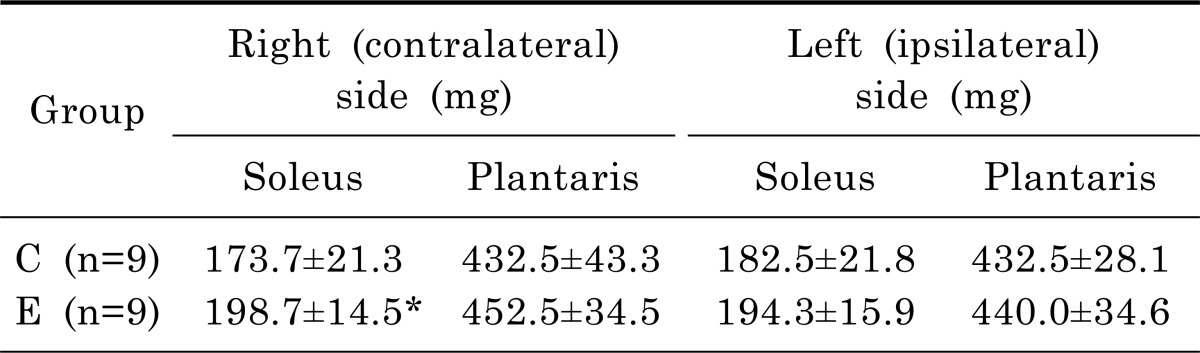
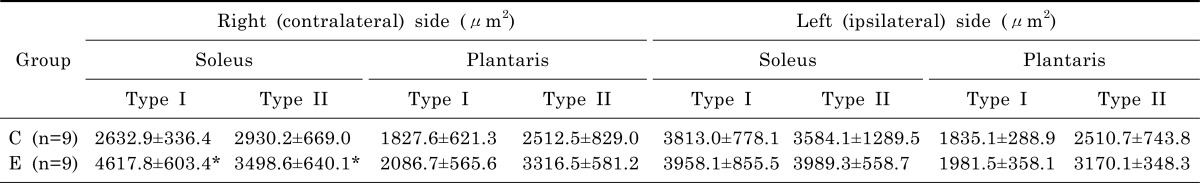
Muscle weights of the exercise and control group are shown in Table 4. The weight of right (contralateral) soleus muscle was 14% greater in the exercise group than in the control group (t=2.192, p=.046). There are no significant differences of right plantaris, left (ipsilateral) soleus, and left plantaris muscles between exercise and control groups. Cross-sections of the soleus and plantaris muscles from rats in the exercise and control groups are shown in Fig. 1. As presented in Table 5, the cross-sectional area of the Type I fiber and Type II of right soleus muscle of the exercise group was greater than that of the control group (t=3.237, p=.010; t=2.866, p=.019).
Table 4.
Muscle weight of hindlimb muscles of control (C) and exercise (E) groups
n, number of animals; C, control with Parkinson's disease; E, exercise with Parkinson's disease.
*Significant difference between E & C groups (p<.05).
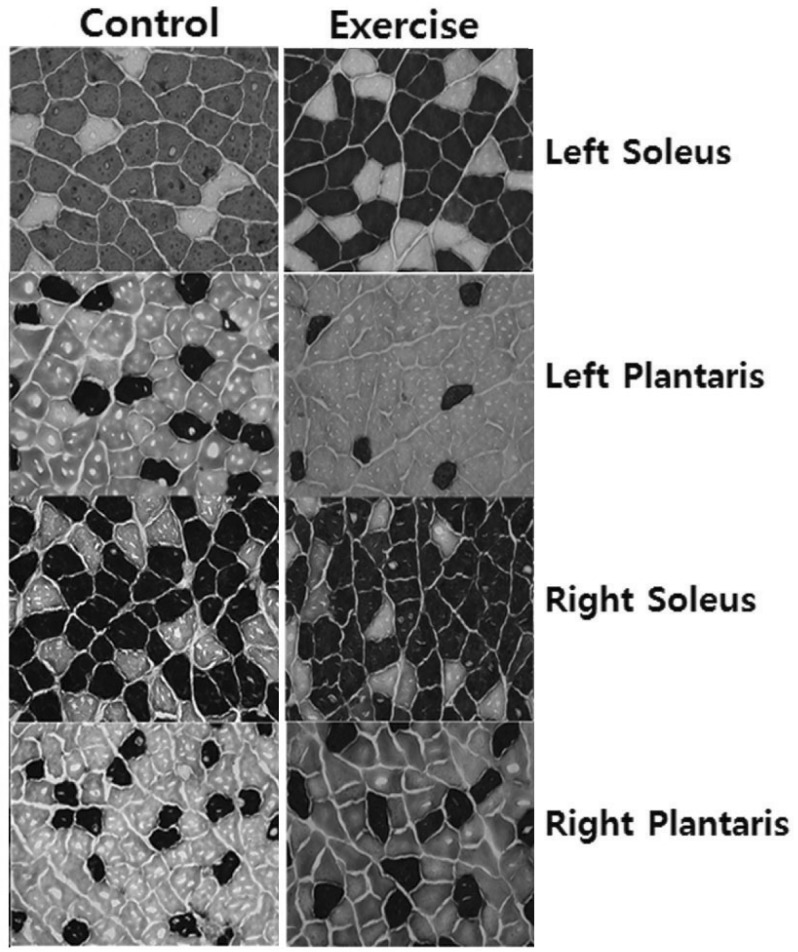
Fig. 1.
Cross-sections of the hindlimb muscles in control (left) and exercise (right) rats. The first line is left (ipsilateral) soleus in control and exercise rats. The second line is left plantaris in control and exercise rats. The third line is right (contralateral) soleus in control and exercise rats. The fourth line is right plantaris in exercise and control rats. Dark=Type I muscle fiber, light=Type II muscle fiber (Myosin ATPase straining, 100×).
Table 5.
Cross-sectional area of hindlimb muscles of control (C) and exercise (E) groups
n, number of animals.
*Significant difference between C & E groups (p<.05).
TH immunohistochemistry and immunoblot
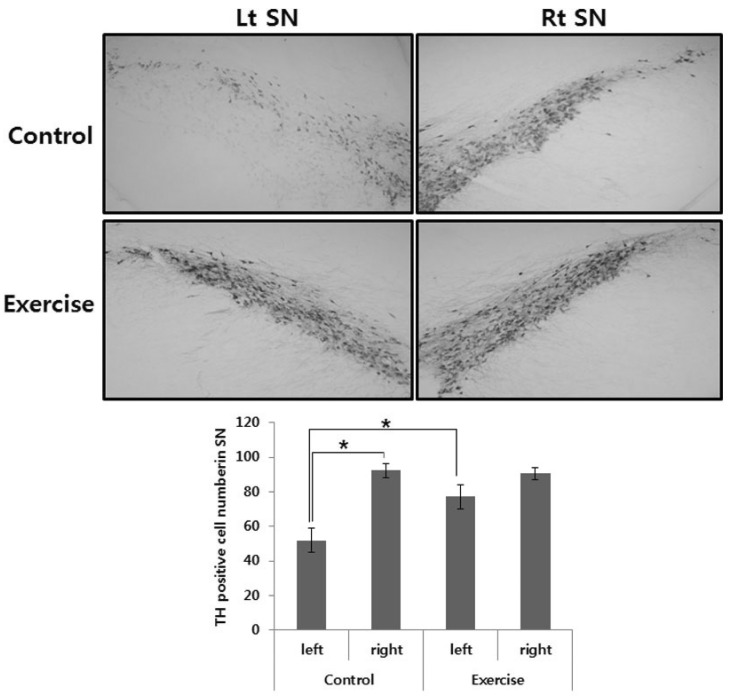
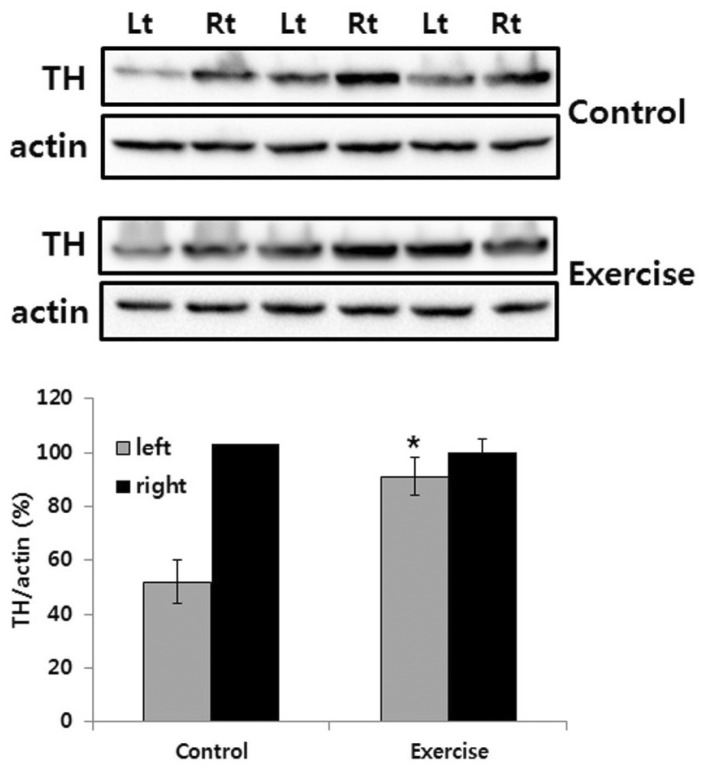
The effects of exercise in 6-OHDA-injected rat were judged by immunohistochemistry of TH-positive DA neurons in the SN and immunoblot analysis of TH in the striatum. Photomicrographs of TH-positive neurons in the SN are shown in Fig. 2. The 6-OHDA-injected lesioned SN had significantly fewer TH positive neurons, as compared with the intact SN (p<.01). However, exercise groups had significantly greater TH positive neurons in the lesioned SN, as compared with the control group (p<.01; Fig. 2). In addition, exercise significantly increased TH expression level in the lesioned striatum, as compared with the control group (p<.01; Fig. 3).
Fig. 2.
TH-specific immunohistochemical staining and the number of TH positive neurons in substantia nigra of unilateral 6-OHDA lesioned Parkinson rat. The data represent the means±SEM. *Significant difference between left and right SN in the control group and control and exercise group of 6-OHDA lesioned side (p<.01).
Fig. 3.
TH-immunoblot in the striatum of unilateral 6-OHDA lesioned Parkinson rat. The striatum was lysed and inmmunoblotted with anti-TH antibody. The intensity of the TH bands was quantitated by densitometry and it was normalized versus the actin. The data are expressed as mean±SEM with 9 rats per group. *Significant difference between control and exercise group of 6-OHDA lesioned side (p<.01).
Phosphorylation of GSK3β and ERK in the striatum
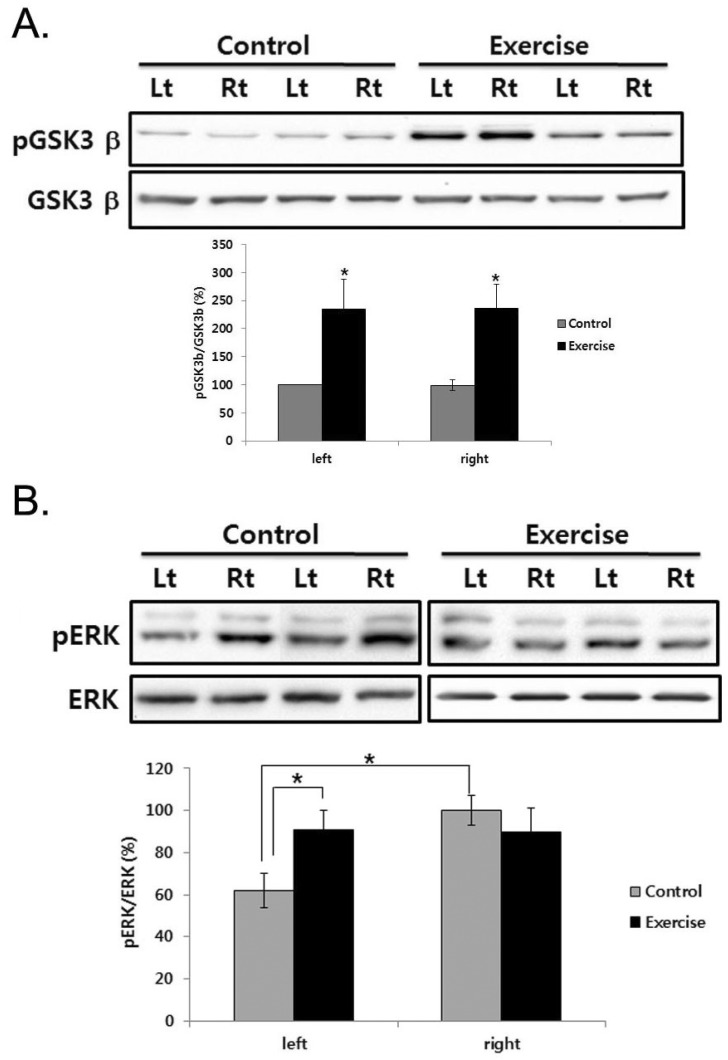
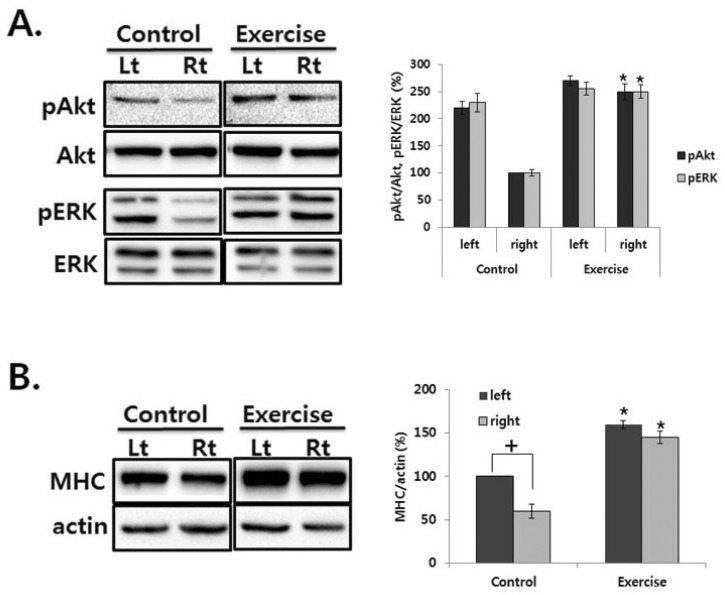
To determine the effect of exercise on the recovery of TH-positive neurons, we observed phosphorylation level of glycogen synthase kinase (GSK) 3β and extracellular signal-regulated kinases (ERKs) in the striatum. There was no significant difference on the GSK3β phosphorylation between lesioned and intact striatum in control group, but exercise significantly increased it in the striatum (Fig. 4A). Moreover, in the lesioned striatum, ERK phosphorylation decreased, but exercise significantly recovered ERK phosphorylation (p<.01; Fig. 4B).
Fig. 4.
ERK and GSK3β phosphorylation in the striatum of unilateral 6-OHDA lesioned Parkinson rat. The striatum was lysed and inmmunoblotted with pGSK3β or GSK3β (A), anti-pERK, ERK (B) antibody. The intensity of the protein bands was quantitated by densitometry and the phosphorylated form was normalized versus the total form. The data are expressed as mean±SEM with 9 rats per group. *Significant difference between left and right SN in the control group and control and exercise group of each side (p<.01).
Phosphorylation of Akt and ERK in the soleus
To examine the signaling pathway activated by exercise on the recovery of atrophied muscle, we examined the phosphorylation level of Akt and ERK in the soleus muscle. In the no treated rat, Akt and ERK phosphorylation decreased in the contralateral soleus muscle, but exercise recovered it (Fig. 5A). To confirm the recovery of atrophied muscle, the expression level of myosin heavy chain (MHC) was examined in the muscle. MHC isoforms have myosin adenosinetriphosphatase activities correlated with the speed of muscle fiber shortening [14]. Therefore, the MHC expression has been used as a phenotypic marker for functional aspects of muscle fibers and has been changed in the regenerated muscle fiber [15]. As shown in Fig. 5B, MHC expression decreased in the contralateral soleus muscle of control rat, but exercise recovered it. Interestingly, exercise induced MHC expression by 1.5 fold in the left soleus muscle compared with that of control (Fig. 5B).
Fig. 5.
ERK and Akt phosphorylation in the soleus muscle of unilateral 6-OHDA lesioned Parkinson rat. (A) The soleus was lysed and inmmunoblotted with each antibody. The intensity of the protein bands was quantitated by densitometry and the phosphorylated form was normalized versus the total form. (B) The soleus was lysed and inmmunoblotted with anti-MHC antibody. The intensity of the protein bands was quantitated by densitometry and it was normalized versus the actin. The data are expressed as mean±SEM with 9 rats per group. †Significant difference between left and right soleus in the control group, *Significant difference between control and exercise group of each side (p<.01).
DISCUSSION
In this study, we demonstrated that exercise significantly increases in the number of tyrosine hydroxylase positive neuron and phosphorylates GSK3β and ERK in the striatum, and recovers atrophied contralateral soleus. Moreover, exercise recovered the decreased level of Akt and ERK phosphorylation in the contralateral soleus muscle.
Rats with unilateral DA depletions (hemi-Parkinson rats) display impairments in their contralateral hindlimbs in adjusting posture and moving [4]. They compensate by supporting themselves mainly on their ipsilateral hindlimb. Thus, their center of gravity is shifted to the ipsilateral side and movement is preferentially directed toward the ipsilateral side, in part to maintain equilibrium and in part to remove weight from the contralateral limbs so that they can enter the swing phase of the stepping cycle. It is proposed that the contralateral limbs may be unable to apply force to adjust posture and produce movement [4]. In addition, we also examined the decreased locomotor activity and contralateral hindlimb soleus muscle atrophy in the hemi-Parkinson rats [7], suggesting that hemi-Parkinson rats display impairments in locomotion activity and it results in muscle atrophy including reduction of muscle protein synthesis and stimulation of muscle protein degradation [16].
Systematic reviews have found that exercise programs which specifically target balance and lower limb muscle strength are effective in preventing falls in the general older population [17,18]. In people with PD, lower limb muscle strength and regular exercise are significantly correlated with physical abilities [19-21], therefore highlighting the role of exercise as an appropriate intervention in this population. Exercise has been shown to improve balance [22,23] and strength [24,25] and cueing training has been shown to improve freezing of gait in people with PD [19].
Several studies have shown that regular aerobic exercise improve the brain volume, besides preventing brain tissue loss when compared older people training aerobically with sedentary by using high-resolution magnetic resonance imaging scans [26] and induce neurotrophic factors that benefit glutamatergic neurons which in turn improves neural learning and function [27] and angiogenic effects [11]. Moreover, it has been reported that exercise promotes neuroplasticity [28] and improves cognitive functioning; especially the executive processes [29]. In addition, exercise using treadmill also demonstrated significant cell proliferation in the brain [30] and might be good for traumatic brain injury rat model by up-regulation of brain-derived neurotropic factor (BDNF) [31] and Parkinson's disease rat model by up-regulation BDNF and glial cell line-derived neurotrophic (GDNF) in the striatum [32]. Previously, it has been reported that BDNF receptors are coupled to glycogen synthase kinase (GSK) 3β phosphorylation in granule neurons through both PKC and PI3K/Akt dependent mechanisms [33] and also ERK activation which is necessary for BDNF-induced dendritic spine density in hippocampal CA1 pyramidal neurons [34]. In this study, we also examined that exercise recovered 6-OHDA-induced dopamine neuron loss and phosphorylated GSK3β and ERK in the striatum. GSK3β is a multifunctional serine/threonine kinase and participates in diverse cellular processes and important signaling pathways linked to a number of medical disorders, such as diabetes, cancer, mood disorders, schizophrenia, and neurodegeneration. Using an in vitro model of PD, 6-OHDA activates GSK3β in cultured human neuroblostoma SH-SY5Y cells as well as rat cerebellar granule neurons [35] but not in vivo 6-OHDA-induced PD model [36]. Moreover, GSK3β mediates 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neuronal death in vitro and in vivo [37,38] and intraperitoneal administration of specific inhibitors for GSK3β, indirubin-30-oxime and AR-A014418 in mice, prevents dopaminergic neurons in the SN from MPTP-induced apoptosis and restores the depletion of striatal dopamine and ameliorates behavioral impairments caused by MPTP [37]. In the present study, we examined the phosphorylation levels of GSK3β and ERK in the striatum after exercise training. Exercise using treadmill also demonstrated significant up-regulation of BDNF and GDNF in the SN and striatum in the PD mouse model [39]. Thus, increased growth factor may phosphorylate GSK3β and ERK in the striatum and SN. Although, in this study, we could not examine the phosphorylation level of GSK3β and ERK in SNpc, it has been reported that growth factor expression in striatum protects 6-OHDA-induced dopamine cell death and improves behavioral performance than that in SN [40]. Thus, this suggests that inactivation of GSK3β in the striatum by exercise-induced growth factor may exert prevention of 6-OHDA-induced dopamine neuron death in SN through nigrostriatal dopamine system.
A consistent abnormality in PD is degeneration of dopaminergic neurons in the SN, leading to a reduction of stratial DA levels. As tyrosine hydroxylase catalyzes the formation of L-DOPA, the rate-limiting step in the biosynthesis of DA, TH-deficiency causes important clinical symptoms in PD [41]. In this study, we examined that exercise recovers 6-OHDA-induced TH cell loss in SN and striatal TH depletion. Moreover, exercise restored the number of amphetamine-induced rotations, which is concert with previous reports that amphetamine-induced rotation has been shown to correlate with both the extent of TH cell loss and the degree of striatal dopamine depletion [42,43].
Skeletal muscle mass is maintained by a delicate balance between protein synthesis and protein breakdown and experiences hypertrophy or atrophy in response to altered functional demands by adjusting either side of this equilibrium [18,44]. Triggered by extracellular signals such as growth factors and mechanical overloading, muscle can increase mass by changing the overall dimensions of its fibers [45,46]. On the other hand, cued by a variety of stimuli ranging from immobilization, clinical application of corticosteroids, cachexia, and microgravity, to normal aging process [47,48], skeletal muscle undergoes significant loss of mass. The signaling pathways that govern muscle hypertrophy and/or atrophy have yet to be fully defined. Recent research has identified Akt and its downstream signal cascades as pivotal regulators of muscle hypertrophy by enhancing protein synthesis and concomitant repression of protein breakdown [49,50]. Moreover, inhibition of extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in vitro and in vivo pronounced atrophy in both slow and fast muscles [51]. In concert with, we also examined the dephosphorylation of Akt and ERK in the atrophied soleus muscle, but exercise recovered that. Thus, these results suggest that exercise may recover hemi PD-induced atrophied muscle by activation of Akt and ERK.
It has been reported that low-intensity exercise could induce hindlimb muscle hypertrophy [52] and preferential atrophy muscle during hindlimb unweighting is a slow-twitch skeletal muscle, soleus in rats and mice [53]. Moreover, previously we reported that contralateral soleus muscle was only atrophied in the PD model [7]. Thus, in the present study, exercise recovers atrophied soleus muscle through increase of muscle mass and cross sectional area of type I and II in PD animal model and there was no difference of percentage changes between type I and Type II in the affected soleus; type I ratio of control (80.9±3.1) and exercise (83.68±3.2).
Our findings indicate that exercise recovers decreased weights and type I & II fiber cross-sectional area of the contralateral soleus as well as TH-positive cells in the 6-OHDA-induced hemi-Parkinson rat model. This study supports that exercise strengthens the weakened muscle as well as damaged brain in the Parkinson rat, which may also be beneficial to slow progression of PD.
ACKNOWLEDGEMENTS
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (810-20090016).
ABBREVIATIONS
- ERK
extracellular signal-regulated kinase
- GSK3β
glycogen synthase kinase 3β
- 6-OHDA
6-hydroxydopamine
- PI3K
phosphatidylinositol 3-kinases
References
- 1.Sethi KD. Clinical aspects of Parkinson disease. Curr Opin Neurol. 2002;15:457–460. doi: 10.1097/00019052-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 2.Mitoma H, Hayashi R, Yanagisawa N, Tsukagoshi H. Characteristics of parkinsonian and ataxic gaits: a study using surface electromyograms, angular displacements and floor reaction forces. J Neurol Sci. 2000;174:22–39. doi: 10.1016/s0022-510x(99)00329-9. [DOI] [PubMed] [Google Scholar]
- 3.Schwarting RK, Huston JP. The unilateral 6-hydroxydopamine lesion model in behavioral brain research. Analysis of functional deficits, recovery and treatments. Prog Neurobiol. 1996;50:275–331. doi: 10.1016/s0301-0082(96)00040-8. [DOI] [PubMed] [Google Scholar]
- 4.Whishaw IQ, Suchowersky O, Davis L, Sarna J, Metz GA, Pellis SM. Impairment of pronation, supination, and body co-ordination in reach-to-grasp tasks in human Parkinson's disease (PD) reveals homology to deficits in animal models. Behav Brain Res. 2002;133:165–176. doi: 10.1016/s0166-4328(01)00479-x. [DOI] [PubMed] [Google Scholar]
- 5.Muir GD, Whishaw IQ. Ground reaction forces in locomoting hemi-parkinsonian rats: a definitive test for impairments and compensations. Exp Brain Res. 1999;126:307–314. doi: 10.1007/s002210050739. [DOI] [PubMed] [Google Scholar]
- 6.Cano-de-la-Cuerda R, Pérez-de-Heredia M, Miangolarra-Page JC, Muñoz-Hellín E, Fernández-de-Las-Peñas C. Is there muscular weakness in Parkinson's disease? Am J Phys Med Rehabil. 2010;89:70–76. doi: 10.1097/PHM.0b013e3181a9ed9b. [DOI] [PubMed] [Google Scholar]
- 7.Kim Y, Choe MA. Effect of decreased locomotor activity on hindlimb muscles in a rat model of Parkinson's disease. J Korean Acad Nurs. 2010;40:580–588. doi: 10.4040/jkan.2010.40.4.580. [DOI] [PubMed] [Google Scholar]
- 8.van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillerson JL, Caudle WM, Reverón ME, Miller GW. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119:899–911. doi: 10.1016/s0306-4522(03)00096-4. [DOI] [PubMed] [Google Scholar]
- 10.Kleim JA, Cooper NR, VandenBerg PM. Exercise induces angiogenesis but does not alter movement representations within rat motor cortex. Brain Res. 2002;934:1–6. doi: 10.1016/s0006-8993(02)02239-4. [DOI] [PubMed] [Google Scholar]
- 11.Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, Konda S, Engberg K, Lauterbur PC, Greenough WT. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–1046. doi: 10.1016/s0306-4522(02)00664-4. [DOI] [PubMed] [Google Scholar]
- 12.Yasuhara T, Hara K, Maki M, Matsukawa N, Fujino H, Date I, Borlongan CV. Lack of exercise, via hindlimb suspension, impedes endogenous neurogenesis. Neuroscience. 2007;149:182–191. doi: 10.1016/j.neuroscience.2007.07.045. [DOI] [PubMed] [Google Scholar]
- 13.Goodwin VA, Richards SH, Taylor RS, Taylor AH, Campbell JL. The effectiveness of exercise interventions for people with Parkinson's disease: a systematic review and meta-analysis. Mov Disord. 2008;23:631–640. doi: 10.1002/mds.21922. [DOI] [PubMed] [Google Scholar]
- 14.Bárány M. ATPase activity of myosin correlated with speed of muscle shortening. J Gen Physiol. 1967;50:197–218. doi: 10.1085/jgp.50.6.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bigard XA, Janmot C, Merino D, Lienhard F, Guezennec YC, D'Albis A. Endurance training affects myosin heavy chain phenotype in regenerating fast-twitch muscle. J Appl Physiol. 1996;81:2658–2665. doi: 10.1152/jappl.1996.81.6.2658. [DOI] [PubMed] [Google Scholar]
- 16.Frimel TN, Kapadia F, Gaidosh GS, Li Y, Walter GA, Vandenborne K. A model of muscle atrophy using cast immobilization in mice. Muscle Nerve. 2005;32:672–674. doi: 10.1002/mus.20399. [DOI] [PubMed] [Google Scholar]
- 17.Glass DJ. Molecular mechanisms modulating muscle mass. Trends Mol Med. 2003;9:344–350. doi: 10.1016/s1471-4914(03)00138-2. [DOI] [PubMed] [Google Scholar]
- 18.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37:1974–1984. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 19.Nallegowda M, Singh U, Handa G, Khanna M, Wadhwa S, Yadav SL, Kumar G, Behari M. Role of sensory input and muscle strength in maintenance of balance, gait, and posture in Parkinson's disease: a pilot study. Am J Phys Med Rehabil. 2004;83:898–908. doi: 10.1097/01.phm.0000146505.18244.43. [DOI] [PubMed] [Google Scholar]
- 20.Inkster LM, Eng JJ, MacIntyre DL, Stoessl AJ. Leg muscle strength is reduced in Parkinson's disease and relates to the ability to rise from a chair. Mov Disord. 2003;18:157–162. doi: 10.1002/mds.10299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pääsuke M, Ereline J, Gapeyeva H, Joost K, Mõttus K, Taba P. Leg-extension strength and chair-rise performance in elderly women with Parkinson's disease. J Aging Phys Act. 2004;12:511–524. doi: 10.1123/japa.12.4.511. [DOI] [PubMed] [Google Scholar]
- 22.Ashburn A, Fazakarley L, Ballinger C, Pickering R, McLellan LD, Fitton C. A randomised controlled trial of a home based exercise programme to reduce the risk of falling among people with Parkinson's disease. J Neurol Neurosurg Psychiatry. 2007;78:678–684. doi: 10.1136/jnnp.2006.099333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nieuwboer A, Kwakkel G, Rochester L, Jones D, van Wegen E, Willems AM, Chavret F, Hetherington V, Baker K, Lim I. Cueing training in the home improves gait-related mobility in Parkinson's disease: the RESCUE trial. J Neurol Neurosurg Psychiatry. 2007;78:134–140. doi: 10.1136/jnnp.200X.097923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hirsch MA, Toole T, Maitland CG, Rider RA. The effects of balance training and high-intensity resistance training on persons with idiopathic Parkinson's disease. Arch Phys Med Rehabil. 2003;84:1109–1117. doi: 10.1016/s0003-9993(03)00046-7. [DOI] [PubMed] [Google Scholar]
- 25.Dibble LE, Hale TF, Marcus RL, Droge J, Gerber JP, LaStayo PC. High-intensity resistance training amplifies muscle hypertrophy and functional gains in persons with Parkinson's disease. Mov Disord. 2006;21:1444–1452. doi: 10.1002/mds.20997. [DOI] [PubMed] [Google Scholar]
- 26.Colcombe SJ, Erickson KI, Raz N, Webb AG, Cohen NJ, McAuley E, Kramer AF. Aerobic fitness reduces brain tissue loss in aging humans. J Gerontol A Biol Sci Med Sci. 2003;58:176–180. doi: 10.1093/gerona/58.2.m176. [DOI] [PubMed] [Google Scholar]
- 27.Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- 28.Kleim JA, Jones TA, Schallert T. Motor enrichment and the induction of plasticity before or after brain injury. Neurochem Res. 2003;28:1757–1769. doi: 10.1023/a:1026025408742. [DOI] [PubMed] [Google Scholar]
- 29.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: a meta-analytic study. Psychol Sci. 2003;14:125–130. doi: 10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 30.Yi SS, Hwang IK, Yoo KY, Park OK, Yu J, Yan B, Kim IY, Kim YN, Pai T, Song W, Lee IS, Won MH, Seong JK, Yoon YS. Effects of treadmill exercise on cell proliferation and differentiation in the subgranular zone of the dentate gyrus in a rat model of type II diabetes. Neurochem Res. 2009;34:1039–1046. doi: 10.1007/s11064-008-9870-y. [DOI] [PubMed] [Google Scholar]
- 31.Griesbach GS, Hovda DA, Gomez-Pinilla F. Exercise-induced improvement in cognitive performance after traumatic brain injury in rats is dependent on BDNF activation. Brain Res. 2009;1288:105–115. doi: 10.1016/j.brainres.2009.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tajiri N, Yasuhara T, Shingo T, Kondo A, Yuan W, Kadota T, Wang F, Baba T, Tayra JT, Morimoto T, Jing M, Kikuchi Y, Kuramoto S, Agari T, Miyoshi Y, Fujino H, Obata F, Takeda I, Furuta T, Date I. Exercise exerts neuroprotective effects on Parkinson's disease model of rats. Brain Res. 2010;1310:200–207. doi: 10.1016/j.brainres.2009.10.075. [DOI] [PubMed] [Google Scholar]
- 33.Ortega F, Pérez-Sen R, Morente V, Delicado EG, Miras-Portugal MT. P2X7, NMDA and BDNF receptors converge on GSK3 phosphorylation and cooperate to promote survival in cerebellar granule neurons. Cell Mol Life Sci. 2010;67:1723–1733. doi: 10.1007/s00018-010-0278-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alonso M, Medina JH, Pozzo-Miller L. ERK1/2 activation is necessary for BDNF to increase dendritic spine density in hippocampal CA1 pyramidal neurons. Learn Mem. 2004;11:172–178. doi: 10.1101/lm.67804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen G, Bower KA, Ma C, Fang S, Thiele CJ, Luo J. Glycogen synthase kinase 3beta (GSK3beta) mediates 6-hydroxydopamine-induced neuronal death. FASEB J. 2004;18:1162–1164. doi: 10.1096/fj.04-1551fje. [DOI] [PubMed] [Google Scholar]
- 36.Yong Y, Ding H, Fan Z, Luo J, Ke ZJ. Lithium fails to protect dopaminergic neurons in the 6-OHDA model of Parkinson's disease. Neurochem Res. 2011;36:367–374. doi: 10.1007/s11064-010-0368-z. [DOI] [PubMed] [Google Scholar]
- 37.Wang W, Yang Y, Ying C, Li W, Ruan H, Zhu X, You Y, Han Y, Chen R, Wang Y, Li M. Inhibition of glycogen synthase kinase-3beta protects dopaminergic neurons from MPTP toxicity. Neuropharmacology. 2007;52:1678–1684. doi: 10.1016/j.neuropharm.2007.03.017. [DOI] [PubMed] [Google Scholar]
- 38.Petit-Paitel A, Brau F, Cazareth J, Chabry J. Involvment of cytosolic and mitochondrial GSK-3beta in mitochondrial dysfunction and neuronal cell death of MPTP/MPP-treated neurons. PLoS One. 2009;4:e5491. doi: 10.1371/journal.pone.0005491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. Eur J Neurosci. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Connor B. Adenoviral vector-mediated delivery of glial cell line-derived neurotrophic factor provides neuroprotection in the aged parkinsonian rat. Clin Exp Pharmacol Physiol. 2001;28:896–900. doi: 10.1046/j.1440-1681.2001.03544.x. [DOI] [PubMed] [Google Scholar]
- 41.Haavik J, Toska K. Tyrosine hydroxylase and Parkinson's disease. Mol Neurobiol. 1998;16:285–309. doi: 10.1007/BF02741387. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler MG, Szechtman H. Relation between motor asymmetry and direction of rotational behaviour under amphetamine and apomorphine in rats with unilateral degeneration of the nigrostriatal dopamine system. Behav Brain Res. 1990;39:123–133. doi: 10.1016/0166-4328(90)90099-z. [DOI] [PubMed] [Google Scholar]
- 43.Iancu R, Mohapel P, Brundin P, Paul G. Behavioral characterization of a unilateral 6-OHDA-lesion model of Parkinson's disease in mice. Behav Brain Res. 2005;162:1–10. doi: 10.1016/j.bbr.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 44.Solomon AM, Bouloux PM. Modifying muscle mass-the endocrine perspective. J Endocrinol. 2006;191:349–360. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 45.Bodine SC, Stitt TN, Gonzalez M, Kline WO, Stover GL, Bauerlein R, Zlotchenko E, Scrimgeour A, Lawrence JC, Glass DJ, Yancopoulos GD. Akt/mTOR pathway is a crucial regulator of skeletal muscle hypertrophy and can prevent muscle atrophy in vivo. Nat Cell Biol. 2001;3:1014–1019. doi: 10.1038/ncb1101-1014. [DOI] [PubMed] [Google Scholar]
- 46.Rommel C, Bodine SC, Clarke BA, Rossman R, Nunez L, Stitt TN, Yancopoulos GD, Glass DJ. Mediation of IGF-1-induced skeletal myotube hypertrophy by PI(3)K/Akt/mTOR and PI(3)K/Akt/GSK3 pathways. Nat Cell Biol. 2001;3:1009–1013. doi: 10.1038/ncb1101-1009. [DOI] [PubMed] [Google Scholar]
- 47.McKinnell IW, Rudnicki MA. Molecular mechanisms of muscle atrophy. Cell. 2004;119:907–910. doi: 10.1016/j.cell.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 48.Price SR. Increased transcription of ubiquitin-proteasome system components: molecular responses associated with muscle atrophy. Int J Biochem Cell Biol. 2003;35:617–628. doi: 10.1016/s1357-2725(02)00385-0. [DOI] [PubMed] [Google Scholar]
- 49.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stitt TN, Drujan D, Clarke BA, Panaro F, Timofeyva Y, Kline WO, Gonzalez M, Yancopoulos GD, Glass DJ. The IGF-1/PI3K/Akt pathway prevents expression of muscle atrophy-induced ubiquitin ligases by inhibiting FOXO transcription factors. Mol Cell. 2004;14:395–403. doi: 10.1016/s1097-2765(04)00211-4. [DOI] [PubMed] [Google Scholar]
- 51.Shi H, Scheffler JM, Zeng C, Pleitner JM, Hannon KM, Grant AL, Gerrard DE. Mitogen-activated protein kinase signaling is necessary for the maintenance of skeletal muscle mass. Am J Physiol Cell Physiol. 2009;296:C1040–C1048. doi: 10.1152/ajpcell.00475.2008. [DOI] [PubMed] [Google Scholar]
- 52.Choe MA, Go JJ, Kwak HK, Baek J, Jung JY, Song YJ, An GJ. Comparison of hypertrophic effects of low-intensity exercise on rat hindlimb muscles between every other day exercise and everyday exercise. J Korean Biol Nurs Sci. 2011;13:1–7. [Google Scholar]
- 53.Thomason DB, Booth FW. Atrophy of the soleus muscle by hindlimb unweighting. J Appl Physiol. 1990;68:1–12. doi: 10.1152/jappl.1990.68.1.1. [DOI] [PubMed] [Google Scholar]