Abstract
Repair- and recombination-defective mutations at two loci (mei-9 and mei-41) of Drosophila melanogaster have been examined for their effects on the induction of chromosome aberrations by x-rays and the formation of sister chromatid exchanges (SCEs). Irradiation of larval neuroblast cells during the S phase with x-rays showed that mutants at both of these loci are about 10 times more sensitive than wild type to the induction of chromosome aberrations. The pattern of induced aberrations was characteristic for each mutant locus: in cells bearing mei-9 mutations most breaks were chromatid deletions, whereas in the presence of mei-41 mutations similar frequencies of chromatid and isochromatid deletions were observed. Furthermore, chromatid interchanges could not be induced in cells carrying mei-9 alleles; therefore these mutations define a step necessary for chromatid rejoining. mei-41 alleles also define a function involved in the formation of chromatid interchanges; total exchanges were less frequent than expected from nonmutant controls; and the proportion of exchanges arising by symmetrical rejoining was markedly reduced. These data indicate that chromatid and isochromatid deletions have different molecular steps in their formation, and that different molecular mechanisms are also involved in the symmetrical and unsymmetrical rejoining in chromatid interchanges. Neuroblast cells of larvae bearing mei-9 and mei-41 alleles were also treated for 13 hr with 5-bromodeoxyuridine at 9 μg/ml in order to differentiate sister chromatids for the scoring of SCEs. Whereas mei-41 had a normal level of SCEs, mei-9 exhibited a frequency of SCEs that was about 70% that of the control. Because both mei-9 and mei-41 mutations result in defective meiotic recombination, these data suggest that they define steps shared by symmetrical interchange formation and meiotic recombination that do not participate in the formation of most SCEs.
Keywords: recombination-defective meiotic mutants, x-ray-induced aberrations in neuroblast cells
Full text
PDF
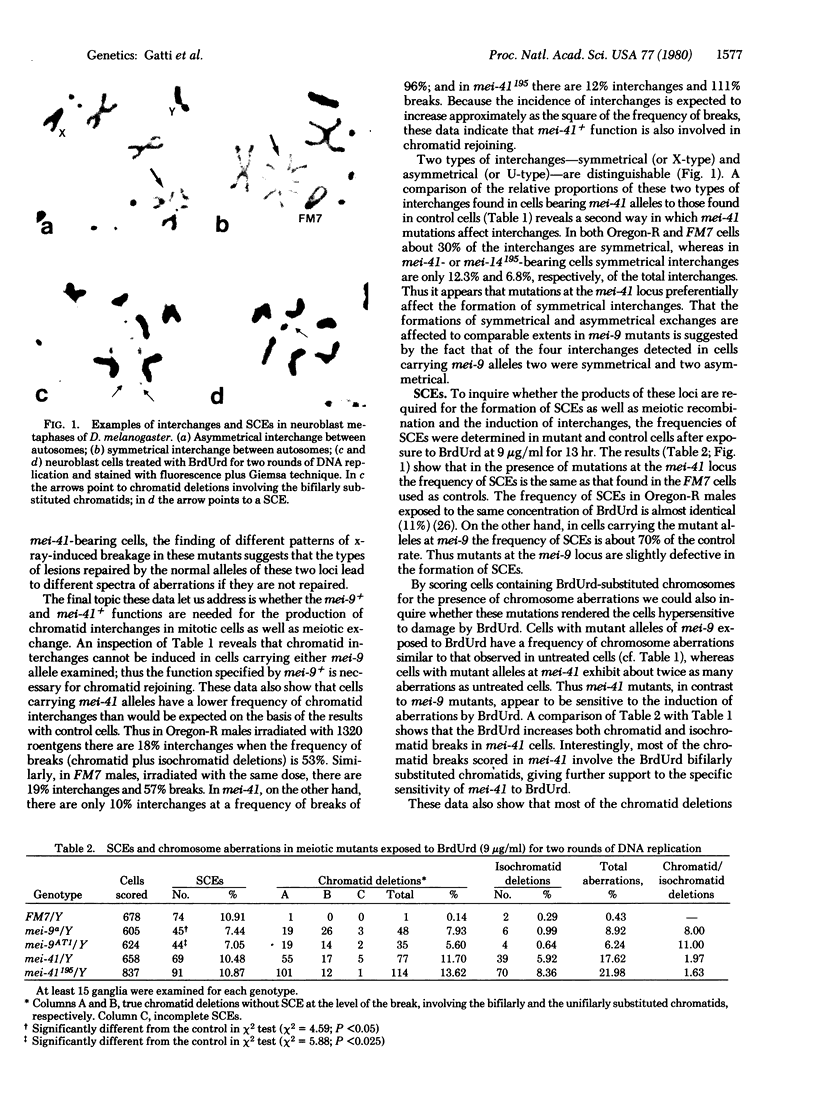


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker B. S., Boyd J. B., Carpenter A. T., Green M. M., Nguyen T. D., Ripoll P., Smith P. D. Genetic controls of meiotic recombination and somatic DNA metabolism in Drosophila melanogaster. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4140–4144. doi: 10.1073/pnas.73.11.4140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., Esposito M. S., Esposito R. E., Sandler L. The genetic control of meiosis. Annu Rev Genet. 1976;10:53–134. doi: 10.1146/annurev.ge.10.120176.000413. [DOI] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T. Genetic analysis of sex chromosomal meiotic mutants in Drosophilia melanogaster. Genetics. 1972 Jun;71(2):255–286. doi: 10.1093/genetics/71.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Carpenter A. T., Ripoll P. The Utilization during Mitotic Cell Division of Loci Controlling Meiotic Recombination and Disjunction in DROSOPHILA MELANOGASTER. Genetics. 1978 Nov;90(3):531–578. doi: 10.1093/genetics/90.3.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker B. S., Smith D. A. The effects of mutagen-sensitive mutants of Drosophila melanogaster in nonmutagenized cells. Genetics. 1979 Jul;92(3):833–847. doi: 10.1093/genetics/92.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Golino M. D., Nguyen T. D., Green M. M. Isolation and characterization of X-linked mutants of Drosophila melanogaster which are sensitive to mutagens. Genetics. 1976 Nov;84(3):485–506. doi: 10.1093/genetics/84.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Golino M. D., Setlow R. B. The mei-9 alpha mutant of Drosophila melanogaster increases mutagen sensitivity and decreases excision repair. Genetics. 1976 Nov;84(3):527–544. doi: 10.1093/genetics/84.3.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd J. B., Setlow R. B. Characterization of postreplication repair in mutagen-sensitive strains of Drosophila melanogaster. Genetics. 1976 Nov;84(3):507–526. doi: 10.1093/genetics/84.3.507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter A. T., Sandler L. On recombination-defective meiotic mutants in Drosophila melanogaster. Genetics. 1974 Mar;76(3):453–475. doi: 10.1093/genetics/76.3.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleaver J. E., Bootsma D. Xeroderma pigmentosum: biochemical and genetic characteristics. Annu Rev Genet. 1975;9:19–38. doi: 10.1146/annurev.ge.09.120175.000315. [DOI] [PubMed] [Google Scholar]
- Galloway S. M., Evans H. J. Sister chromatid exchange in human chromosomes from normal individuals and patients with ataxia telangiectasia. Cytogenet Cell Genet. 1975;15(1):17–29. doi: 10.1159/000130495. [DOI] [PubMed] [Google Scholar]
- Gatti M. Genetic control of chromosome breakage and rejoining in Drosophila melanogaster: spontaneous chromosome aberrations in X-linked mutants defective in DNA metabolism. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1377–1381. doi: 10.1073/pnas.76.3.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M., Pimpinelli S., De Marco A., Tanzarella C. Chemical induction of chromosome aberrations in somatic cells of Drosophila melanogaster. Mutat Res. 1975 Dec;33(2-3):201–212. doi: 10.1016/0027-5107(75)90196-7. [DOI] [PubMed] [Google Scholar]
- Gatti M., Santini G., Pimpinelli S., Olivieri G. Lack of spontaneous sister chromatid exchanges in somatic cells of Drosophila melanogaster. Genetics. 1979 Feb;91(2):255–274. doi: 10.1093/genetics/91.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatti M., Tanzarella C., Olivieri G. Analysis of the chromosome aberrations induced by x-rays in somatic cells of Drosophila melanogaster. Genetics. 1974 Aug;77(4):701–719. doi: 10.1093/genetics/77.4.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- German J., Crippa L. P., Bloom D. Bloom's syndrome. III. Analysis of the chromosome aberration characteristic of this disorder. Chromosoma. 1974;48(4):361–366. doi: 10.1007/BF00290993. [DOI] [PubMed] [Google Scholar]
- Kato H. Mechanisms for sister chromatid exchanges and their relation to the production of chromosomal aberrations. Chromosoma. 1977 Feb 3;59(3):179–191. doi: 10.1007/BF00292776. [DOI] [PubMed] [Google Scholar]
- Kato H. Spontaneous and induced sister chromatid exchanges as revealed by the BUdR-labeling method. Int Rev Cytol. 1977;49:55–97. doi: 10.1016/s0074-7696(08)61947-6. [DOI] [PubMed] [Google Scholar]
- Kato H., Stich H. F. Sister chromatid exchanges in ageing and repair-deficient human fibroblasts. Nature. 1976 Apr 1;260(5550):447–448. doi: 10.1038/260447a0. [DOI] [PubMed] [Google Scholar]
- Latt S. A., Stetten G., Juergens L. A., Buchanan G. R., Gerald P. S. Induction by alkylating agents of sister chromatid exchanges and chromatid breaks in Fanconi's anemia. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4066–4070. doi: 10.1073/pnas.72.10.4066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen T. D., Boyd J. B., Green M. M. Sensitivity of drosophila mutants to chemical carcinogens. Mutat Res. 1979 Nov;63(1):67–77. doi: 10.1016/0027-5107(79)90104-0. [DOI] [PubMed] [Google Scholar]
- Nguyen T. D., Green M. M., Boyd J. B. Isolation of two X-linked mutants in Drosophila melanogaster which are sensitive to gamma-rays. Mutat Res. 1978 Jan;49(1):139–143. doi: 10.1016/0027-5107(78)90086-6. [DOI] [PubMed] [Google Scholar]
- REVELL S. H. The accurate estimation of chromatid breakage, and its relevance to a new interpretation of chromatid aberrations induced by ionizing radiations. Proc R Soc Lond B Biol Sci. 1959 Sep 1;150:563–589. doi: 10.1098/rspb.1959.0043. [DOI] [PubMed] [Google Scholar]
- Schroeder T. M., German J. Bloom's syndrome and Fanconi's anemia: demonstration of two distinctive patterns of chromosome disruption and rearrangement. Humangenetik. 1974;25(4):299–306. doi: 10.1007/BF00336905. [DOI] [PubMed] [Google Scholar]
- Smith P. D. Mutagen sensitivity of Drosophila melanogaster. I. Isolation and preliminary characterization of a methyl methanesulphonate-sensitive strain. Mutat Res. 1973 Nov;20(2):215–220. doi: 10.1016/0027-5107(73)90191-7. [DOI] [PubMed] [Google Scholar]
- Smith P. D. Mutagen sensitivity of Drosophila melanogaster. III. X-linked loci governing sensitivity to methyl methanesulfonate. Mol Gen Genet. 1976 Nov 24;149(1):73–85. doi: 10.1007/BF00275962. [DOI] [PubMed] [Google Scholar]
- Wigglesworth D. J., Savage J. R. A comparison of the geometric configurations adopted by radiation-induced chromatid interchanges in animals and plants. Mutat Res. 1977 Jul;44(1):71–85. doi: 10.1016/0027-5107(77)90116-6. [DOI] [PubMed] [Google Scholar]
- Wolff S., Bodycote J. The induction of chromatid deletions in accord with the breakage-and-reunion hypothesis. Mutat Res. 1975 Jul;29(1):85–91. doi: 10.1016/0027-5107(75)90023-8. [DOI] [PubMed] [Google Scholar]
- Wolff S., Bodycote J., Thomas G. H., Cleaver J. E. Sister chromatid exchange in xeroderma pigmentosum cells that are defective in DNA excision repair or post-replication repair. Genetics. 1975 Oct;81(2):349–355. doi: 10.1093/genetics/81.2.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff S. Sister chromatid exchange. Annu Rev Genet. 1977;11:183–201. doi: 10.1146/annurev.ge.11.120177.001151. [DOI] [PubMed] [Google Scholar]