Abstract
Significant advancements in percutaneous treatment of coronary artery disease have been achieved with the introduction of bare metal stents. They have two major drawbacks: acute/subacute stent thrombosis, successfully managed with antiplatelet therapy immediately after stent implantation; and in-stent restenosis, prevention of which has been achieved with the development of drug-eluting stents. Drug-eluting stents have become preferred therapy for patients undergoing coronary artery intervention, though reports of late stent thrombosis have led to uncertainty about the duration of antiplatelet therapy after drug-eluting stents placement. Much controversy remains regarding perioperative management of patients with these devices, presenting for surgery or other invasive procedures. The purpose of this review is to provide an overview of the changing culture of coronary artery stenting, in addition to discussing perioperative management strategies and controversies surrounding coronary stents and antiplatelet therapy. A comprehensive literature search of MEDLINE was conducted using as keywords: antiplatelet therapy, non-coronary surgery, drug-eluting stents, and stent thrombosis. There is no definite standard of care for the perioperative management of drug-eluting stents in patients with drug-eluting stents. However, there is a growing understanding of the importance of continuation of drug-eluting stents in the perioperative period in order to prevent stent thrombosis along with a concern about the possibility of increased bleeding. Appropriate timing of surgery after coronary artery stenting, team approach to the perioperative management of such patients with involvement of cardiologist, anesthesiologist, and surgeon, and development of an individual plan for each patient, weighing that patient’s risk of thrombosis vs the risk of bleeding, could improve patient safety and optimize outcome.
Keywords: antiplatelet therapy, non-coronary surgery, drug-eluting stents, stent thrombosis
Introduction
In September of 1977, Gruentzig performed the first coronary angioplasty as a nonsurgical method for coronary artery revascularization on a 40-year-old patient in Zurich, Switzerland [1].
The angioplasty in fact induces a “controlled injury” to the coronary vessel and has two major limitations - acute vessel closure (6%-8%) and restenosis (30%-50%).
The pathophysiology of acute vessel closure after angioplasty involves denudation of the endothelium of the coronary artery followed by rapid accumulation of fibrin and platelets, disruption of the atheromatous plaque with intimal dissection and medial tearing, and elastic recoil. Restenosis involves smooth muscle proliferation and neointimal hyperplasia [2, 3].
In an attempt to overcome these problems, bare metal stents (BMS) were introduced into clinical practice in 1986. BMS are metallic scaffolds deployed within a diseased coronary artery segment to optimize the lumen integrity by tacking dissection flaps against the vessel wall and providing mechanical lumen patency. Two large clinical trials, the Belgium Netherlands Stent Arterial Revascularization Therapies Study (BENESTENT) and the North American Stent Restenosis Study (STRESS) [3, 4], showed BMS significantly decrease the incidence of target-lesion revascularization from 25%-35% with percutaneous coronary angioplasty (PTCA) to 10%-15% with stenting. The success in treatment of acute vessel closure came with a price - increased rates of acute (24h) and subacute (24h to 30 days) stent thrombosis, which was addressed with aggressive anticoagulation attempts.
The BENESTENT and the STRESS study reported subacute stent thrombosis of 3.5% and 3.4% respectively despite the complex anticoagulation regimens used (dextran, aspirin, dipyridamole, heparin, and warfarin) [3, 4].
The introduction of intravascular ultrasound, high pressure balloons during stent deployment, and the establishment of dual antiplatelet therapy (DAPT) after stent placement contributed to the decrease of BMS thrombosis (currently 1.2%) [5, 6].
The usefulness of a dual antiplatelet therapy was demonstrated by the PCI-CURE study in which 2658 patients with acute coronary syndrome (ACS) underwent percutaneous coronary intervention (PCI). Patients were randomly assigned to one-year treatment with clopidogrel and aspirin (ASA) or placebo and ASA. In this study, an overall 31% reduction (p = 0.002) of cardiovascular mortality or myocardial infarction (MI) rate was observed in the clopidogrel group [7].
The difference between both groups appears during the first 3 months, and stays constant or slightly increases up to 12 months. The results of the PCI-CURE study provided the basis for recommending the institution of DAPT of clopidogrel and ASA for patients presenting with ACS, including the patients treated with stents.
The purpose of this review is to provide an overview of the changing culture of coronary artery stenting, in addition to discussing perioperative management strategies and controversies surrounding coronary stents and antiplatelet therapy. A comprehensive literature search of MEDLINE was conducted using as keywords: antiplatelet therapy, non-coronary surgery, drug-eluting stents, and stent thrombosis. Over 250 relevant articles were found, 88 of which we have cited and discussed in this article based on their specific relevance to perioperative management, perioperative bleeding, and perioperative thrombosis in patients with drug eluting stents (DES) on DAPT presenting for invasive procedures, mechanisms of stent thrombosis.
Antiplatelet agents
Currently, there are three categories of antiplatelet agents in use: acetylsalicylic acid (ASA), platelet P2Y12 receptor inhibitors (clopidogrel, prasugrel, ticagrelor), and platelet GPIIb-IIIa inhibitors (eptifibatide, tirofiban, abciximab).
ASA is recommended for primary prevention only for diabetic patients with risk of cardiovascular disease [8]. When used for secondary prevention, ASA decreases the MI rate by 30% [9].
After a coronary event, ASA is a lifelong therapy [10]. In their meta-analyses of 50,279 patients for secondary prevention for coronary artery disease, Biondi-Zoccai et al. [11] showed that the cardiac complication rate was three times higher after ASA withdrawal and increased even more in patients with coronary stents [8, 12].
There was, on average, a 10.6-day period between withdrawal from ASA and thrombotic events (8.5 days for coronary symptoms). P2Y12 inhibitors include the thienopyridines, clopidogrel and prasugrel, and the cyclopentyl triazolopyrimidine ticagrelor. Clopidogrel, a pro-drug, is metabolized to active metabolite in the liver in a two-step process by CYP3A4/3A5 and CYP2B6/1A2/2C9/2C19 esterases. Clopidogrel decreases the risk of MI in unstable angina by 18% and the risk of coronary stent thrombosis by 30% [7]. Addition of clopidogrel to ASA decreases the relative risk in the combined end point of cardiovascular death, MI, or stroke by 20% [13].
Prasugrel, also a pro-drug, converts to active metabolite more rapidly, in only one step (CYP3A4 dependent). The metabolite achieves 2.2 times higher level than that of clopidogrel. In phase III clinical trials (TRILOGY-ACS), prasugrel, when compared to clopidogrel, produced a statistically significant reduction of 19% in the primary endpoint of cardiovascular death, nonfatal MI, or nonfatal stroke in the UA/NSTEMI population (p=0.0004) [14]. A 34% decline was observed in urgent target vessel revascularization (p<0.001) and a 42% reduction in heart attack with subsequent death from cardiovascular causes (p=0.02). Ticagrelor is the first approved reversible P2Y12 receptor antagonist. Approval was based on the results of the PLATO (Platelet Inhibition and Patient Outcomes) trial, a large (18,624 patients in 43 countries) head-to-head patient outcomes study of ticagrelor versus clopidogrel, both given in combination with ASA and other standard therapy. PLATO showed treatment with ticagrelor for 12 months was associated with a 21% RRR (Relative Risk Reduction) in death (4% vs. 5.1%; 1.1% ARR; p=0.001) and a 16% RRR in MI compared to clopidogrel (5.8% vs. 6.9%; 1.1% ARR (Absolute Risk Reduction); p<0.005) [15].
Approval of ticagrelor in the U.S. was delayed due to lack of efficacy in the pre-specified subgroup of patients from North America. Two additional analyses were performed by the Duke Clinical Research Institute and AstraZeneca. Though analyses were not able to rule out the possibility of chance as an explanation for the north American subgroup, it was shown that high dose ASA ≥300 mg/day was used far more often in the U.S. than in the rest of the world (53.6% vs. 1.7%). Furthermore, the lowest risk of cardiovascular death, MI, or stroke with ticagrelor compared with clopidogrel is associated with a low maintenance dose of concomitant ASA [15]. As a result, the approval came with a black box warning stating that daily ASA doses above 100 mg decrease effectiveness of the medication. It is also contraindicated in patients with a history of hemorrhagic stroke because of increased risk of bleeding.
Ticagrelor does not require metabolic activation for its clinical effects, has only one active metabolite. The Food and Drug Administration (FDA) recommends stopping ticagrelor 5 days prior to surgical procedures. Even though it reversibly binds to platelet P2Y12 receptors, there is currently no known reversible agent for ticagrelor and it is not expected to be dialyzable. Dyspnea, requiring discontinuation of the treatment, was observed in 14% of the ticagrelor-treated patients compared with 8% in the clopidogrel group [15].
In their new guidelines, The American College of Chest Physicians suggests Ticagrelor 90mg twice daily plus low dose ASA over clopidogrel 75mg plus low dose ASA for patients in the first year after an ACS who have undergone PCI with stent placement [16]. This is the first time that clinical treatment guidelines have specifically suggested the use of ticagrelor over clopidogrel.
Platelet GP IIb/IIIa inhibitors, including tirofiban, hydrochloride, and eptifibatide, block the cross-linking of platelets to fibrinogen, thus inhibiting formation of bridges between the activated platelets and thrombus formation. They are used for the prevention of immediate thrombosis of coronary stents in the first 24-48 hours after PCI [17].
Transition from BMS to DES
Restenosis continues to be the “weak point” of the BMS, occurring at a rate of 20%-25% within 6 months of implantation and peaking at 3 months after the stent implantation [18]. It results in ACS in about 35% of the patients [19] and repeat revascularization of the restenotic lesions in 60%-80% [20]. BMS restenose because the stent struts traumatize the vascular wall and provoke an inflammatory response. This response is followed by an exaggerated proliferation within the media and adventitia, which produces significant neointimal proliferation and occlusion of the stent [19]. In patients with co-morbidities and complex coronary lesions, the response is much more frequent [21]. Techniques to treat stent restenosis include PTCA, atherectomy, repeat stenting, and brachytherapy (intra-coronary delivery of a radioactive isotope) [22]. Failure using these techniques was almost 30%, with recurrent restenosis after in-stent PTCA up to 85% and thrombotic occlusion after brachytherapy up to 15.6% [23,24,25].
Successful prevention of in-stent restenosis was achieved with the development of DES. Coating the BMS with a polymer (containing slowly released antiproliferative material that suppresses the neointimal hyperplasia) decreased the in-stent restenosis from 20% to 4%-6% and significantly decreased of the rate of re-intervention [26, 27].
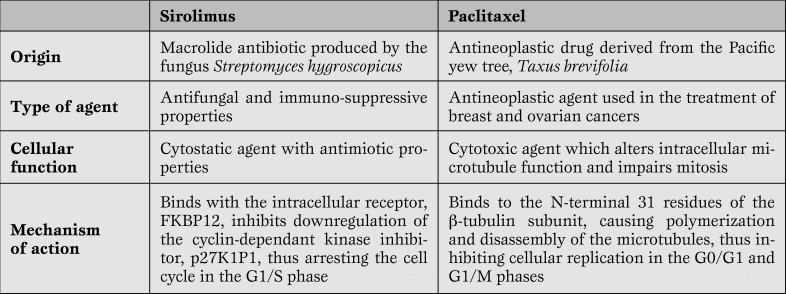
The first generation DES uses sirolimus and paclitaxel as antiproliferative agents to suppress the vascular smooth muscle cell migration and proliferation. Differences between the two agents are presented in Table 1.
Table 1.
Comparison of Sirolimus and Paclitaxel.
Sirolimus is completely released from the polymer within 4 to 6 weeks, while only 10% of the paclitaxel is released within 90 days (the other 90% remains sequestered indefinitely).
DES were approved by the FDA in April of 2003 (sirolimus-eluting DES) and March of 2004 (paclitaxel DES). The FDA approval was based on the results of randomized trials that involved selected patient populations [28,29,30,31,32,33,34,35]. The indications included patients with symptomatic ischemic disease due to de novo lesions of length <30 mm (sirolimus eluting stents) and <28 mm (paclitaxel eluting stents) in native coronary arteries with reference vessel diameter of >2.5 mm to <3.5 mm (<3.7 mm for paclitaxel stents). Clinical trials investigating the restenosis of stents found a 74% reduction of restenosis at 4 years of implantation [36].
As a result, the use of DES significantly increased (up to 85% of all stents placed) in the U.S. and Europe in 2005 [37]. In 60% of the cases, the DES were used “off label” in high-risk populations (diabetics, patients with ACS, low ejection fraction, or renal failure), high-risk lesions (bifurcating lesions, long ones, small vessel lesions, in stent lesions, multiple lesions, left main disease, saphenous vein graft lesions), or other conditions that were excluded from the initial trials [38].
Stent thrombosis and DES
In 2003, 290 cases of subacute stent thrombosis occurring after sirolimus DES implantation were reported to the FDA along with a 20% mortality rate [39].
The goal of the Basel Stent Kosten Effektivitäts Trial-Late Thrombotic Events (BASKET-LATE) study was to determine the true incidence of late stent thrombosis, MI, and death in 746 patients randomized to receive DES or BMS. Patients on DAPT for 6 months without any adverse cardiac events had clopidogrel stopped and were followed for an additional 12 months. Results showed the following:
late stent thrombosis-related events (death and MI) occurred two to three times more frequently in patients with DES than those with BMS;
late stent thrombosis carried a four times higher risk of cardiac death/MI (p< 0.00010);
late stent thrombosis and its complications occurred up to 1 year after clopidogrel discontinuation [18].
Authors concluded that while DES use in 100 patients avoids five target lesion revascularization events at 6 months, it unfortunately leads to 3.3 late (within 18 months) deaths or MI. At the 55th World Congress of Cardiology, two meta-analyses were presented, showing a significant increase in the rate of total mortality and Q wave MI in DES compared to BMS at after 12 months and up to 3 years [40, 41].
Experimental models of DES demonstrate incomplete healing, fibrin deposition, and inflammatory cells, indicating a hypersensitivity reaction [2, 42] while BMS demonstrate complete endothelialization at 28 days. Sirolimus and paclitaxel have shown to impair endothelial function both within the stent and in the distal coronary artery, leading to delayed arterial healing of the stent itself, as well as enhancing the risk for distal arterial ischemia and coronary occlusion [43]. In addition, they enhance expression of the endothelial tissue factor, which creates a prothrombotic state.
Despite equal stenosis severity and follow-up duration, patients with DES, compared to BMS, more frequently have collaterals insufficient to prevent ischemia during occlusion. The most powerful histological predictor of stent thrombosis has been incomplete endothelial coverage of the stent [44].
Several studies and registries have identified clinical predictors for delayed endothelial coverage and thrombosis of DES. ACS, left ventricular ejection fraction <30%, treatment of bifurcating lesions, renal insufficiency, and diabetes have shown to be strong predictors of stent thrombosis [41, 45,46,47,48,49,50]. The strongest independent clinical predictor is the premature discontinuation of the clopidogrel therapy [48,49,50].
The concerns about late stent thrombosis resulted in an emergency FDA advisory panel meeting in December 2006, which reassured that DES in the studied “on-label” settings appear safe and efficacious, but warned that data regarding safety and efficacy in “off-label” situations is not available and will likely not match results seen in the lower-risk “on-label” settings [10]. Not surprisingly, use of DES dropped precipitously to approximately 60% within months.
Multiple registry studies have since shown that “off-label” DES use is indeed associated with a roughly two- to threefold increase in clinical adverse events, including stent thrombosis, compared with “on-label” use [51,52,53]. Despite these findings, it is clear that DES remain superior to BMS even in high-risk “off-label” situations.
Recently, a comprehensive meta-analysis of almost 10,000 randomized controlled trial patients and over 180,000 observational study patients confirmed the overall benefit of DES over BMS in both “on-label” and “off-label” populations. Evidence showed trends to reduced (randomized trials) or significantly reduced (observational studies) death and MI, and dramatic significant reductions in target vessel revascularization (regardless of study type) [54].
Antiplatelet therapy and des
Controversy surrounding the DES late thrombosis issue creates a controversy about the length of antiplatelet therapy in patients with DES. The initial recommendations made by the FDA/American college of cardiology (ACC)/American heart association (AHA)/Society for Cardiovascular Angiography Interventions (SCAI) and the stent manufacturers were completely arbitrary. They advised patients to remain on DAPT for a minimum of 3 months after the implantation of sirolimus DES and 6 months after paclitaxel DES followed by life-long ASA therapy. In 2005, a focused update of the ACC/AHA/SCAI PCI guidelines recommended that all patients who receive a DES should be given clopidogrel for at least 12 months in the absence of an increased risk of bleeding.
With the growing number of publications concerning the safety of DES, the FDA published a scientific advisory in January of 2007, endorsed by 5 major professional societies: AHA/ACC/SCAI, the American College of Surgeons (ACS), and the American Dental Association (ADA). Once again, the importance of 12 months DAPT after placement of DES and life-long ASA therapy was emphasized. Once further, in the 2011 ACCF/AHA/SCAI guidelines for PCI, the role of DAPT for prevention of thrombosis in patients with stents was strongly re-enforced with class IB recommendation for treatment with P2Y12 inhibitor for at least 12 months after PCI with DES in addition to indefinite therapy with ASA and class II B recommendation for consideration of DAPT beyond 12 months [55].
The ideal duration of antiplatelet therapy is still unknown [54, 56]. DAPT trial, which is currently ongoing, compares 12 versus 30 months of DAPT among 15 000 patients treated with DES. This trial is powered to assess the primary efficacy endpoints of differences in stent-thrombosis rates and major adverse cardiovascular/cerebrovascular events (MACCE), with a primary safety endpoint of major bleeding.
Currently, around 60% of patients undergoing PCI receive DES and are placed on DAPT for at least one year. It is suggested that 5% of these patients will require non-cardiac surgery during this time [57], posing a unique challenge during the perioperative period.
DES management - Non-cardiac surgery
The decision to stop antiplatelet medications before invasive procedures in order to decrease the risk of bleeding may expose patients with DES to increased risk for stent thrombosis, MI, and cardiac death. Conversely, continuing antiplatelet therapy in order to prevent stent thrombosis may expose patients to increased risk of bleeding and need for transfusions during invasive procedures.
Current literature on the use of antiplatelet agents in surgery reports the average surgical blood loss increase by ASA is approximately 2.5% - 20%.
In non-cardiac surgery, a meta analysis of 474 studies on the impact of low dose ASA on surgical blood loss showed that ASA alone increases the average intraoperative hemorrhagic risk by a factor of 1.5, but does not increase mortality and morbidity [58]. Possible exceptions may be intracranial neurosurgery and transurethral prostatectomy where ASA has been a contributing factor to fatal outcomes [12, 59].
The major side effect of clopidogrel is increased risk of spontaneous hemorrhage by 38% (incidence 1%-2%) [7]. In non-cardiac surgery increased bleeding has been described in transbronchial biopsy and pacemaker and defibrillator implantation [60, 61]. Prasugrel is a 10 times more potent platelet inhibitor than clopidogrel; however, it is associated with a statistically significant increase in non-CABG (coronary artery bypass grafting) major bleeding (2.4% vs. 1.8%, p=0.03) and fatal bleeding (0.4% vs. 0.1%, p=0.002) compared to clopidogrel.
Although ticagrelor is a reversible P2Y12 inhibitor, the PLATO trial showed higher incidence of non-CABG related bleeding (RR 1.18) and significantly increased incidence of fatal intracranial bleeding (RR 10.95) [15]. With DAPT, the bleeding time increases three- to fourfold [62] over ASA alone, and surgical blood loss increases by an average of 30% to 50% [63]. Significant bleeding in patients on DAPT undergoing different non-cardiac surgical procedures has been described [64,65,66]. However, other case reports and series have not found such association [57, 67,68,69].
Some surgical procedures are associated with significant mortality and morbidity if bleeding is encountered, such as intracranial or spine surgery, where even a small amount of bleeding can cause brain or cord compression and irreversible brain or cord damage. Chassot et al. noted increased mortality and morbidity after intracranial surgery in patients on clopidogrel therapy. The surgical bleeding and transfusion rates in the rest of the surgical procedures, although increased by 50%, were not associated with an increase in mortality and morbidity [63].
Risk of perioperative thrombosis
The incidence of major coronary adverse events after PCI is estimated around 4% to 5%, and 20% to 45% of these events can result in death [56, 70]. Patients are most vulnerable immediately after a PCI because the stenotic lesion is transformed into an unstable area due to the rupture of its endothelial covering. When undergoing surgery during this early period, the rate of mortality (30%) and morbidity (20-40%) is 5 to 10 times higher than matched patients undergoing the same operation under maximal medical therapy or after appropriate delay [68, 71].
Premature discontinuation of antiplatelet therapy has been found to be the strongest predictor of stent thrombosis, carrying significant risk of mortality and morbidity even when the discontinuation is not associated with surgery [38, 48, 72]. As long as DES have not endothelialized, the patient is absolutely dependent on the antiplatelet medications for stent thrombosis prevention. Surgery itself produces a prothrombotic and proinflammatory state that increases the risk of stent thrombosis.
The stress response to surgery includes sympathetic activation and cytokine release that promotes shear stress on arterial plaques, enhanced vascular reactivity conductive of vasospasm, increased platelet activation, and increased hypercoagulability [73, 74].
Perioperative management
Currently, there is no definite standard of care for the perioperative management of patients with coronary stents, though deep understanding of the mechanisms of DES and the indications for antiplatelet therapy could ensure patient safety and optimize outcome.
A survey conducted on 374 interventional cardiologists found that although there is agreement among interventional cardiologists on the optimum delay for surgery after stenting, on the need for BMS or balloon angioplasty alone if early noncardiac surgery is needed, and on treatment of perioperative thrombosis, there is significant inconsistency on the optimum antiplatelet therapy for patients who need surgery early after stent implantation [75]. If the physicians most intimately involved with the management of patients with DES do not agree on how to manage antiplatelet therapy perioperatively, we may speculate the chance of having consensus among anesthesiologists, surgeons, internists, and other interventional physicians on this topic is minimal. It is likely that the full implications of stopping antiplatelet therapy are not fully appreciated, and a reasonable fear of bleeding predominates [76].
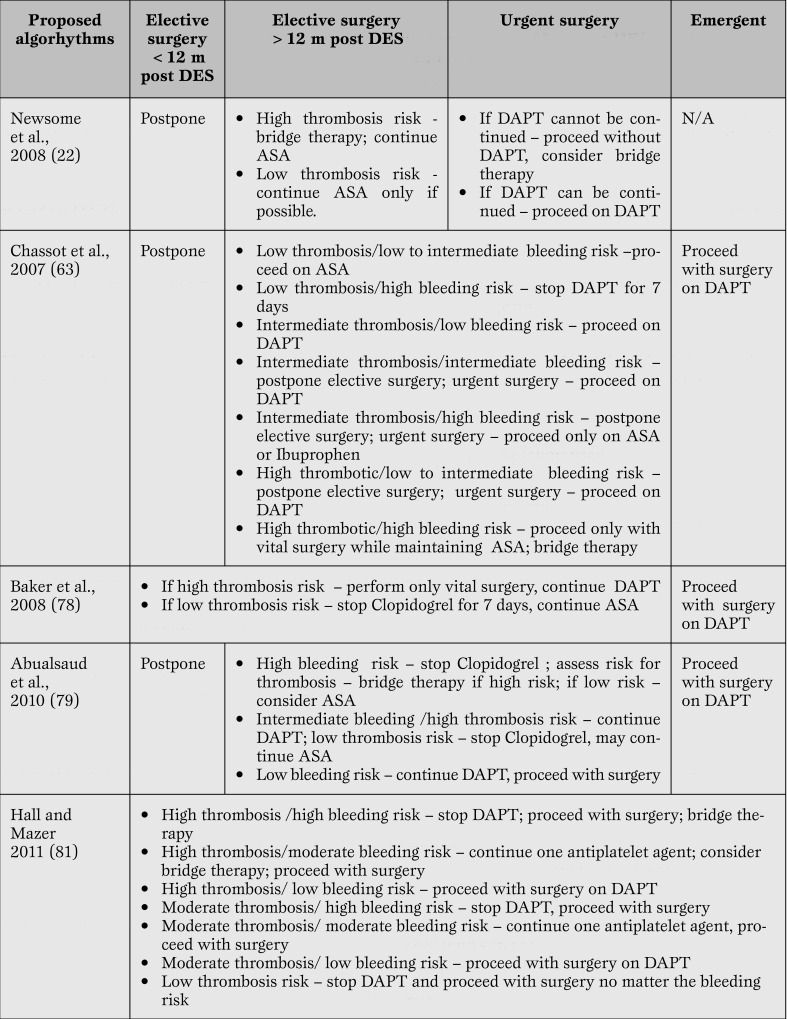
Different algorithms for perioperative antiplatelet therapy management in patients with stents have been suggested [75, 77,78,79,80,81], but there are no prospective studies evaluating those algorithms. Table 2 summarizes recent recommendations on the decision to proceed with antiplatelet therapy and/or surgical intervention, taking into account bleeding risk. Until such studies are conducted and evidence-based guidelines are established, every institution should have well-publicized policies and guidelines to manage these patients. Patients with stents, especially DES, should be identified early in the preoperative work-up. Each case should be managed on an individual basis and the risk and consequences of stent thrombosis should be weighed against those of perioperative bleeding.
Table 2.
Recommendations for intervention based on bleeding risk. DES = drug eluting stents; ASA=aspirin; DAPT = dual antiplatelet therapy.
According to the 2011 ACCF/AHA/SCAI Guidelines, even before considering stent implantation, patients should be evaluated for possibility of surgery in the following 12 months and should not be treated with DES if such possibility exists [55]. Percutaneous angioplasty or BMS, requiring a minimum of 4 to 6 weeks of antiplatelet therapy, or medical management only if the surgery cannot be delayed, should be considered instead. Routine prophylactic coronary revascularization should not be performed in patients with stable coronary artery disease before non-cardiac surgery because of possible significant harm to the patient [55, 56].
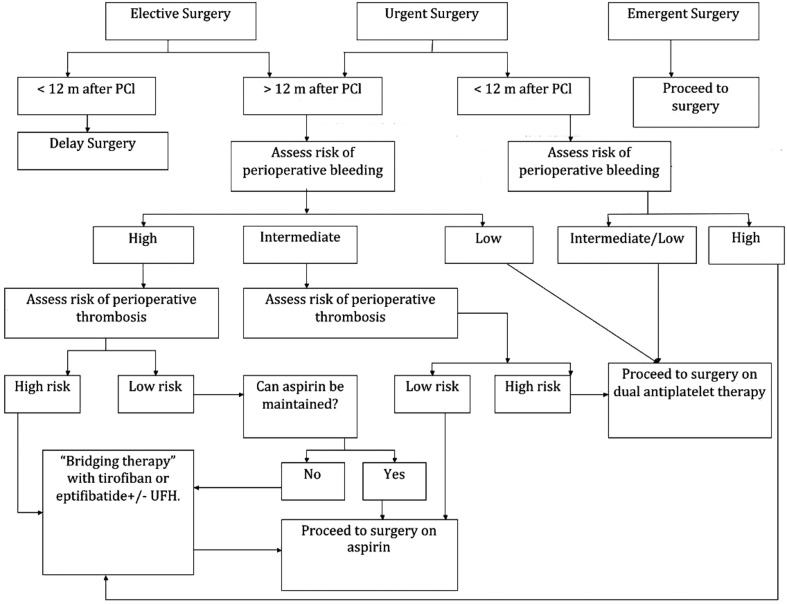
Once patients present for surgery with a stent in place, consideration should be given to the electiveness of the surgical procedure. Figure 1 summarizes a chain of interventions based on three different surgical scenarios.
Figure 1.
Perioperative management flow chart.
Elective surgery should not be performedwithin 4 to 6 weeks of stent placement or within 12 months of DES placement in patients who’s antiplatelet therapy will need to be discontinued perioperatively [55, 56]. Brancati et al. assessed the perioperative outcome of patients undergoing non-cardiac surgery after coronary stent implantation in a single center registry. After multivariable analysis, the predictor of primary endpoint, defined as perioperative occurrence of major adverse events, was the time interval between stenting and surgery with a statistically significant increase in the number of events when surgery was performed within 6 weeks of BMS placement [82]. There was no difference in the major bleeding between the groups with different antiplatelet regimens. Once again, these results supported the AHA/ACC recommendations on timing of non-cardiac surgery after stent implantation.
If a patient with DES presents for an elective procedure more than one year after the stent placement and still on DAPT, the management of the antiplatelet medications will depend on the bleeding risk of the specific surgical procedure and on the complexities of the DES (thrombotic risk). Chassot et al. grouped the surgical procedures into three groups according to their bleeding risk [63]:
Low bleeding risk surgical procedures that usually do not require blood transfusion, such as peripheral, plastic, and minor general surgery; biopsies; minor orthopedic procedures; minor ENT (ear-nose-throat) procedures; endoscopies; anterior chamber of the eye surgeries; or dental extractions and dental surgery.
Intermediate bleeding risk surgical procedures that frequently require blood transfusions. Examples can be visceral surgery; cardiovascular surgery; major orthopedic, ENT, and reconstructive surgery; or endoscopic urology.
High bleeding risk possible bleeding in a closed space such as intracranial neurosurgical procedures; spinal canal surgery; or posterior chamber of the eye surgery.
There is a growing agreement that DAPT needs to be continued indefinitely in patients with complex stent placing procedures, such as stenting of bifurcating lesions, left main stents, overlapping stents, stents within stents, small vessel stents, multiple stents, saphenous vein graft stents, chronically occluded stents, or in patients with co-morbidities, such as diabetes, low ejection fraction, end stage renal disease, malignancies, advanced age, or with resistance to antiplatelet medications and a history of stent occlusion/thrombosis [56].
For surgical procedures with low risk for bleeding, DAPT should be continued throughout. Intermediate-bleeding-risk surgical procedures should be approached on a case-by-case basis.
In patients with complex stent procedures and co-morbidities, the DAPT should be continued despite the risk for increased bleeding. In all other patients, clopidogrel and ticagrelor can be stopped 5 days before surgery and prasugrel 7 days before surgery, while ASA should be continued. DAPT should be restarted as soon as possible postoperatively (ideally within 24 hours) with a loading dose of 300 mg - 600 mg clopidogrel, or prasugrel 60 mg, or ticagrelor 180 mg [57, 83]. High-bleeding-risk surgical procedures present a challenge. Patients with 12 months completed of DAPT and low risk for thrombosis of the coronary artery stents can stop clopidogrel and ticagrelor 5 days prior to surgery and prasugrel 7 days before surgery while continuing ASA. ASA discontinuation even more than one year after DES placement may lead to stent thrombosis [83, 84].
For patients with coronary stents with high risk for thrombosis presenting for high-risk bleeding surgical procedures, “bridge” therapy has been suggested. Use of short-acting GP IIb/IIIa inhibitors, such as tirofiban or eptifibatide, has been proposed as a bridge between the time of the thienopyridine discontinuation and surgery. Either medication is given as infusion and requires patient admission to the hospital 3 days after the discontinuation of thienopyridines. Infusion is stopped 4 to 6 hours prior to surgical procedure and restarted as soon as possible after the surgery upon agreement between cardiology and surgery [85, 86]. Usually, heparin infusion commences, though there is no evidence supporting that heparin offers efficient protection in high-risk coronary situations [85]. Nonsteroidal anti-inflammatory drugs (NSAIDs), such as ibuprofen, inhibit COX-1, as ASA does. Since action is reversible and platelet function is completely recovered within 24 hours of their discontinuation, NSAIDs are suggested as an alternative to ASA [63].
However, all of these “bridging techniques” are controversial, with little data published to support use, and are associated with an increased cost.
Cangrelor, an investigational parenteral, reversible, direct P2Y12 platelet inhibitor with its extremely short (5 to 9 minutes) half-life, may present an alternative for “bridge” therapy in the near future. In the recently published results from the BRIDGE (Maintenance of platelet inhibition with Cangrelor) trial, cangrelor was effective at maintaining platelet inhibition in patients on thienopyridines who required bypass surgery [87]. This prospective, randomized, double-blind, placebo-controlled, multicenter trial evaluated 210 patients on thienopyridine therapy awaiting CABG. The thienopyridine was stopped and patients were randomized to treatment with cangrelor or placebo for at least 48 hours which then was stopped 1 to 6 hours prior to surgery.
The cangrelor group had low levels of platelet reactivity throughout the treatment period compared to patients on placebo. Excessive CABG related bleeding occurred in 11.8% of patients on cangrelor vs 10.4% in patients on placebo. There was no difference in major bleeding prior to CABG, although minor bleeding was slightly higher in the cangrelor group [87].
In surgical procedures where even a small postoperative hemorrhage can have disastrous consequences, such as intracranial surgery, spinal surgery in the medullary canal, and surgery of the posterior chamber of the eye, both P2Y12 inhibitors and ASA may need to be discontinued 7 days prior to surgery. In such cases “bridge” therapy may be considered.
Management of patients with stents in need of urgent surgical procedures depends on the time interval between DES placement and the surgical procedure. If the time is less than one year (or less than one month for BMS), then the DAPT has to be continued throughout, except for surgeries in enclosed spaces. Less invasive surgical techniques and alternative treatments should be considered in such patients. If patients present for an urgent surgical procedure more than 12 months after the DES placement, the same paths as elective surgical procedures should be followed. ASA should be continued throughout in most cases.
Future developments of DES management
Current efforts are directed towards creating reversible short-acting platelet inhibitors that can be used in the perioperative period, creating DES with more predictable endothelialization, and developing point-of-care tests of platelet function that can help the perioperative management of patients on antiplatelet therapy.
Even though both the CHAMPION PLATFORM study and the CHAMPION PCI trial, which compared clopidogrel and cangrelor, were terminated early for the lack of efficacy end points, cangrelor may prove valuable in the perioperative period as a bridge therapy as shown by the results of the BRIDGE trial [87]. Although this path still involves hospital admission ahead of planned surgery and requires IV infusion which will be associated with increased cost, cangrelor may provide better platelet inhibition than that of the currently proposed combination of GPIIb/IIIa inhibitors and heparin.
A new generation DES was introduced in 2008: Zotarolimus DES system, which uses phosphorylchlorine-base, biocompatible polymer and the Everolimus DES, approved in July 2008. Recently, the results of the ISAR-TEST trial were published showing that a polymer-free dual-drug sirolimus- and probucol-eluting stents are non-inferior to the second generation Zotarolimus-eluting stent [88]. New platforms (cobalt-chromium and platinum-chromium), new delivery systems, new polymers allowing better biocompatibility and/or flexibility and will attempt to decrease the rate of late stent thrombosis and expand stent indications.
In February 2012, the FDA approved the first DES for use in patients with diabetes based on the results of the RESOLUTE trial - The Resolute Integrity Zotarolimus stent. Several days later, based on the results of the HORIZONS - AMI trial, the FDA approved the first DES for treatment of acute myocardial infarction - the ION paclitaxel eluting stent. Alternatively to the traditional BMS and DES, a simple chemical coating may be effective in preventing thrombotic events, shortening the duration of required antiplatelet therapy, and allowing for safe perioperative discontinuation of antiplatelet agents.
Coatings such as titanium-nitride-oxide, dimethyl sulfoxide, CD34 antibodies and stents containing an integrilin-binding cyclic Arg-Gly-Asp peptide are being explored. In recent years, there has also been a significant effort in developing point-of-care tests for assessment of the effects of the antiplatelet drugs for guidance of the perioperative management of patients on antiplatelet therapy. The gold standard is still light transmittance aggregometry, but the test requires significant sample preparation, special equipment, and trained personnel and is difficult to use in everyday practice.
Plateletworks, VerifyNow, and thromboelastography platelet mapping are some current available tests that are not as reliable as the light transmittance aggregometry and still require special equipment and trained personnel.
Recommendations
Understanding the risks of stopping antiplatelet therapy by the healthcare professionals is of paramount importance.
Use of a multidisciplinary team approach, including the interventional cardiologist, anesthesiologist, and surgeon, to guide perioperative management is imperative.
Invasive procedures on patients with DES, at high risk for stent thrombosis, and DAPT discontinued should be performed in centers with around-the-clock invasive cardiology services as the primary option.
However, smaller institutions may not have such coverage, and should not be fully excluded from performing invasive procedures due to the overall high volume of stent implantation.
Outpatient surgery on such patients should not be performed, and ideally, patients should be kept in the hospital for at least 48 hours for cardiac monitoring or until antiplatelet medications are restarted.
Acknowledgments
The authors would like to acknowledge K. Hudec for contributions to this manuscript.
Footnotes
Source of Support Nil.
Conflict of interest None declared.
Cite as: Dimitrova G, Tulman DB, Bergese SD. Perioperative management of antiplatelet therapy in patients with drug-eluting stents. HSR Proceedings in Intensive Care and Cardiovascular Anesthesia 2012; 4(3): 153-167
References
- Mueller R L, Sanborn T A. The history of interventional cardiology: cardiac catheterization, angioplasty, and related interventions. Am Heart J. 1995;129:146–172. doi: 10.1016/0002-8703(95)90055-1. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Hong M K, Virmani R. et al. Granulomatous \'foreign body reactions\' contribute to exaggerated in-stent restenosis. Coron Artery Dis. 1999;10:9–14. doi: 10.1097/00019501-199901000-00002. [DOI] [PubMed] [Google Scholar]
- Serruys P W, de Jaegere P, Kiemeneij F. et al. A comparison of balloon-expandable-stent implantation with balloon angioplasty in patients with coronary artery disease. Benestent study group. N Engl J Med. 1994;331:489–495. doi: 10.1056/NEJM199408253310801. [DOI] [PubMed] [Google Scholar]
- Fischman D L, Leon M B, Baim D S. et al. A randomized comparison of coronary-stent placement and balloon angioplasty in the treatment of coronary artery disease. Stent Restenosis Study Investigators. N Engl J Med. 1994;331:496–501. doi: 10.1056/NEJM199408253310802. [DOI] [PubMed] [Google Scholar]
- Colombo A, Hall P, Nakamura S. et al. Intracoronary stenting without anticoagulation accomplished with intravascular ultrasound guidance. Circulation. 1995;91:1676–1688. doi: 10.1161/01.cir.91.6.1676. [DOI] [PubMed] [Google Scholar]
- Bertrand M E, Legrand V, Boland J. et al. Randomized multicenter comparison of conventional anticoagulation versus antiplatelet therapy in unplanned and elective coronary stenting. The full anticoagulation versus aspirinand ticlopidine (fantastic) study. Circulation. 1998;98:1597–1603. doi: 10.1161/01.cir.98.16.1597. [DOI] [PubMed] [Google Scholar]
- Mehta S R, Yusuf S, Peters R J. et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- Pignone M, Alberts M J, Colwell J A. et al. Aspirin for primary prevention of cardiovascular events in people with diabetes: a position statement of the American Diabetes Association, a scientific statement of the American Heart Association, and an expert consensus document of the American College of Cardiology Foundation. Circulation. 2010;121:2694–2701. doi: 10.1161/CIR.0b013e3181e3b133. [DOI] [PubMed] [Google Scholar]
- Harrington R A, Becker R C, Ezekowitz M. et al. Antithrombotic therapy for coronary artery disease: the seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126:5135S–5148S. doi: 10.1378/chest.126.3_suppl.513S. [DOI] [PubMed] [Google Scholar]
- Grines C L, Bonow R O, Casey D E Jr. et al. Prevention of premature discontinuation of dual antiplatelet therapy in patients with coronary artery stents: a science advisory from the American Heart Association, American College of Cardiology, Society for Cardiovascular Angiography and Interventions, American College of Surgeons, and American Dental Association, with representation from the American College of Physicians. Circulation. 2007;115:813–818. doi: 10.1161/CIRCULATIONAHA.106.180944. [DOI] [PubMed] [Google Scholar]
- Biondi-Zoccai G G, Lotrionte M, Agostini P. et al. A systematic review and meta-analysis on the hazards of discontinuing or not adhering to aspirin among 50, 279 patients at risk for coronary artery disease. Eur Heart J. 2006;27:2667–2674. doi: 10.1093/eurheartj/ehl334. [DOI] [PubMed] [Google Scholar]
- Palmer J D, Sparrow O C, Iannotti F. Postoperative hematoma: a 5-year survey identification of avoidable risk factors. Neurosurgery. 1994;35:1061–1064. doi: 10.1227/00006123-199412000-00007. [DOI] [PubMed] [Google Scholar]
- Mehta S R, Yusuf S, Peters R J. et al. Effects of pretreatment with clopidogrel and aspirin followed by long-term therapy in patients undergoing percutaneous coronary intervention: the PCI-CURE study. Lancet. 2001;358:527–533. doi: 10.1016/s0140-6736(01)05701-4. [DOI] [PubMed] [Google Scholar]
- Chin C T, Roe M T, Fox K A. et al. TRILOGY ACS Steering Committee. Study design and rationale of a comparison of prasugrel and clopidogrel in medically managed patients with unstable angina/non-ST-segment elevation myocardial infarction: the TaRgeted platelet Inhibition to cLarify the OptimalstrateGy to medicallY manage Acute Coronary Syndromes (TRILOGY ACS) trial. Am Heart J. 2010;160:16–22. doi: 10.1016/j.ahj.2010.04.022. [DOI] [PubMed] [Google Scholar]
- Mahaffey K W, Wojdyla D M, Carroll K. et al. Ticagrelor compared with clopidogrel by geographic region in the platelet inhibition and patient outcomes (PLATO) trial. Circulation. 2011;124:544–554. doi: 10.1161/CIRCULATIONAHA.111.047498. [DOI] [PubMed] [Google Scholar]
- Eikelboom J W, Hirsh J, Spencer F A. et al. Antiplatelet drugs: Antithrombotic therapy and Prevention of thrombosis, 9th edition: American College of Chest Physicians; Evidence-Based Clinical Practice Guidelines. CHEST. 2012;141:89–119. doi: 10.1378/chest.11-2293. [Suppl. 2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montalescot G, Barragan P, Wittenberg O. et al. Platelet glycoprotein IIb/IIIa inhibition with coronary stenting for acute myocardial infarction. N Engl J Med. 2001;344:1895–1903. doi: 10.1056/NEJM200106213442503. [DOI] [PubMed] [Google Scholar]
- Woods T C, Marks A R. Drug-eluting stents. Ann Rev Med. 2004;55:169–178. doi: 10.1146/annurev.med.55.091902.105243. [DOI] [PubMed] [Google Scholar]
- Chen M S, John J M, Chew D P. et al. Bare metal stent restenosis is not a benign clinical entity. Am Heart J. 2006; 151: 1260-4. 2006;151:1260–1264. doi: 10.1016/j.ahj.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Hoffmann R, Mintz G S. Coronary in-stent restenosis - predictors, treatment and prevention. Eur Heart J. 2000;21:1739–1749. doi: 10.1053/euhj.2000.2153. [DOI] [PubMed] [Google Scholar]
- Tcheng J E, Talley J D, O\'Shea J C. et al. Clinical pharmacology of higher dose eptifibatide in percutaneous coronary intervention (the PRIDE study). Am J Cardiol. 2001; 88: 1097-102. 2001;88:1097–1102. doi: 10.1016/s0002-9149(01)02041-0. [DOI] [PubMed] [Google Scholar]
- Newsome L T, Kutcher M A, Royster R L. Coronary artery stents: Part I. Evolution of percutaneous coronary intervention. AnesthAnalg. 2008;107:552–569. doi: 10.1213/ane.0b013e3181732049. [DOI] [PubMed] [Google Scholar]
- Yokoi H, Kimura T, Nakagawa Y. Long-term clinical and quantitative angiographic follow-up after the Palmaz-Schatz stent restenosis. J Am Coll Cardiol. 1995; 27: 224. 1995;27:224. [Google Scholar]
- Lemos P A, Serruys P W, Sousa J E. Drug-eluting stents: cost versus clinical benefit. Circulation. 2003;107:3003–3007. doi: 10.1161/01.CIR.0000078025.19258.28. [DOI] [PubMed] [Google Scholar]
- Drenth D J, Veeger N J, Winter J B. et al. A prospective randomized trial comparing stenting with off-pump coronary surgery for high-grade stenosis in the proximal left anterior descending coronary artery: three-year follow-up. J Am Coll Cardiol. 2002;40:1955–1960. doi: 10.1016/s0735-1097(02)02536-6. [DOI] [PubMed] [Google Scholar]
- Arjomand H, Turi Z G, McCormick D, Goldberg S. Percutaneous coronary intervention: historical perspectives, current status, and future directions. Am Heart J. 2003;146:787–796. doi: 10.1016/S0002-8703(03)00153-4. [DOI] [PubMed] [Google Scholar]
- Sousa J E, Serruys P W, Costa M A. New frontiers in cardiology: drug-eluting stents: Part II. Circulation. 2003;107:2383–2389. doi: 10.1161/01.CIR.0000069331.67148.2F. [DOI] [PubMed] [Google Scholar]
- Morice M C, Serruys P W, Sousa J E. et al. Randomized Study with the Sirolimus-Coated BxVelocity Balloon-Expandable Stent in the Treatment of Patients with de Novo Native Coronary Artery Lesions. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002; 346: 1773-80. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- Moses J W, Leon M B, Popma J J. et al. Sirolimus-eluting stents versus standard stents in patients with stenosis in a native coronary artery. N Engl J Med. 2003;349:1315–1323. doi: 10.1056/NEJMoa035071. [DOI] [PubMed] [Google Scholar]
- Schofer J, Schluter M, Gershlick A H. et al. Sirolimus-eluting stents for treatment of patients with long atherosclerotic lesions in small coronary arteries: double-blind, randomised controlled trial (E-SIRIUS). Lancet. 2003;362:1093–1099. doi: 10.1016/S0140-6736(03)14462-5. [DOI] [PubMed] [Google Scholar]
- Schampaert E, Cohen E A, Schluter M. et al. The Canadian study of the sirolimus-eluting stent in the treatment of patients with long de novo lesions in small native coronary arteries (C-SIRIUS). J Am CollCardiol. 2004;43:1110–1115. doi: 10.1016/j.jacc.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Grube E, Silber S, Hauptmann K E. et al. TAXUS I: six- and twelve-month results from a randomized, double-blind trial on a slow-release paclitaxel-eluting stent for de novo coronary lesions. Circulation. 2003;107:38–42. doi: 10.1161/01.cir.0000047700.58683.a1. [DOI] [PubMed] [Google Scholar]
- Colombo A, Drzewiecki J, Banning A. et al. Randomized study to assess the effectiveness of slow- and moderate-release polymer-based paclitaxel-eluting stents for coronary artery lesions. Circulation. 2003;108:788–794. doi: 10.1161/01.CIR.0000086926.62288.A6. [DOI] [PubMed] [Google Scholar]
- Stone G W, Ellis S G, Cox D A. et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N Engl J Med. 2004;350:221–231. doi: 10.1056/NEJMoa032441. [DOI] [PubMed] [Google Scholar]
- Comparison of a polymer-based paclitaxel-eluting stent with a bare metal stent in patients with complex coronary artery disease: a randomized controlled trial. JAMA. 2005;294:1215–1223. doi: 10.1001/jama.294.10.1215. [DOI] [PubMed] [Google Scholar]
- Stone G W, Moses J W, Ellis S G. et al. Safety and efficacy of sirolimus- and paclitaxel-eluting coronary stents. N Engl J Med. 2007;356:998–1008. doi: 10.1056/NEJMoa067193. [DOI] [PubMed] [Google Scholar]
- Kandzari D E, Roe MT M T, Ohman E M. et al. Frequency, predictors, and outcomes of drug-eluting stent utilization in patients with high-risk non-ST-segment elevation acute coronary syndromes. Am J Cardiol. 2005;96:750–755. doi: 10.1016/j.amjcard.2005.05.015. [DOI] [PubMed] [Google Scholar]
- Jaffe R, Strauss B H. Late and very late thrombosis of drug-eluting stents: evolving concepts and perspectives. J Am Coll Cardiol. 2007;50:119–127. doi: 10.1016/j.jacc.2007.04.031. [DOI] [PubMed] [Google Scholar]
- FDA Public Health Web Notification: final update of information for physicians on sub-acute thromboses (SAT) and hypersensitivity reactions with use of the cordis CYPHER? sirolimus-eluting coronary stent. U.S. Food and Drug Administration. 2004 [http://www.fda.gov/cdrh/safety/cypher3.html]
- Camenzind E, Steg P G, Wijns W. Controversies in cardiovascular medicine. A cause for concern. Circulation. 2007;115:1440–1455. doi: 10.1161/CIRCULATIONAHA.106.666800. [DOI] [PubMed] [Google Scholar]
- Pfisterer M, Brunner-La Rocca H P, Buser P T. et al. Late clinical events after clopidogrel discontinuation may limit the benefit of drug-eluting stents: an observational study of drug-eluting versus bare-metal stents. J Am CollCardiol. 2006;48:2584–2591. doi: 10.1016/j.jacc.2006.10.026. [DOI] [PubMed] [Google Scholar]
- Farb A, Burke A P, Kolodgie F D, Virmani R. Pathological mechanisms of fatal late coronary stent thrombosis in humans. Circulation. 2003;108:1701–1706. doi: 10.1161/01.CIR.0000091115.05480.B0. [DOI] [PubMed] [Google Scholar]
- Luscher T F, Steffel J, Eberli F R. et al. Drug-eluting stent and coronary thrombosis: biological mechanisms and clinical implications. Circulation. 2007;115:1051–1058. doi: 10.1161/CIRCULATIONAHA.106.675934. [DOI] [PubMed] [Google Scholar]
- Finn A V, Joner M, Nakazawa G. et al. Pathological correlates of late drug-eluting stent thrombosis: strut coverage as a marker of endothelialization. Circulation. 2007;115:2435–2441. doi: 10.1161/CIRCULATIONAHA.107.693739. [DOI] [PubMed] [Google Scholar]
- Iakovou I. Incidence, predictors, and outcome of thrombosis after successful implantation of drug-eluting stents. JAMA. 2005;293:2126–2130. doi: 10.1001/jama.293.17.2126. [DOI] [PubMed] [Google Scholar]
- Ong A T, McFadden E P, Regar E. et al. Late angiographic stent thrombosis (LAST) events with drug-eluting stents. J Am Coll Cardiol. 2005;45:2088–2092. doi: 10.1016/j.jacc.2005.02.086. [DOI] [PubMed] [Google Scholar]
- Gurbel P A, Kandzari D E. Stent thrombosis associated with first-generation drug-eluting stents: issues with antiplatelet therapy. Neth Heart J. 2007;15:148–150. doi: 10.1007/BF03085971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park D W, Park S W, Park K H. et al. Frequency of and risk factors for stent thrombosis after drug-eluting stent implantation during long-term follow-up. Am J Cardiol. 2006;98:352–356. doi: 10.1016/j.amjcard.2006.02.039. [DOI] [PubMed] [Google Scholar]
- Spertus J A, Kettelkamp R, Vance C. et al. Prevalence, predictors, and outcomes of premature discontinuation of thienopyridine therapy after drug-eluting stent placement: results from the PREMIER registry. Circulation. 2006;113:2803–2809. doi: 10.1161/CIRCULATIONAHA.106.618066. [DOI] [PubMed] [Google Scholar]
- Ellis S G, Colombo A, Grube E. et al. Incidence, timing, and correlates of stent thrombosis with the polymeric paclitaxel drug-eluting stent: a TAXUS II, IV, V, and VI meta-analysis of 3,445 patients followed for up to 3 years. J Am CollCardiol. 2007;49:1043–1051. doi: 10.1016/j.jacc.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Win H K, Caldera A E, Maresh K. et al. Clinical outcomes and stent thrombosis following off-label use of drug-eluting stents. JAMA. 2007;297:2001–2009. doi: 10.1001/jama.297.18.2001. [DOI] [PubMed] [Google Scholar]
- Beohar N, Davidson C J, Kip K E. et al. Outcomes and complications associated with off-label and untested use of drug-eluting stents. JAMA. 2007;297:1992–2000. doi: 10.1001/jama.297.18.1992. [DOI] [PubMed] [Google Scholar]
- Applegate R J, Sacrinty M T, Kutcher M A. et al. "Off-label" stent therapy: 2-year comparison of drug-eluting versus bare-metal stents. J Am CollCardiol. 2008;51:607–614. doi: 10.1016/j.jacc.2007.08.064. [DOI] [PubMed] [Google Scholar]
- Kirtane A J, Gupta A, Iyengar S. et al. Safety and efficacy of drug-eluting and bare-metal stents: a comprehensive meta-analysis of randomized trials and observational studies. Circulation. 2009;119:3198–3206. doi: 10.1161/CIRCULATIONAHA.108.826479. [DOI] [PubMed] [Google Scholar]
- Levine G N, Bates E R, Blankenship J C. et al. 2011 ACCF/AHA/SCAI Guidelines for Percutaneous Coronary Intervention: Executive Summary. Circulation. 2011;124:2574–2609. doi: 10.1161/CIR.0b013e31823a5596. [DOI] [PubMed] [Google Scholar]
- Fleisher L A, Beckman J A, Brown K A. et al. ACC/AHA 2007 guidelines on perioperative cardiovascular evaluation and care for noncardiac surgery. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). Circulation. 2007;116:1971–1996. doi: 10.1161/CIRCULATIONAHA.107.185700. [DOI] [PubMed] [Google Scholar]
- Vicenzi M N, Meislitzer T, Heitzinger B. et al. Coronary artery stenting and non-cardiac surgery-a prospective outcome study. Br J Anaesth. 2006;96:686–693. doi: 10.1093/bja/ael083. [DOI] [PubMed] [Google Scholar]
- Burger W, Chemnitius J M, Kneissl G D, Rucker G. Low-dose aspirin for secondary cardiovascular prevention - cardiovascular risks after its perioperative withdrawal versus bleeding risks with its continuation: review and meta-analysis. J Intern Med. 2005;257:399–414. doi: 10.1111/j.1365-2796.2005.01477.x. [DOI] [PubMed] [Google Scholar]
- Thurston A V, Briant S L. Aspirin and post-prostatectomy haemorrhage. Br J Urol. 1993;71:574–576. doi: 10.1111/j.1464-410x.1993.tb16027.x. [DOI] [PubMed] [Google Scholar]
- Ernst A, Eberhardt R, Wahidi M. et al. Effect of routine clopidogrel use on bleeding complications after transbronchial biopsy in humans. Chest. 2006; 129: 734-7. 2006;129:734–737. doi: 10.1378/chest.129.3.734. [DOI] [PubMed] [Google Scholar]
- Wiegand U K H, LeJeune D, Boguschewski F. Pocket hematoma after pacemaker or implantable cardioverter defibrillator surgery. Chest. 2004; 126: 1177-86. 2004;126:1177–1186. doi: 10.1378/chest.126.4.1177. [DOI] [PubMed] [Google Scholar]
- Payne D A, Hayes P D, Jones C I. et al. Combined therapy with clopidogrel and aspirin significantly increases the bleeding time through a synergistic antiplatelet action. J Vasc Surg. 2002;35:1204–1209. doi: 10.1067/mva.2002.122027. [DOI] [PubMed] [Google Scholar]
- Chassot P G, Delabays A, Spahn D R. Perioperative antiplatelet therapy; the case for continuing therapy in patients at risk for myocardial infarction. Br J Anaesth. 2007;99:316–328. doi: 10.1093/bja/aem209. [DOI] [PubMed] [Google Scholar]
- Moore M, Power M. Perioperative hemorrhage and combined clopidogrel and aspirin therapy. Anesthesissology. 2004;101:792–794. doi: 10.1097/00000542-200409000-00030. [DOI] [PubMed] [Google Scholar]
- Cuthill J A, Young S, Green I A, Oldroyd K. Anesthetic considerations in a parturient with critical coronary artery disease and a drug-eluting stent presenting for caesarian section. Int J ObstetAnesth. 2005;14:167–171. doi: 10.1016/j.ijoa.2004.10.010. [DOI] [PubMed] [Google Scholar]
- Davies B R. Combined aspirin and clopidogrel in cataract surgical patients: a new risk factor for ocular hemorrhage? Br J Ophthalmol. 2004;88:1226–1227. doi: 10.1136/bjo.2004.045997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S H, Fasseas P, Orford J L. et al. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am CollCardiol. 2003;42:234–240. doi: 10.1016/s0735-1097(03)00622-3. [DOI] [PubMed] [Google Scholar]
- Sharma A K. Major noncardiac surgery following coronary stenting. When is it safe to operate? Catheter CardiovascInterv. 2004; 63: 141-5. 2004;63:141–145. doi: 10.1002/ccd.20124. [DOI] [PubMed] [Google Scholar]
- Schouten O, van Domburg R T, Bax J J. et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am CollCardiol. 2007;49:122–124. doi: 10.1016/j.jacc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- de Souza D G, Baum V C, Ballert N M. Late thrombosis of a drug-eluting stent presenting in the perioperative period. Anesthesiology. 2007;106:1057–1059. doi: 10.1097/01.anes.0000265168.52501.c7. [DOI] [PubMed] [Google Scholar]
- Kaluza G L, Joseph J, Lee J R. et al. Catastrophic outcomes of non-cardiac surgery soon after coronary stenting. J Am CollCardiol. 2000;35:1288–1294. doi: 10.1016/s0735-1097(00)00521-0. [DOI] [PubMed] [Google Scholar]
- Collet J P, Montalescot G, Blanchet B. et al. Impact of prior use or recent withdrawal of oral antiplatelet agents on acute coronary syndromes. Circulation. 2004; 110: 2361-7. 2004;110:2361–2367. doi: 10.1161/01.CIR.0000145171.89690.B4. [DOI] [PubMed] [Google Scholar]
- Schouten O, van Domburg R T, Bax J J. et al. Noncardiac surgery after coronary stenting: early surgery and interruption of antiplatelet therapy are associated with an increase in major adverse cardiac events. J Am Coll Cardiol. 2007;49:122–124. doi: 10.1016/j.jacc.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Lo B, Fijnheer R, Castigliego D. et al. Activation of hemostasis after coronary artery bypass grafting with or without cardiopulmonary bypass. AnesthAnalg. 2004;99:634–640. doi: 10.1213/01.ANE.0000130257.64006.5C. [DOI] [PubMed] [Google Scholar]
- Khair T, Garcia B, Banerjee S, Brilakis E S. Contemporary approaches to perioperative management of coronary stents and to preoperative coronary revascularization: a survey of 374 interventional cardiologists. CardiovascRevascMed. 2011;12:99–104. doi: 10.1016/j.carrev.2009.09.007. [DOI] [PubMed] [Google Scholar]
- Patterson L, Hunter D, Mann A. Appropriate waiting time for noncardiac surgery following coronary stent insertion: views of Canadian anesthesiologists. Can J Anesthesiol. 2005;52:440–441. doi: 10.1007/BF03016295. [DOI] [PubMed] [Google Scholar]
- Mollman H, Nef H M, Hamm C W, Elsasser A. How to manage patients with need for antiplatelet therapy in the setting of (un-)planned surgery. Clin Res Cardiol. 2008;98:8–15. doi: 10.1007/s00392-008-0718-x. [DOI] [PubMed] [Google Scholar]
- Metzler H, Huber K, Kozek-Langenecker S. Anaesthesia in patients with drug-eluting stents. Curr Opin Anaesthesiol. 2008;21:55–59. doi: 10.1097/ACO.0b013e3282f357b6. [DOI] [PubMed] [Google Scholar]
- Abualsaud A O, Eisnberg M J. Perioperative management of patients with drug eluting stents. J Am CollCardiolIntv. 2010;3:131–142. doi: 10.1016/j.jcin.2009.11.017. [DOI] [PubMed] [Google Scholar]
- Newsome L T, Weller R S. Coronary artery stents: II. Perioperative considerations and management. AnesthAnalg. 2008;107:570–590. doi: 10.1213/ane.0b013e3181731e95. [DOI] [PubMed] [Google Scholar]
- Hall E, Mazer C D. Antiplatelet drugs: A review of their pharmacology and management in the perioperative period. AnesthAnalg. 2011;112:292–318. doi: 10.1213/ANE.0b013e318203f38d. [DOI] [PubMed] [Google Scholar]
- Brancati M F, Giammarinaro M, Burzotta F. et al. Outcome of non-cardiac surgery after stent implantation in the DES era: results of the surgery after stent (SAS) era. J Inv Card. 2011;23:44–49. [PubMed] [Google Scholar]
- Murphy J T, Fahy B G. Thrombosis of sirolimus-eluting coronary stent in the postanesthesia care unit. Anesth Analg. 2005;101:971–973. doi: 10.1213/01.ANE.0000175821.42010.9F. [DOI] [PubMed] [Google Scholar]
- McFadden E E P, Stabile E, Regar E. et al. Late thrombosis in drug-eluting coronary stents after discontinuation of antiplatelet therapy. Lancet. 2004;364:1519–1521. doi: 10.1016/S0140-6736(04)17275-9. [DOI] [PubMed] [Google Scholar]
- Newsome L T, Kutcher M A, Gandhi S K. et al. A protocol for the perioperative management of patients with intracoronary drug-eluting stents. APSF Newsletter. 2006-0;21:81–82. [Google Scholar]
- Savonitto S, Caracciolo M, Cattaneo M, DE Servi S. Management of patients with recently implantedcoronary stents on dual antiplatelet therapy who need to undergo major surgery. J Thromb Haemost. 2011;9:2133–2142. doi: 10.1111/j.1538-7836.2011.04456.x. [DOI] [PubMed] [Google Scholar]
- Angiolillo D J, Firstenberg M S, Price M J. et al. Bridging antiplatelet therapy with cangrelor in patients undergoing cardiac surgery. JAMA. 2012;307:265–274. doi: 10.1001/jama.2011.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massburg S, Byrne R A, Kastrati A A. et al. Polymer-free sirolimus- and probucol-eluting versus new generation zotarolimus-eluting stents in coronary artery disease: the Intracoronary Stenting and Angiographic Results: Test Efficacy of Sirolimus- and Probucol-Eluting versus Zotarolimus-eluting Stents (ISAR-TEST) trial. Circulation. 2011;124:624–632. doi: 10.1161/CIRCULATIONAHA.111.026732. [DOI] [PubMed] [Google Scholar]