Abstract
Age-related memory loss is considered to commence at middle-age and coincides with reduced adult hippocampal neurogenesis and neurotrophin levels. Consistent physical activity at midlife may preserve brain-derived neurotrophic factor (BDNF) levels, new cell genesis and learning. In the present study, 9-month-old female C57Bl/6J mice were housed with or without a running wheel and injected with bromodeoxyuridine (BrdU) to label newborn cells. Morris water maze learning, open field activity and rotarod behavior were tested 1 and 6 months after exercise onset. Here we show that long-term running improved retention of spatial memory and modestly enhanced rotarod performance at 15 months of age. Both hippocampal neurogenesis and mature BDNF peptide levels were elevated after long-term running. Thus, regular exercise from the onset and during middle-age may maintain brain function.
Keywords: Neurogenesis, BDNF, Cognition, Locomotor Activity, Exercise
INTRODUCTION
Memory deteriorates during the course of normal aging. In humans, this process begins in early adulthood and progresses linearly from age 20 to age 80 (Park et al., 2002). From middle age onwards, age related cognitive, anatomical and physiological deficits begin to appear (Granger et al., 1996; Lynch et al., 2006). The hippocampus is particularly vulnerable in this regard. Rodent studies have shown reduced dendritic branching, spine density and decreased vascularization (Black et al., 1990; Geinismann et al., 1992; Sonntag et al., 1997; Driscoll et al., 2003; Mattson and Magnus, 2006). In addition, adult neurogenesis (Kuhn et al., 1996), brain-derived neurotrophin factor (BDNF) and trkB levels (Silhol et al., 2005) as well as BDNF-mediated synaptic plasticity (Rex et al., 2006) are reduced. Concomitantly, hippocampal-dependent memory function deteriorates with age (Erickson and Barnes, 2003). Moreover, age-related deficits in spatial navigation tasks, such as the Morris water maze (Morris et al., 1982) occur earlier in females than males (Markowska, 1999; Frick et al., 2000).
Accumulating evidence indicates that exercise improves hippocampal function, even in aged animals. Spatial learning is enhanced in old mice following voluntary wheel running (van Praag et al., 2005; Barrientos et al., 2011) or treadmill exercise (Albeck et al., 2006; Aquiar et al., 2011). Physical activity also increased dentate gyrus new cell number and neuronal differentiation though to a lesser extent than in young animals (van Praag et al., 2005; Kohman et al., 2011). In another study, voluntary exercise reversed age-related decline in cell proliferation but failed to reach the level of younger animals (Kronenberg et al., 2006). Exercise increases BDNF expression in the hippocampus (Cotman and Berchtold, 2002) and in the dentate gyrus in particular (Farmer et al., 2004) in young rodents. Interestingly, exercise in aging animals was not as effective at increasing BDNF levels as in young rodents (Adlard et al., 2005) or had no effect after 4 weeks of voluntary wheel running (Barrientos et al., 2011; see however Aquiar et al., 2011).
In the present study, we aimed to determine the effects of long-term running, commenced in adult (9-month-old) mice on adult hippocampal neurogenesis, BDNF levels, spatial learning and motor behavior. Here we show that consistent voluntary exercise maintains the retention of spatial memory (after 6 months of running), enhances hippocampal neurogenesis and increases mature BDNF peptide levels (following 8 months of exercise) upon middle-age. Motor behavior in the open field and rotarod showed trends towards improvements with running. Predominantly, exercise at the onset and throughout middle-age in female mice benefits cognition. These beneficial effects may be mediated at least in part by enhanced hippocampal neurogenesis and neurotrophin levels.
METHODS
Mice and General Experimental Procedures
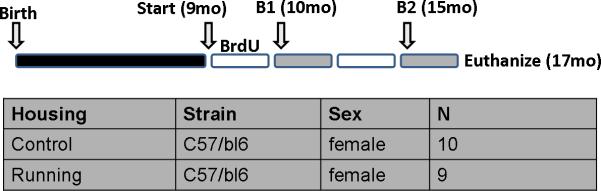
Female C57Bl/6J mice (8-weeks-old) were purchased from the Jackson Labs (Bar Harbor, ME). The mice were maintained on a standard NIH-07 diet (Harlan-Tekland, Indianapolis, IN) with free access to water during a 12-hour light/12-hour dark cycle. Animals were group housed until the start of the experiment at 9 months of age when mice where housed individually and randomly assigned to control (n=10) or running (Run, n=9) groups (Figure 1). Mice in the running group were housed with a running wheel and distance run was recorded daily (Clocklab, Coulborn Instruments, Whitehall, PA). In order to analyze the survival of newborn cells, bromodeoxyuridine (BrdU) (50 mg/kg) was injected i.p. for the first 10 days of individual housing. Mice were then subjected to 2 series of behavior testing at 10-months and 15-months of age. The weights of the mice were recorded at monthly intervals.
Figure 1.
Timeline of short and long-term running effects on behavior and neurogenesis. Animals were housed with a running wheel from 9 months of age. In order to analyze the survival of newborn cells, bromodeoxyuridine (BrdU) (50 mg/kg) was injected i.p. for the first 10 days of individual housing. After 1 month (B1) and 6 months (B2) an identical battery of behavioral testing was performed. Once the second testing session was complete, the animals were euthanized at 17 months of age. The table below shows the housing conditions, mouse strain used, sex and the number of mice per group at the beginning of the experiment.
Eight months after the start of the study animals were deeply anesthetized by isoflurane inhalation and perfused with 0.1M phosphate buffered saline. Animals were decapitated and brains were immediately removed. The right hemisphere was placed in 4% paraformaldehyde for 48 hrs, followed by equilibration in 30% sucrose. Tissue was sectioned coronally (40 μm) on a freezing microtome (Thermo-Fisher) and stored at -20° C in cryoprotectant solution. The left hemisphere was dissected and frozen on dry ice for western blot analysis of mature BDNF and later stored at -80°C. All animal procedures were done in accordance with the National Institute of Health Animal Care and Use Committee.
Morris Water Maze
Mice were trained in the Morris water maze (Morris et al., 1982) to find a platform hidden 5 mm below the surface of a pool (1.40-m diameter) filled with water made opaque with white nontoxic paint. Starting points were changed daily for each trial. A trial lasted either until the mouse had found the platform or for a maximum of 60 sec. Mice rested on the platform for 10 sec after each trial. If the mouse did not find the platform in 60 sec it was gently guided towards it and left there for 10 sec. Mice were trained with 4 trials per day over 6 days. Upon completion of training, the platform was removed for 60-sec probe trials at 4 hr (6 month time-point) and 24 hr (1 month and 6 month time-points) after the last training session. Latency to find the platform, swim speed and time spent in quadrants were recorded semi-automatically by a video tracking system (Anymaze, Stoelting Co.).
Open Field
Animals from the 2 groups were randomized and tested on 2 consecutive days in an open field arena (27.3 × 27.3 cm, height 20.3 cm) (Med Associates Inc., Georgia, VT). Animals were placed in the center of the arena at the beginning of the testing paradigm and were left undisturbed for 30 min. All testing occurred between 08:00 and 11:00 hrs on the day of testing. The center zone was defined as a 10.2 cm square equidistant from the peripheral walls. Each arena had a black floor and walls where xy movements were monitored by two sets of pulsed-modulated infrared photobeams. This tracking system recorded data directly and measured total distance traveled in the arena and in each zone (Med Associates Inc., Georgia, VT).
Rotarod
Five min trials on an accelerating paradigm from 3 to 30 rpm were used for testing on the rotarod. The mice were placed on the rotating rod and the latency to fall and the number of falls was recorded. This was repeated 3 times consecutively and the averages were used as measures of strength and locomotor ability.
Bromodeoxyuridine immunohistochemistry and cell counts
A 1:6 series of free-floating coronal sections (40μm) was washed in TBS and pre-incubated with 0.6% H2O2 for 30 min. After rinsing, the sections were incubated in 2N HCl at 37° C for 30 min to denature DNA and then neutralized in 0.1 M borate buffer for 10 min at room temperature. After thorough washing, the sections were blocked with TBS++ (3% Donkey Serum, 0.05 M TBS, 0.5% Triton-X 100) for 30 min at room temperature and incubated with rat anti-BrdU (1:200, Accurate Chemical Westbury NY) overnight at 4°C. Thereafter, the sections were washed and immersed in biotin-SP-conjugated donkey anti-rat secondary IgG (1:250, Jackson ImmunoResearch, West Grove, PA) followed by 2 hours in ABC reagent (1:800, Vectastain Elite; Vector Laboratories, Burlingame, CA). The sections were then incubated with the substrate 3, 3'-Diaminobenzidine (DAB) (Sigma, St. Louis, MO) for 5 min to visualize the cells that had incorporated BrdU. BrdU+ cells were counted in a 1:6 series of the first 7 sections starting at the rostral dentate gyrus (240 μm apart) through a 20X objective (Olympus, BX51).
The volume of the dentate gyrus for each group of animals was determined by DAPI staining a 1:6 series of sections and outlining the granular cell layer (GCL) and subgranular zone (SGZ) on a microscope equipped with Stereoinvestigator (Microbrightfield). Seven sections were outlined making boundary contour tracings to determine the area of the dentate gyrus at each level. Area values were used by the Cavalieri method to determine hippocampal volume.
Double immunofluorescence staining for cell fate analysis
Free floating sections (1:6 series) were simultaneously incubated with primary antibodies against BrdU (1: 100 Accurate Chemical Westbury NY) and the neuronal marker NeuN (1:100 Millipore, Billerica, MA) after the denaturation, neutralization, washing and blocking steps described above. Antibodies were diluted in TBS++ and then sections were incubated for 48 hr at 4° C. After rinses with TBS and blocking in TBS++, sections were co-incubated with donkey anti-rat Alexa Fluor 488 (1:250, Molecular Probes, Carlsbad, CA) and donkey anti-mouse Alexa Fluor 568 dyes (1:250, Jackson ImmunoResearch, West Grove, PA) for 2 hours at room temperature. Fluorescent signals were imaged with a Zeiss LSM 510 confocal laser-scanning microscope. Confocal and z-stacked images were used to determine the percentage of BrdU-positive cells co-labeling with NeuN. Based on the total number of BrdU-positive cells analyzed, the percentage of BrdU-positive cells with a neuronal phenotype was calculated. Based on this percentage, the total number of BrdU+/NeuN+ cell numbers could be calculated from the total number of BrdU+ cells.
Doublecortin immunohistochemistry
Migrating neuroblasts located within the GCL were identified by immunohistochemistry. A 1:12 series of sections were stained for the presence of microtubule-associated protein doublecortin (DCX) (1:800, Santa Cruz Biotechnology, Inc. ) for 1 hr at room temperature and overnight at 4° C. After washing thoroughly, sections were reacted with biotinylated donkey anti-goat secondary antibody (1:500, Jackson ImmunoResearch) with a tyramide amplification step. Chromogen development with DAB (20 mg/100 ml TB, 0.01% H2O2) was carried out for 20 min.
Quantification of DCX cells was carried out by manually counting and classifying each cell in the suprapyramidal and infrapyramidal blades of the hippocampus using an Olympus BH-2 at 400x total magnification.
BDNF Western Blot
To assay mature BDNF peptide levels, hippocampal tissue was homogenized in 400 μl of a 1 X RIPA buffer containing protease inhibitors (Complete Mini, Roche Diagnostics) using pestles and microtubes (ISC BioExpress) and then sonicated for 10 sec (Ultrasonic Processor, Model GE70). After incubation at room temperature for 10 min, the lyzed samples were centrifuged at room temperature for 10 min and the supernatants collected. The protein concentrations were then measured using the Bradford method (Bio-Rad). Following this, the lysates were reduced with 5X DTT at 70°C for 1 hr to break the strong disulfide bonds of BDNF. The samples were subsequently diluted to a final concentration of 3 μg/μl with the lysis buffer used above and a 4X LDS NuPAGE sample buffer (Invitrogen). Before electrophoresis, the samples were heated to 90°C for 5 min, rapidly cooled on ice for 1 min and then equilibrated to room temperature for 10 min. The samples were loaded onto a 4-12% NuPAGE Bis-Tris gel. The electrophoresis was carried out in 1 X MES buffer with added antioxidant and the proteins in the gel were transferred to an Immobilon-FL PVDF membrane (Millipore) using NuPAGE transfer buffer (Invitrogen). The polyclonal rabbit BDNF antibody (Santa Cruz Biotechnology, Inc.) and an infrared-labeled goat anti-rabbit secondary antibody (Li-Cor Biosciences) were used to detect BDNF on the membrane. The polyclonal rabbit β-tubulin antibody (Li-Cor Biosciences) was used a loading control. A positive control of human recombinant BDNF was ran alongside our samples. For quantification purposes, the membranes were scanned by a Li-Cor Odyssey Infrared Scanner and the intensities of the BDNF bands were normalized to those of β-tubulin.
Statistical Analysis
All statistical analyses were carried out using either Statview (Abacus Corporation) or GraphPad Prism. For Morris water maze latency, one way analysis of variance (ANOVA) with repeated measures was performed followed by Fisher's post-hoc tests for individual days. For the time in quadrants, a one-way ANOVA was performed on each group followed by Fisher's post-hoc tests. For two-group comparisons, unpaired student's t-tests were carried out.
RESULTS
Behavioral Testing, Running Distance and Weights
Animals underwent a battery of behavioral tasks to investigate the effects of long-term running during middle age. These tasks tested spatial learning and memory, anxiety, and motor abilities following short-term (1-month) and prolonged (6-months) exercise. The average distance run per day by the mice in this study was 4.0 ± 0.5 km. The weights of the mice [at the onset (26.6 + 0.9 gr, Controls; 26.4 ± 0.4 gr, Runners) and after 5 months of running (30.6 ±1.6 gr, Controls; 27.7 ±0.9 gr, Runners)] did not differ between groups at any time point during the experiment (F(1,16)=1.59, p=0.22).
Morris Water Maze
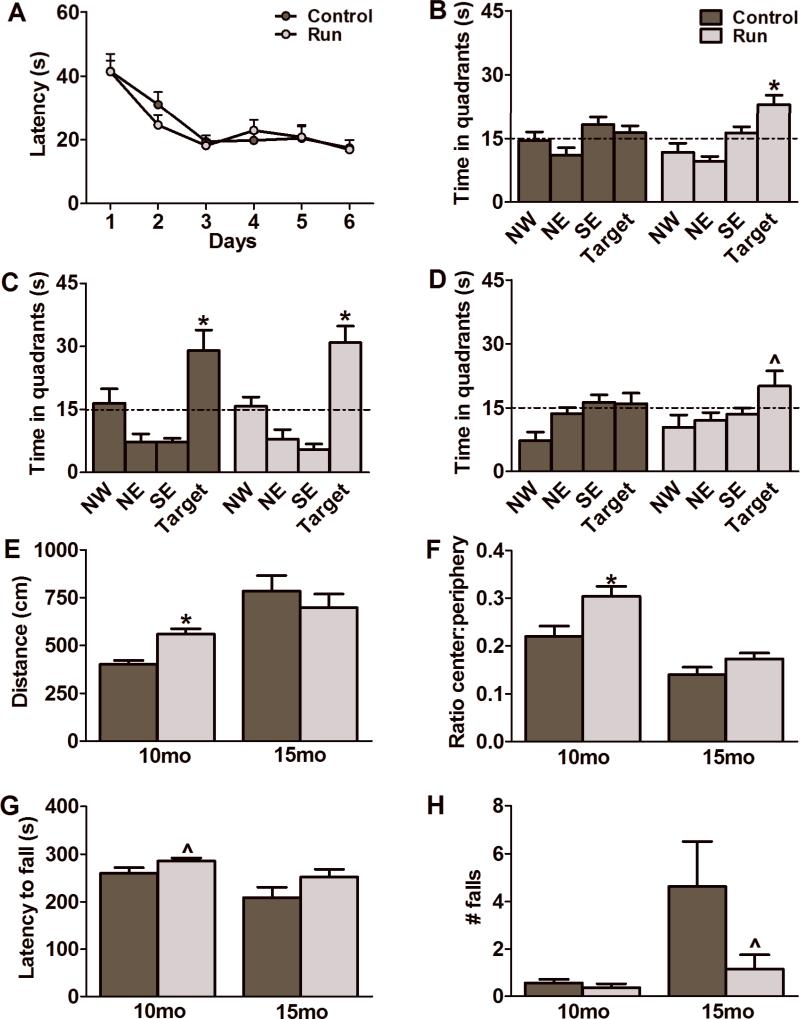
To assess spatial learning and memory, mice were tested in the Morris water maze. There were no observable differences between groups after 1 month of treatment (Fig. 2A). All animals in the study learned the task to criterion within 6 days (F(1,16)=0.05, p=0.83) with both groups remembering equally well the location of the platform in a probe test 24 hrs after the last training session (Controls: F(3,36)=10.72, p<0.0001; Runners: F(3,32)=19.57, p<0.0001; Fig. 2C). After 6 months of running, mice were trained to a different platform location. There was no differences between groups in acquisition of the task over 6 days of training (F(1,16)=0.71, p=0.41, data not shown). However, during the probe tests, only the running group showed a significant preference for the target quadrant at 4-hr after the last training session (Controls: F(3,32)=2.81, p=0.06; Runners: F(3,32)=10.87, p<0.0001; Fig. 2B). Specific comparisons showed that time spent in the platform quadrant by the runners differed from all the other quadrants (p<0.05). At the 24-hr probe test the runners showed a trend towards preference for the target quadrant (F(3,32)=2.74, p=0.06; Fig. 2D), whereas controls did not do so. No differences in swim speed or path length were detected between groups (data not shown).
Figure 2.
Effects of short and long-term running paradigms on cognitive, locomotor and anxiety-related behaviors. Morris water maze training (A) and a 24-hr probe trial (C), to test for retrieval of spatial memory, after 1 month of running showed no difference between the groups (N=9-10 per group). At 6 months after housing with a running wheel, the runners, unlike the controls, showed a clear preference for the target quadrants on the 4-hr (B) and the 24-hr (D) probe trials (N=9 per group). At the 1 month timepoint, runners displayed significant improvements on performance in the open field in terms of distance travelled (E) and a greater percentage of distance travelled in the center versus the peripheral area of the arena (F) compared to controls (N=9-10 per group) . These effects were no longer apparent after 6 months of running (E,F) (N=9 per group). Though not significantly different, the rotarod performance of the runners indicated strong trends towards an increase in the latency to fall (G) at 1 month (N=9-10 per group) and a decrease in the number of falls (H) at 6 months (N=9 per group) compared to controls. Data represents the mean ± SEM. (*p<0.05; ^ represents a strong trend). Dashed lines in B, C and D represent 15 seconds or one quarter of the time allotted for completion of the probe trial.
Open Field
The open field assay measures anxiety-related behaviors as well as motor capacity. In the open field, running animals showed increased distance travelled in the arena (t(10)=4.63: p=0.0009) as well as an increased ratio of distance travelled in the center to distance travelled in the periphery (t(10)=2.83: p=0.02) compared to controls after 1 month (Fig. 2E,F). This indicates that the runners were less anxious, travelling more in the center of the arena as opposed to the peripheral area. Interestingly, there was no significant difference between groups at 6 months for both of these parameters (Distance: t(10)=0.78: p=0.45; Ratio: t(10)=1.69: p=0.12; Fig. 2E,F).
Rotarod
Rotarod performance was assessed after 1 month and 6 months of housing with a running wheel to test for strength and motor abilities. Runners showed trends towards an increase in latency to fall at 1 month (t(17)=1.85: p=0.08; Fig. 2G) and a decrease in the number of falls at 6 months of age (t(16)=1.76: p=0.09; Fig. 2H). This finding, taken together with the open field data, suggests an improved locomotor ability after short and long-term running.
Long-term survival of BrdU labeled cells in the dentate gyrus
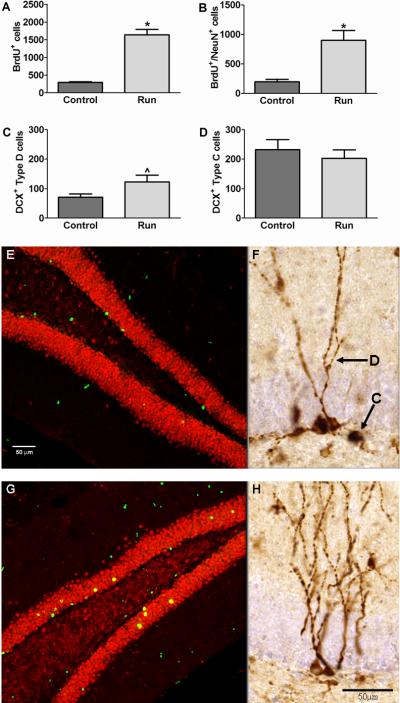
The thymidine analog BrdU was injected for the first 10 days after housing with a running wheel to label dividing neural progenitor cells. After 8 months of treatment, BrdU labeled cells were counted in the GCL to determine survival of these cells. A significant difference was found between the groups. Specifically running elevated the number of surviving BrdU+ cells (t(16)=8.65: p<0.0001; Fig. 3A). There was no difference between groups in the volume of the GCL of the dentate gyrus (Controls: 10576358±53376 μm2; Runners: 1156982±70911 μm2 ; t(16)=0.91: p=0.38).
Figure 3.
Consistent running results in increased hippocampal neurogenesis. The number of BrdU+ cells (A) as well as the total number of new neurons (B) was significantly increased in runners as compared to controls (N=5-9 per group) . The number of type D cells in the suprapyramidal layer of the dentate gyrus (C) in the runners was greater than controls but it did not reach significance (N=9 per group). There was no difference in type C cells in the suprapyramidal layer of the dentate gyrus between the groups (N=9 per group) (D). Examples of photomicrographs of BrdU+/NeuN+ labeling of newborn neurons in controls (E) and runners (G). Photomicrographs displaying doublecortin (DCX) expressing immature neurons in the dentate gyrus of control (F) and running (H) mice. Arrows and labels indicate the type D and type C cells analyzed in this study. Scale bars = 50μm. Data represents the mean ± SEM. (*p<0.05; ^ represents a strong trend).
Neurogenesis in the dentate gyrus
Double-labeling of cells for BrdU and the neuronal marker NeuN showed no difference in the percentage of cells labeled with both markers (Controls: 63.17±10.76 %; Runners: 58.00±5.59 %; t(9)=0.40: p=0.70). However, the total number of new neurons was higher in the running than the control group (t(9)=4.47: p=0.002; Fig. 3B). Representative images from each group are shown in Fig. 3 E and G.
Postmitotic hippocampal neuroblasts
Microtubule associated protein DCX is found in migratory neuroblasts in the hippocampus (Brown et al., 2003). It has been shown previously that DCX+ neuroblasts have multiple developmental stages and that postmitotic neurons can be identified by branching dendrites in the molecular layer. We distinguished morphologically different DCX+ cells based on previously identified stages of neuronal differentiation (Plümpe et al., 2006). Pre-mitotic DCX+ cells have shorter and less developed dendrites (type C). Mature post-mitotic DCX+ cells (type D) have at least 1 dendrite that extends into the GCL. To be classified as post-mitotic the dendrite must have 1 or more branch point at the intersection with the molecular layer or have a delicate dendritic tree branching within the GCL, as described previously (Oomen et al., 2010).
Type C & D cells were counted in the GCL. For D cells, a trend towards significance was observed. Runners had more type D cells in the suprapyramidal blade of the dentate gyrus compared to controls (t(16)=1.99: p=0.06; Fig. 3C). There was no significant difference in type D cells in the infrapyramidal blade (Controls: 72.00±8.25; Runners: 82.67±10.67; t(16)=0.79: p=0.44). The type C cells in the suprapyramidal blade (t(16)=0.65: p=0.52; Fig. 3D) or the infrapyramidal blade (Controls: 148.00±34.70; Runners: 182.70±17.18; t(16)=0.89: p=0.38) did not differ between the groups. Representative images from each group are shown in Figs. 3 F and H with arrows indicating examples of type C and D cells.
BDNF levels in the hippocampus
Our data indicate that mature BDNF peptide, as measured by western blot, was increased in the hippocampus of long-term runners as compared to controls (t(15)=3.36: p=0.004; Fig. 4A,B).
Figure 4.
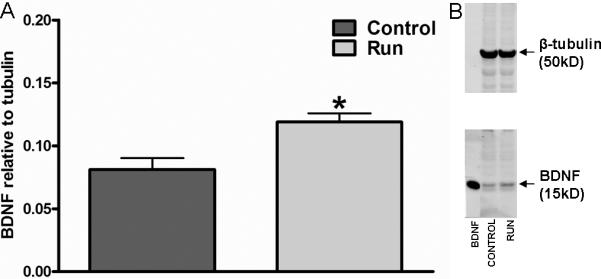
Long-term running leads to an increase in hippocampal levels of mature BDNF peptide. Runners have increased levels of mature BDNF peptide compared to controls after 8 months of running (A). Data were normalized to β-tubulin loading controls. (B) Image of immunoblot showing increased mature BDNF peptide levels in the runners. BDNF band is a positive control. Data represents the mean ± SEM. (*p<0.05).
DISCUSSION
The results from the present study show that consistent exercise starting at adult age (9 months-old) through middle age in female C57Bl/6 mice improves spatial memory (at 15 months-old), and increases adult hippocampal neurogenesis and mature BDNF peptide levels (at 17 months-old). In addition, modest enhancements of motor behavior in the open field and rotarod were observed. Our data are consistent with reported beneficial effects of shorter exercise protocols in young and aged animals and suggest that long-term running may protect against the onset of age-related memory decline.
Initial testing of the mice in the Morris water maze after one month of running at 10 months of age, the time-point at which some studies have suggested that memory deficits begin to appear in C57Bl/6J mice (Magnusson, 1997) did not reveal any differences between the sedentary and running mature adult mice in either acquisition or retention of the task. Both groups showed a clear preference for the target quadrant during the probe trial. At this age, the mice may not demonstrate memory impairments yet or the 4-trial per day training paradigm may not have been challenging enough. Previous research in young adult rodents applied a 2-trial per day training paradigm to demonstrate enhanced memory function in running mice (van Praag et al., 1999) and rats (Vaynman et al., 2004). Upon re-testing in the water maze, when mice were middle-aged (15-months-old), sedentary and running mice learned the task at equal rates, consistent with other research in middle-aged female C57Bl/6J mice (Frick et al., 2000). However, only the runners showed a significant retention of the platform location after completion of training. Thus, onset of memory deficits at middle-age may be prevented or delayed by running.
Upon testing in the open field, runners differed from controls with regard to total and center distance traveled at the first behavioral testing time-point. Runners traveled a greater distance in the arena and in the center compared to the periphery. Running is generally considered to have anti-depressant effects (Salmon et al., 2001) and the increased center distance suggests it is also anxiolytic (Salam et al., 2009). Upon re-testing at 15 months there was no longer a difference between control and running mice, likely due to the increased activity in the sedentary group, which is consistent with an increase in open field activity observed in 17-month-old C57Bl/6J mice by other researchers (Frick et al., 2000). A trend towards improved performance in the rotarod was also observed at both time-points. In particular, at the 15 month time-point the runners showed a trend towards less falls, suggesting that exercise may help prevent or delay the onset of motor frailty with aging. This finding could not be accounted for by differences in weights of the mice. These results support benefits of exercise in aged mice for motor function (Valdez et al., 2009).
To begin to evaluate potential mechanisms underlying the observed behavioral improvements as a result of exercise we evaluated both hippocampal neurogenesis and neurotrophin levels. Increasing evidence suggests that adult neurogenesis (Altman et al., 1965; Zhao et al., 2008; Lucassen et al., 2010) plays an important role in learning and memory (Aimone et al., 2011). Adult neurogenesis declines with aging (Kuhn et al., 1996; Heine et al., 2004; Olariu et al., 2007) and can be reversed to some extent with voluntary wheel running (van Praag et al., 2005; Kronenberg et al., 2006; Kohman et al., 2011), possibly due to peripheral factors delivered to the brain with running (Fabel et al., 2003; Kobilo et al., 2011; Villeda et al., 2011). Interestingly, BrdU labeling in 9-month-old adult animals revealed that the new neurons generated survived until 17 months, supporting previous research pertaining to long-term survival of new neurons (Kempermann et al., 2003), and were enhanced in number by exercise. Furthermore, runners showed a trend towards increased DCX+ ‘type D’ cells (Plümpe et al., 2006) in the suprapyramidal blade of the dentate gyrus. More DCX+ cells have been found in the suprapyramidal blade than in the infrapyramidal blade in sedentary young adult (2-month-old) but not in middle-aged (10-month-old) mice (Jinno, 2010). Functional activation of the adult generated neurons as measured by immediate early gene expression is more pronounced in the supra- as compared to the infrapyramidal blade (Ramirez-Amaya et al., 2006). Thus, benefits of running for learning and memory may be influenced by regional neurogenic enhancements. One possible caveat to our study is the fact that the experiments were performed on cycling females. Considering the prolonged exercise paradigm during the onset of mouse menopause between 13-16 months of age (Nelson et al., 1982), and the reported effects of estrous on dentate gyrus cell proliferation, but not new cell survival (Tanapat et al., 1999), this is likely not a factor in our results.
Mature BDNF peptide levels were found to be elevated after 8 months of wheel running, suggesting that the duration of running is important. These findings extend research in young rodents showing that exercise enhances neurotrophin gene expression and protein levels, in particular in the hippocampus (Neeper et al., 1995; Cotman and Berchtold 2002). Previous studies with shorter periods of exercise (one month) had suggested that running was not effective at increasing BDNF levels in aging animals (Adlard et al., 2005; Barrientos et al., 2011; see however Aquiar et al., 2011). BDNF is a key trophic factor that plays critical roles in both synaptic plasticity and cell survival (Black, 1999). Exercise-induced elevated BDNF levels may enhance hippocampal synaptic function (Vaynman et al., 2004) and support the survival of newly born neurons (Farmer et al., 2004; Li et al., 2008; Choi et al., 2009). Indeed, recent research showed that BDNF plays a role in cognitive effects of exercise in humans and is associated with increased hippocampal volume (Erickson et al., 2011). Further research may be needed to determine the effects of running in both young and aged animals on pro-BDNF levels as well as other neurotrophins relevant to adult neurogenesis and synaptic plasticity, such as NT-3 (Shimazu et al., 2006).
In conclusion, long-term voluntary exercise during middle-age improves spatial memory, enhances hippocampal neurogenesis and increases mature BDNF peptide levels. In the open field a transient anxiolytic effect was observed and rotarod performance showed trends towards improvements with running. Thus, exercise starting at the onset and continued throughout middle-age benefits cognition, mood and motor coordination in female mice.
ACKNOWLEDGMENTS
This research was supported in part by the Intramural Research Program, National Institute on Aging, NIH. We thank Kriti Gandhi and Zejun Wang for technical assistance. We thank Dr. Qing-Rong Liu and Dr. Yavin Shaham for assistance and advice on western blot BDNF analysis, and Dr. Marian Joels (RMI Utrecht, The Netherlands) for helpful discussions. MM and PJL are supported by the EU (NEURAD), HSN, NWO and ISAO.
REFERENCES
- Adlard PA, Perreau VM, Cotman CW. The exercise-induced expression of BDNF within the hippocampus varies across life-span. Neurobiol Aging. 2005;26:511–520. doi: 10.1016/j.neurobiolaging.2004.05.006. [DOI] [PubMed] [Google Scholar]
- Aimone JB, Deng W, Gage FH. Resolving new memories: a critical look at the dentate gyrus, adult neurogenesis, and pattern separation. Neuron. 2011;70:589–596. doi: 10.1016/j.neuron.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albeck DS, Sano K, Prewitt GE, Dalton L. Mild forced treadmill exercise enhances spatial learning in the aged rat. Behav Brain Res. 2006;168:345–348. doi: 10.1016/j.bbr.2005.11.008. [DOI] [PubMed] [Google Scholar]
- Altman J, Das GD. Autoradiographic and histology evidence of postnatal hippocampal neurogenesis in rats. J Comp Neurol. 1965;124:319–335. doi: 10.1002/cne.901240303. [DOI] [PubMed] [Google Scholar]
- Aquiar AS, Jr, Castro AA, Moreira EL, Glaser V, Santos AR, Tasca CI, Latini A, Prediger RD. Short bouts of mild-intensity physical exercise improve spatial learning and memory in aging rats: Involvement of hippocampal plasticity via AKT, CREB and BDNF signaling. Mech Ageing Dev. 2011 doi: 10.1016/j.mad.2011.09.005. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Crysdale NY, Chapman TR, Ahrendsen JT, Day HE, Campeau S, Watkins LR, Patterson SL, Maier SF. Little exercise, big effects: reversing aging and infection-induced memory deficits, and underlying processes. J Neurosci. 2011;31:11578–11586. doi: 10.1523/JNEUROSCI.2266-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black IB. Trophic regulation of synaptic plasticity. J Neurobiol. 1999;41:108–118. [PubMed] [Google Scholar]
- Black JE, Isaacs KR, Anderson BJ, Alcantara AA, Greenough WT. Learning causes synaptogenesis, whereas motor activity causes angiogenesis, in cerebellar cortex of adult rats. Proc Natl Acad Sci USA. 1990;87:5568–5572. doi: 10.1073/pnas.87.14.5568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Choi SH, Li Y, Parada LF, Sisodia SS. Regulation of hippocampal progenitor cell survival, proliferation and dendritic development by BDNF. Mol Neurodegener. 2009;4:52. doi: 10.1186/1750-1326-4-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotman CW, Berchtold NC. Exercise: a behavioral intervention to enhance brain health and plasticity. Trends Neurosci. 2002;25:295–301. doi: 10.1016/s0166-2236(02)02143-4. [DOI] [PubMed] [Google Scholar]
- Driscoll I, Hamilton DA, Petropoulos H, Yeo RA, Brooks WM, Baumgartner RN, Sutherland RJ. The aging hippocampus: cognitive, biochemical and structural findings. Cereb Cortex. 2003;13:1344–1351. doi: 10.1093/cercor/bhg081. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Erickson KI, Voss MW, Prakash RS, Basak C, Szabo A, Chaddock L, Kim JS, Heo S, Alves H, White SM, Wojcicki TR, Mailey E, Vieira VJ, Martin SA, Pence BD, Woods JA, McAuley E, Kramer AF. Exercise training increases size of hippocampus and improves memory. Proc Natl Acad Sci U S A. 2011;108:3017–3022. doi: 10.1073/pnas.1015950108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabel K, Fabel K, Tam B, Kaufer D, Baiker A, Simmons N, Kuo CJ, Palmer TD. VEGF is necessary for exercise-induced adult hippocampal neurogenesis. Eur J Neurosci. 2003;18:2803–2812. doi: 10.1111/j.1460-9568.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- Farmer J, Zhao X, van Praag H, Wodtke K, Gage FH, Christie BR. Effects of voluntary exercise on synaptic plasticity and gene expression in the dentate gyrus of adult male Sprague-Dawley rats in vivo. Neuroscience. 2004;124:71–79. doi: 10.1016/j.neuroscience.2003.09.029. [DOI] [PubMed] [Google Scholar]
- Frick KM, Burlingame LA, Arters JA, Berger-Sweeney J. Reference memory, anxiety and estrous cyclicity in C57BL/6NIA mice are affected by age and sex. Neuroscience. 2000;95:293–307. doi: 10.1016/s0306-4522(99)00418-2. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Granger R, Deadwyler S, Davis M, Moskovitz B, Kessler M, Rogers G, Lynch G. Facilitation of glutamate receptors reverses an age-associated memory impairment in rats. Synapse. 1996;22:332–337. doi: 10.1002/(SICI)1098-2396(199604)22:4<332::AID-SYN4>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- Heine VM, Maslam S, Joëls M, Lucassen PJ. Prominent decline of newborn cell proliferation, differentiation, and apoptosis in the aging dentate gyrus, in absence of an age-related hypothalamus-pituitary-adrenal axis activation. Neurobiol Aging. 2004;25:361–375. doi: 10.1016/S0197-4580(03)00090-3. [DOI] [PubMed] [Google Scholar]
- Jinno S. Decline in adult neurogenesis during aging follows a topographic pattern in the mouse hippocampus. J Comp Neurol. 2011;519:451–66. doi: 10.1002/cne.22527. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Gast D, Kronenberg G, Yamaguchi M, Gage FH. Early determination and long-term persistence of adult-generated new neurons in the hippocampus of mice. Development. 2003;130:391–399. doi: 10.1242/dev.00203. [DOI] [PubMed] [Google Scholar]
- Kobilo T, Yuan C, van Praag H. Endurance factors improve hippocampal neurogenesis and spatial memory in mice. Learn Mem. 2011;18:103–107. doi: 10.1101/lm.2001611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohman RA, Deyoung EK, Bhattacharya TK, Peterson LN, Rhodes JS. Wheel running attenuates microglia proliferation and increases expression of a proneurogenic phenotype in the hippocampus of aged mice. Brain Behav Immun. 2011 doi: 10.1016/j.bbi.2011.10.006. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg G, Bick-Sander A, Bunk E, Wolf C, Ehninger D, Kempermann G. Physical exercise prevents age-related decline in precursor cell activity in the mouse dentate gyrus. Neurobiol Aging. 2006;27:1505–1513. doi: 10.1016/j.neurobiolaging.2005.09.016. [DOI] [PubMed] [Google Scholar]
- Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Luikart BW, Birnbaum S, Chen J, Kwon CH, Kernie SG, Bassel-Duby R, Parada LF. TrkB regulates hippocampal neurogenesis and governs sensitivity to antidepressive treatment. Neuron. 2008;59:399–412. doi: 10.1016/j.neuron.2008.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucassen PJ, Meerlo P, Naylor AS, van Dam AM, Dayer AG, Fuchs E, Oomen CA, Czéh B. Regulation of adult neurogenesis by stress, sleep disruption, exercise and inflammation: Implications for depression and antidepressant action. Eur Neuropsychopharmacol. 2010;20:1–17. doi: 10.1016/j.euroneuro.2009.08.003. [DOI] [PubMed] [Google Scholar]
- Lynch G, Rex CS, Gall CM. Synaptic plasticity in early aging. Ageing Res Rev. 2006;5:255–280. doi: 10.1016/j.arr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Magnusson KR. Influence of dietary restriction on ionotropic glutamate receptors during aging in C57B1 mice. Mech Ageing Dev. 1997;95:187–202. doi: 10.1016/s0047-6374(97)01884-8. [DOI] [PubMed] [Google Scholar]
- Markowska AL. Sex dimorphisms in the rate of age-related decline in spatial memory: relevance to alterations in the estrous cycle. J Neurosci. 1999;19:8122–8133. doi: 10.1523/JNEUROSCI.19-18-08122.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Magnus T. Ageing and neuronal vulnerability. Nat Rev Neurosci. 2006;7:278–294. doi: 10.1038/nrn1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris RG, Garrud P, Rawlins JN, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Neeper SA, Gómez-Pinilla F, Choi J, Cotman C. Exercise and brain neurotrophins. Nature. 1995;373:109. doi: 10.1038/373109a0. [DOI] [PubMed] [Google Scholar]
- Nelson JF, Felicio LS, Randall PK, Sims C, Finch CE. A longitudinal study of estrous cyclicity in aging C57BL/6J mice: I. Cycle frequency, length and vaginal cytology. Biol Reprod. 1982;27:327–339. doi: 10.1095/biolreprod27.2.327. [DOI] [PubMed] [Google Scholar]
- Olariu A, Cleaver KM, Cameron HA. Decreased neurogenesis in aged rats results from loss of granule cell precursors without lengthening of the cell cycle. J Comp Neurol. 2007;501:659–667. doi: 10.1002/cne.21268. [DOI] [PubMed] [Google Scholar]
- Oomen CA, Soeters H, Audureau N, Vermunt L, van Hasselt FN, Manders EM, Joëls M, Lucassen PJ, Krugers H. Severe early life stress hampers spatial learning and neurogenesis, but improves hippocampal synaptic plasticity and emotional learning under high-stress conditions in adulthood. J Neurosci. 2010;30:6635–6645. doi: 10.1523/JNEUROSCI.0247-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Lautenschlager G, Hedden T, Davidson NS, Smith AD, Smith PK. Models of visuospatial and verbal memory across the adult life span. Psychol. Aging. 2002;17:299–320. [PubMed] [Google Scholar]
- Plümpe T, Ehninger D, Steiner B, Klempin F, Jessberger S, Brandt M, Römer B, Rodriguez GR, Kronenberg G, Kempermann G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006;7:77. doi: 10.1186/1471-2202-7-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rex CS, Lauterborn JC, Lin CY, Kramár EA, Rogers GA, Gall CM, Lynch G. Restoration of long-term potentiation in middle-aged hippocampus after induction of brain-derived neurotrophic factor. J Neurophysiol. 2006;96:677–685. doi: 10.1152/jn.00336.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salam JN, Fox JH, Detroy EM, Guignon MH, Wohl DF, Falls WA. Voluntary exercise in C57 mice is anxiolytic across several measures of anxiety. Behav Brain Res. 2009;197:31–40. doi: 10.1016/j.bbr.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Salmon P. Effects of physical exercise on anxiety, depression, and sensitivity to stress: a unifying theory. Clin Psychol Rev. 2001;21:33–61. doi: 10.1016/s0272-7358(99)00032-x. [DOI] [PubMed] [Google Scholar]
- Shimazu K, Zhao M, Sakata K, Akbarian S, Bates B, Jaenisch R, Lu B. NT-3 facilitates hippocampal plasticity and learning and memory by regulating neurogenesis. Learn Mem. 2006;13:307–315. doi: 10.1101/lm.76006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silhol M, Bonnichon V, Rage F, Tapia-Arancibia L. Age-related changes in brain-derived neurotrophic factor and tyrosine kinase receptor isoforms in the hippocampus and hypothalamus in male rats. Neuroscience. 2005;132:613–624. doi: 10.1016/j.neuroscience.2005.01.008. [DOI] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Tanapat P, Hastings NB, Reeves AJ, Gould E. Estrogen stimulates a transient increase in the number of new neurons in the dentate gyrus of the adult female rat. J Neurosci. 1999;19:5792–5801. doi: 10.1523/JNEUROSCI.19-14-05792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valdez G, Tapia JC, Kang H, Clemenson GD, Jr, Gage FH, Lichtman JW, Sanes JR. Attenuation of age-related changes in mouse neuromuscular synapses by caloric restriction and exercise. Proc Natl Acad Sci U S A. 2010;107:14863–14868. doi: 10.1073/pnas.1002220107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Christie BR, Sejnowski TJ, Gage FH. Running enhances neurogenesis, learning, and long-term potentiation in mice. Proc Natl Acad Sci USA. 1999;96:13427–13431. doi: 10.1073/pnas.96.23.13427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaynman S, Ying Z, Gomez-Pinilla F. Hippocampal BDNF mediates the efficacy of exercise on synaptic plasticity and cognition. Eur J Neurosci. 2004;20:2580–2590. doi: 10.1111/j.1460-9568.2004.03720.x. [DOI] [PubMed] [Google Scholar]
- Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park JS, Couillard-Després S, Aigner L, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T. The ageing systemic milieu negatively regulates neurogenesis and cognitive function. Nature. 2011;477:90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao C, Deng W, Gage FH. Mechanisms and functional implications of adult neurogenesis. Cell. 2008;132:645–660. doi: 10.1016/j.cell.2008.01.033. [DOI] [PubMed] [Google Scholar]