Abstract

Assays armed with catalytic signal amplification have arisen as superior systems for ultrasensitive detection of analytes. Here we describe a conceptually new enzyme assay based on cat-ELISA, catalytic assay using enzyme-linked click chemistry assay (cat-ELCCA), where an enzyme-linked azide is utilized to arm the assay with catalytic fluorescence signal amplification. Using this assay technology, we have developed the first potentially high-throughput screen for the recently disclosed acyltransferase, ghrelin O-acyltransferase (GOAT).
Keywords: alkynes, azides, enzymes, peptides
Assays armed with catalytic signal amplification have arisen as superior systems for ultrasensitive detection of analytes.[1,2] In the biocatalysis field, while fluorogenic and colorimetric enzyme assays designed to examine cleavage reactions have taken advantage of this strategy,[3] many important enzyme classes, including acyltransferases, have mechanistically been excluded from such analytical techniques. As a result, the visualization of activity for these enzymes (i.e. addition of a post-translational modification) typically relies on autoradiographic methods, which are often not high-throughput and are hazardous, precluding library-based screening against these targets.[4] For example, in the acyltransferase field, [3H]-labeled fatty acids have traditionally been utilized to detect protein fatty acid acylation using liquid scintillation counting, although exceptions have been reported.[5] Despite these efforts, high-throughput, fluorescence-based assays still remain highly sought after in this field.
As an alternative approach, several groups have recently reported the use of click chemistry-based approaches utilizing azido-and alkynyl-tagged fatty acids for the nonradioactive detection of protein fatty-acylation in living cells.[6] In specific, the use of CuI-catalyzed Huisgen [3+2] cycloaddition chemistry[7] has proved to be a robust detection method for such efforts. Interestingly, while click chemistry has found widespread success in activity-labeling approaches such as those described above, bioconjugation efforts[8] and enzyme inhibitor discovery,[9] it has yet to be utilized in the development of high-throughput screening assays, despite its biological and chemical compatibility and near quantitative yields.[7c] Herein, we describe our efforts toward the development of a click chemistry-based, high-throughput screen to monitor acyltransferase activity.
Inspired by enzyme immunoassays,[10] in particular cat-ELISA (catalytic assay using ELISA),[11] we sought to design an acyltransferase assay that takes advantage of the catalytic signal amplification obtained with enzyme-linked detection antibodies. In cat-ELISA (Figure 1A), a solid-supported substrate is first treated with a catalytic antibody (or enzyme) to obtain a product, which is subsequently bound with a product-specific antibody. This antibody is then detected with an enzyme-linked secondary antibody, the enzyme of which catalyzes the turnover of a fluorescent or colorimetric substrate in solution to provide signal amplification necessary for sensitive quantitation.
Figure 1.

Comparison of A) cat-ELISA and B) catalytic assay using enzyme-linked click chemistry assay (cat-ELCCA). E = enzyme. S = substrate.
Taking a similar approach, we proposed to immobilize an acyltransferase-recognized, biotinylated peptide fragment to a streptavidin microtiter plate. This immobilized peptide would then be incubated with an alkynyl-tagged fatty acid in the presence of the enzyme (Figure 1B). Following this enzyme-catalyzed acyl transfer, the tagged fatty acid ester would then be converted to the enzyme-linked 1,2,3-triazole using an azido-tagged enzyme via CuI-catalyzed Huisgen [3+2] cycloaddition chemistry.[12] Subsequent addition of a fluorogenic substrate of the linked enzyme would provide catalytic signal amplification. To keep with the terminology of cat-ELISA, we have named this assay technology catalytic assay using enzyme-linked click chemistry assay (cat-ELCCA).
As a model enzyme to demonstrate this technology, we chose to examine the recently disclosed acyltransferase, ghrelin-O-acyltransferase (GOAT).[13] This membrane-bound O-acyltransferase (MBOAT)[14] family member catalyzes the n-octanoylation of ghrelin (Figure 2), a 28-amino acid growth hormone-releasing peptide implicated in feeding, weight gain and the regulation of energy homeostasis, and a leading anti-obesity drug target.[15] Importantly, the functions of this peptide hormone are solely dependent on its n-octanoylation at Ser-3 by GOAT.[16] Of further significance, ghrelin is the only known peptide to contain an n-octanoyl post-translational modification,[16] thus, this peptide is most likely the only substrate for GOAT and inhibitors of this enzyme should be highly specific. As our group has previously examined a vaccine-based approach for sequestering[17] and/or catalyzing the hydrolysis[18] of ghrelin using monoclonal antibodies as anti-obesity strategies, we were also interested in establishing a small molecule-based program targeting GOAT since currently no validated chemical probes for this enzyme have been reported.[19a]
Figure 2.
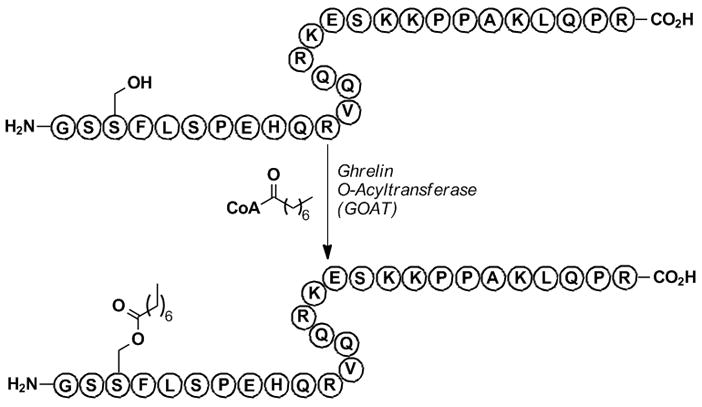
GOAT-catalyzed n-octyanoyl transfer to ghrelin.
To date, only two in vitro assay systems have been reported for screening GOAT activity.[19] Similar to other acyltransferase assays, both rely on autoradiographic methods and neither is amenable to high-throughput screening. Since we envisioned establishing a library-based screening effort against GOAT and the current screening assays impede such efforts, we wanted to use our cat-ELCCA approach to design a robust assay for GOAT. Our assay design is shown in Figure 3. In brief, a biotinylated ghrelin peptide immobilized on a streptavidin-modified microtiter plate would be incubated with GOAT and alkynyl-tagged n-octynoyl-CoA for the acyl transfer reaction; the wells would then be subjected to click chemistry conditions. For the azide-labeled enzyme, we chose horseradish peroxidase (HRP), as activated HRP derivatives for bioconjugation and fluorescent/colorimetric substrates for this enzyme are commercially available. Following the click reaction, the HRP-triazole product would be detected using amplex red as a fluorogenic substrate in the presence of hydrogen peroxide to provide catalytic fluorescence signal amplification of the octynoyl ester-HRP triazole product concentration.
Figure 3.
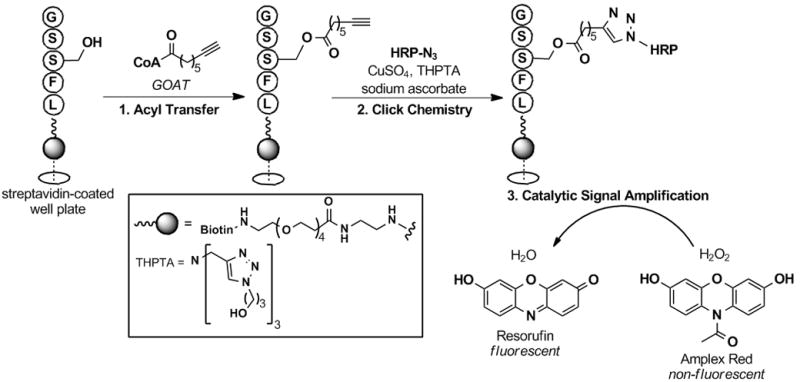
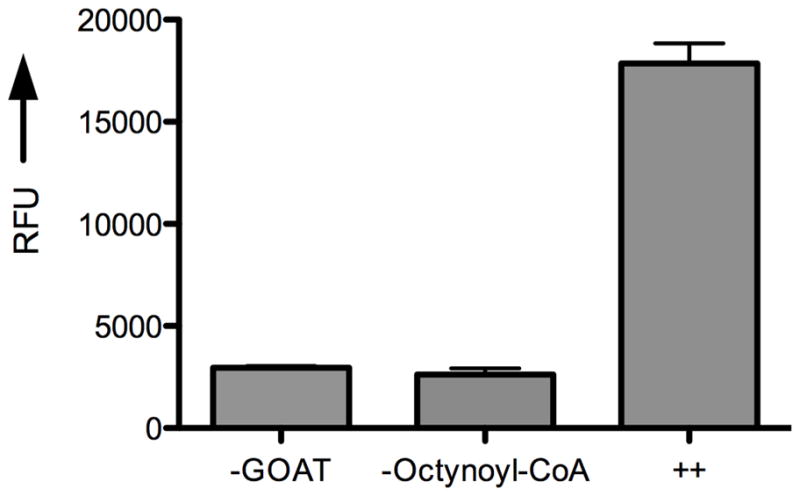
cat-ELCCA for screening GOAT activity. A) Assay design. B) Proof-of-concept data. ++ = Sample treated with both GOAT-containing membranes and n-octynoyl-CoA. RFU = normalized relative fluorescence units at 585 nm.
To test the applicability of cat-ELCCA to the GOAT problem, we first isolated the GOAT enzyme. As previously described,[19a] Sf9 insect cells were infected with a recombinant baculovirus encoding mouse GOAT for protein overexpression. Since GOAT is a transmembrane protein and efforts to purify this enzyme to homogeneity have previously failed,[19a] the crude GOAT-containing membrane fraction was isolated by ultracentrifugation techniques, and the resultant membrane fraction was used as such for all subsequent assays. The acyltransferase activity of the isolated membrane-bound GOAT was confirmed with a control reaction using ghrelin(1–28) and n-octanoyl-CoA. Based on known HPLC traces of n-octanoyl-ghrelin(1–28) and the unacylated peptide, the activity of GOAT in this crude extract was confirmed (data not shown). Additionally, no octanoyl transfer was observed in uninfected Sf9 cell membranes (data not shown).
Next, we turned our attention toward the preparation of suitable ghrelin and azido-HRP substrates. For the immobilized ghrelin substrate, we chose a ghrelin(1–5) pentapeptide containing a C-terminal biotin, which was synthesized using solid phase peptide synthesis (see Supporting Information). Ghrelin(1–5) was chosen as this has been shown to be the minimum recognition sequence for both GOAT activity[19a] and ghrelin’s receptor, growth hormone secretagogue receptor 1a (GHS-R1a),[20] and is synthetically tractable for high-throughput efforts. As anticipated, this ghrelin substrate was found to be recognized by GOAT (HPLC data not shown). To prepare the azido-tagged HRP, we used an aldehyde-functionalized peroxidase as an amine-reactive HRP, and this activated HRP was treated with 11-azido-3,6,9-trioxaundecan-1-amine to obtain azido-tagged HRP (see Supporting Information).
With all of the assay components in hand, we then set out to test our assay design hypothesis. The biotinylated ghrelin(1–5)pentapeptide was first immobilized to the wells of a streptavidin-coated microtiter plate (96-well, black), which was to be followed with the GOAT-catalyzed acyl transfer reaction. Here, the solid-supported ghrelin substrate was first incubated with membrane-bound GOAT (~50 μg) in HEPES buffer (50 mM pH 7.0) for 5 min at 37 °C. Palmitoyl-CoA (50 μM) and n-octynoyl-CoA (1 μM) were then added to the mixture to initiate the reaction. Palmitoyl-CoA was included as a competing fatty acid thioester to reduce any background hydrolysis of n-octynoyl-CoA due to esterases in the crude membrane extract.[19a] The click reaction was then performed with azido-tagged HRP (Figure 3A). It is important to note here that in model studies all attempts to use the commercially available tris[(1-benzyl-1H-1,2,3-triazol-4-yl)methyl]amine (TBTA)[21] ligand at low concentrations of substrate failed; however, use of the more hydrophilic ligand, tris[(3-hydroxypropyl-1H-1,2,3-triazol-4-yl)methyl]amine (THPTA) (Figure 3A),[22] recently disclosed by the Finn group resulted in consistently successful coupling regardless of substrate concentration (data not shown). After the click reaction, the wells were treated with amplex red and hydrogen peroxide, and the resulting resorufin fluorescence was measured (λex = 560 nm, λem = 585 nm). As shown in Figure 3B, our assay design was successful, and under these conditions a 7.5-fold fluorescence enhancement (raw fluorescence data) was observed over wells not treated with GOAT or n-octynoyl-CoA. It is important to note here that this is the first time that GOAT has been shown to accept an alkynyl-CoA substrate.
Given these successful results, we next wanted to characterize the GOAT-catalyzed acyl transfer reaction using our cat-ELCCA system. We first examined the time dependence of n-octanoyl transfer to the immobilized ghrelin(1–5)pentapeptide. As Figure 4A shows, the formation of n-octynoyl-ghrelin(1–5)pentapeptide was linear with time for ~2.0 min. The assay was also linear with respect to the amount of GOAT-containing membranes (up to ~25 μg) added to the wells (Figure 4B). It is important to note here that beyond the range shown in Figure 4B, a slight decrease in product formation was observed, which is likely due to an increased concentration of competing esterases in the crude membrane preparation. As Figures 4C and 4D show, the assay also exhibited saturation kinetics with respect to the concentrations of n-octynoyl-CoA and immobilized ghrelin(1–5)pentapeptide.
Figure 4.


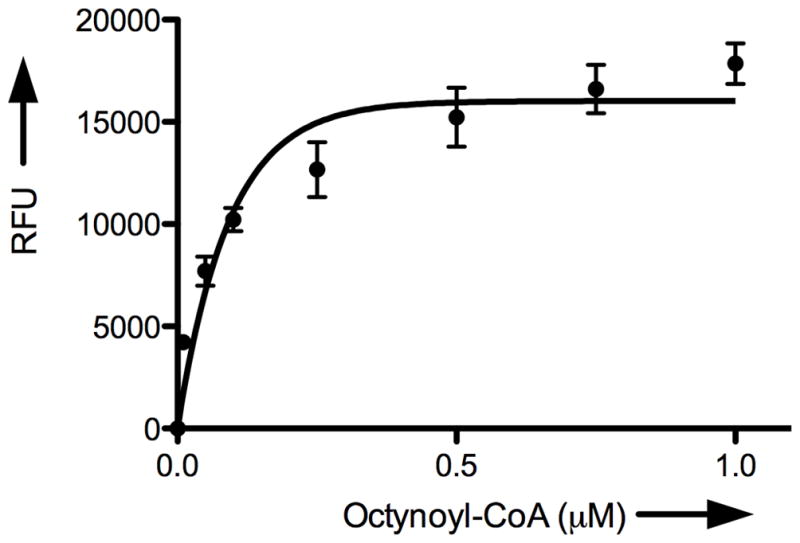
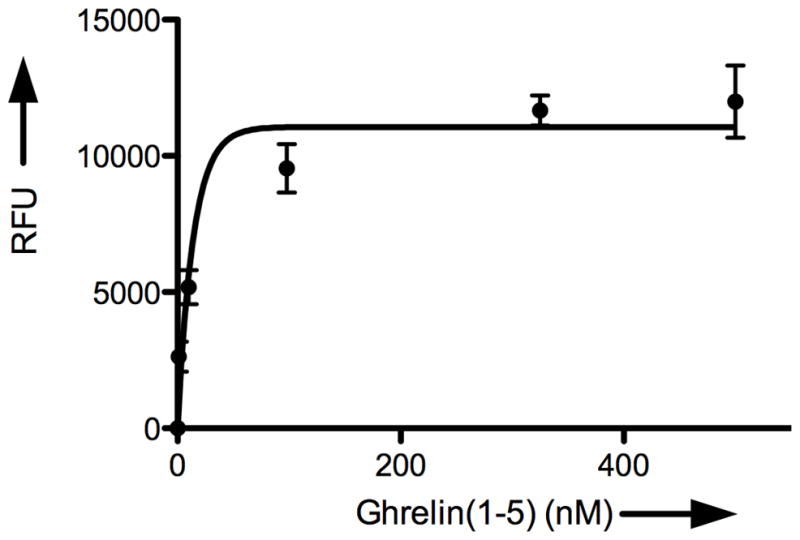
Characterization of GOAT acyltransferase activity using cat-ELCCA. A) Time dependence. B) Dependence on the amount of GOAT membranes. C) Dependence on n-octynoyl-CoA concentration. D) Dependence on immobilized ghrelin(1–5)pentapeptide concentration. RFU = normalized relative fluorescence units at 585 nm.
Because we envision that this assay will be used to screen for antagonists against this potential anti-obesity target, it was also important to determine the apparent Km values for GOAT. Initial velocities were measured for varying concentrations of n-octynoyl-CoA and immobilized ghrelin(1–5)pentapeptide. Nonlinear regression (GraphPad Prism v5.0a) showed that the data fit Michaelis-Menten kinetics (see Supporting Information). The apparent Km values were 67.5 nM and 99.8 nM for n-octynoyl- CoA and the immobilized ghrelin(1–5)pentapeptide, respectively. In addition, to assess the suitability of cat-ELCCA for high-throughput screening, we also determined the signal-to-noise ratio (S/N), signal-to-background ratio (S/B) and Z′ factor, a statistical parameter for scoring assay performance.[23] The S/N ratio was 24, the S/B ratio was 3.5 and the Z′ factor was 0.63 (see Supporting Information), indicating the potential of our assay for high-throughput screening. Most important of these statistical indicators is the Z′ factor, which is reflective of both dynamic range and data variation associated with the signal measurement, and Z′ values between 0.5 and 1 are typically regarded as excellent assays.
In summary, we have described a conceptually new enzyme assay based on cat-ELISA, catalytic assay using enzyme-linked click chemistry assay, or cat-ELCCA. By utilizing an enzyme-linked click chemistry substrate, we are able to arm the assay with catalytic signal amplification necessary for ultrasensitive detection. Using this assay technology, we developed a screening system for the acyltransferase of ghrelin, ghrelin O-acyltransferase (GOAT), that is efficient, sensitive, inexpensive, and importantly, obviates the need for radioisotopes. As GOAT has been implicated as a potential anti-obesity target and much more is still to be learned about this recently disclosed MBOAT family member, we envision that cat-ELCCA will be useful for discovering antagonists and chemical probes for this enzyme, a goal that we are currently pursuing. Finally, as the immobilized peptide and alkynyl substrates are modular, cat-ELCCA should find additional uses with other fatty acid acyltransferases, such as myristoyltransferases and palmitoyltransferases, other biologically relevant acyltransferases (e.g. histone acetyltransferases, N-acetyltransferases) and the discovery of previously unidentified proteins with acyltransferase activity.
Experimental Section
Membrane-bound GOAT (~50 μg) and HEPES buffer (50 mM, pH 7.0) were added to wells of a black, 96-well Reacti-Bind™ Streptavidin High-Binding Capacity microtiter plate containing immobilized ghrelin(1–5)pentapeptide, and the plate was incubated at 37 °C for 5 min. Palmitoyl-CoA (50 μM) and n-octynoyl-CoA (1.0 μM) were then added to the mixture to initiate the enzyme-catalyzed reaction (total volume = 100 μL), and the plate was incubated at 37 °C for another 5 min. Negative controls did not contain either membrane-bound GOAT or n-octynoyl-CoA. The GOAT reaction mixture was then removed, and the wells were washed with TBST (3 × 200 μL) followed by PBS (pH 7.4) (3 × 200 μL). Fresh PBS (pH 7.4), HRP-N3 (1.0 μM), CuSO4 (100 μM), THPTA (500μM) and sodium ascorbate (5 mM) were then added (total volume = 100 μL), and the plate was covered and allowed to shake at 25°C for 2 h. The click reaction mixture was then removed, and the wells were washed again with TBST (3 × 200 μL) and PBS (pH 7.4) (3 × 200 μL). For detection, 100 μL of amplex red/H2O2 solution (QuantaRed Enhanced Chemifluorescent HRP Substrate kit) was added to each well, and the fluorescence (λex = 560 nm, λem = 585 nm) was measured over time (~10 min) on a SpectraMax M2e Microplate Reader (Molecular Devices). A standard curve was generated using known concentrations of HRP-N3 in the reaction buffer.
Supplementary Material
Footnotes
This work was supported by The Skaggs Institute for Chemical Biology. A.L.G. also thanks for a NIH Postdoctoral fellowship (F32-DK083179). We are grateful to Damian Ekiert for assistance with baculovirus expression, Prof. M. G. Finn for helpful discussions and Vu Hong for donation of THPTA.
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Blaedel WJ, Boguslaski RC. Anal Chem. 1978;50:1026–1032. [Google Scholar]
- 2.a) Zhu L, Lynch VM, Anslyn EV. Tetrahedron. 2004;60:7267–7275. [Google Scholar]; b) Wu Q, Anslyn EV. J Am Chem Soc. 2004;126:14682–14683. doi: 10.1021/ja0401038. [DOI] [PubMed] [Google Scholar]; c) Wu Q, Anslyn EV. J Mater Chem. 2005;15:2815–2819. [Google Scholar]; d) Houk RJT, Anslyn EV. New J Chem. 2007;31:729–735. [Google Scholar]; e) Gianneschi NC, Nguyen ST, Mirkin CA. J Am Chem Soc. 2005;127:1644–1645. doi: 10.1021/ja0437306. [DOI] [PubMed] [Google Scholar]; f) Masar MS, III, Gianneschi NC, Oliveri CG, Stern CL, Nguyen ST, Mirkin CA. J Am Chem Soc. 2007;129:10149–10158. doi: 10.1021/ja0711516. [DOI] [PubMed] [Google Scholar]; g) Graf N, Göritz M, Krämer R. Angew Chem. 2006;118:4117–4119. doi: 10.1002/anie.200504319. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2006;45:4013–4015. doi: 10.1002/anie.200504319. [DOI] [PubMed] [Google Scholar]; h) Graf N, Krämer R. Chem Commun. 2006:4375–4376. doi: 10.1039/b611319b. [DOI] [PubMed] [Google Scholar]; i) Song F, Garner AL, Koide K. J Am Chem Soc. 2007;129:12354–12355. doi: 10.1021/ja073910q. [DOI] [PubMed] [Google Scholar]; j) Garner AL, Koide K. J Am Chem Soc. 2008;130:16472–16473. doi: 10.1021/ja8065539. [DOI] [PubMed] [Google Scholar]; k) Garner AL, Song F, Koide K. J Am Chem Soc. 2009;131:5163–5171. doi: 10.1021/ja808385a. [DOI] [PubMed] [Google Scholar]
- 3.a) Rotman B. Proc Natl Acad Sci U S A. 1961;47:1981–1991. doi: 10.1073/pnas.47.12.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Reymond JL, Fluxá VS, Maillard N. Chem Commun. 2009:34–46. doi: 10.1039/b813732c. [DOI] [PubMed] [Google Scholar]
- 4.Resh MD. Methods. 2006;40:191–197. doi: 10.1016/j.ymeth.2006.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Varner AS, De Vos ML, Creaser SP, Peterson BR, Smith CD. Anal Biochem. 2002;308:160–167. doi: 10.1016/s0003-2697(02)00212-9. [DOI] [PubMed] [Google Scholar]; b) Varner AS, Ducker CE, Xia Z, Zhuang Y, De Vos ML, Smith CD. Biochem J. 2003;373:91–99. doi: 10.1042/BJ20021598. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lada AT, Davis M, Kent C, Chapman J, Tomoda H, Omura S, Rudel LL. J Lipid Res. 2004;45:378–386. doi: 10.1194/jlr.D300037-JLR200. [DOI] [PubMed] [Google Scholar]; d) Ducker CE, Griffel LK, Smith RA, Keller SN, Zhuang Y, Xia Z, Diller JD, Smith CD. Mol Cancer Ther. 2006;5:1647–1659. doi: 10.1158/1535-7163.MCT-06-0114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.a) Hang HC, Geutjes EJ, Grotenberg G, Pollington AM, Bijlmakers MJ, Ploegh HL. J Am Chem Soc. 2007;129:2744–2745. doi: 10.1021/ja0685001. [DOI] [PubMed] [Google Scholar]; b) Raghaven A, Charron G, Flexner J, Hang HC. Bioorg Med Chem Lett. 2008;18:5982–5986. doi: 10.1016/j.bmcl.2008.09.083. [DOI] [PubMed] [Google Scholar]; c) Martin BR, Cravatt BF. Nat Methods. 2009;6:135–138. doi: 10.1038/nmeth.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Charron G, Zhang MM, Yount JS, Wilson J, Raghaven A, Shamir E, Hang HC. J Am Chem Soc. 2009;131:4967–4975. doi: 10.1021/ja810122f. [DOI] [PubMed] [Google Scholar]; e) Hannoush RN, Arenas-Ramirez N. ACS Chem Biol. 2009;4:581–587. doi: 10.1021/cb900085z. [DOI] [PubMed] [Google Scholar]; f) Zhang MM, Tsou LK, Charron G, Raghaven A, Hang HC. Proc Natl Acad Sci, U S A. 2010;107:8627–8632. doi: 10.1073/pnas.0912306107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew Chem. 2002;114:2708–2711. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]; b) Tornoe CW, Christensen C, Meldal M. J Org Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]; c) Meldal M, Tornoe CW. Chem Rev. 2008;108:2952–3015. doi: 10.1021/cr0783479. [DOI] [PubMed] [Google Scholar]
- 8.Wang Q, Chan TR, Hilgraf R, Fokin VV, Sharpless KB, Finn MG. J Am Chem Soc. 2003;125:3192–3193. doi: 10.1021/ja021381e. [DOI] [PubMed] [Google Scholar]
- 9.a) Krasinski A, Radic Z, Manetsch R, Raushel J, Taylor P, Sharpless KB, Kolb HC. J Am Chem Soc. 2005;127:6686–6692. doi: 10.1021/ja043031t. [DOI] [PubMed] [Google Scholar]; b) Mocharla VP, Colasson B, Lee LV, Röper S, Sharpless KB, Wong CH, Kolb HC. Angew Chem. 2005;117:118–122. doi: 10.1002/anie.200461580. [DOI] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2005;44:116–120. [Google Scholar]; c) Srinivasan R, Li J, Ling Ng S, Kalesh KA, Yao SQ. Nat Protoc. 2007;2:2655–2664. doi: 10.1038/nprot.2007.323. [DOI] [PubMed] [Google Scholar]
- 10.a) Wisdom GB. Clin Chem. 1976;22:1243–1255. [PubMed] [Google Scholar]; b) Gosling JP. Clin Chem. 1990;36:1406–1427. [PubMed] [Google Scholar]
- 11.Tawfik DS, Green BS, Chap R, Sela M, Eshhar Z. Proc Natl Acad Sci U S A. 1993;90:373–377. doi: 10.1073/pnas.90.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Immobilized substrates have previously been utilitzed in click chemistry see Bryan MC, Fazio F, Lee H-K, Huang C-Y, Chang A, Best MD, Calarese DA, Blixt O, Paulson JC, Burton D, Wilson IA, Wong C-H. J Am Chem Soc. 2004;126:8640–8641. doi: 10.1021/ja048433f.
- 13.a) Yang J, Brown MS, Liang G, Grishin NV, Goldstein JL. Cell. 2008;132:387–396. doi: 10.1016/j.cell.2008.01.017. [DOI] [PubMed] [Google Scholar]; b) Gutierrez JA, Solenberg PJ, Perkins DR, Willency JA, Knierman MD, Jin Z, Witcher DR, Luo S, Onyia JE, Hale JE. Proc Natl Acad Sci U S A. 2008;105:6320–6325. doi: 10.1073/pnas.0800708105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofmann K. Trends Biochem Sci. 2000;25:111–112. doi: 10.1016/s0968-0004(99)01539-x. [DOI] [PubMed] [Google Scholar]
- 15.a) Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa Nature. 1999;402:656–660. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]; b) Tschöp MH, Smiley DL, Heiman ML. Nature. 2000;407:908–913. doi: 10.1038/35038090. [DOI] [PubMed] [Google Scholar]; c) Castaneda TR, Tong J, Datta R, Culler M, Tschöp MH. Frontiers Neuroendocrinol. 2010;31:44–60. doi: 10.1016/j.yfrne.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 16.Kojima M, Kangawa K. Physiol Rev. 2005;85:495–522. doi: 10.1152/physrev.00012.2004. [DOI] [PubMed] [Google Scholar]
- 17.Zorrilla EP, Iwasaki S, Moss JA, Chang J, Otsuji J, Inoue K, Meijler MM, Janda KD. Proc Natl Acad Sci U S A. 2006;103:13226–13231. doi: 10.1073/pnas.0605376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mayorov AV, Amara N, Chang JY, Moss JA, Hixon MS, Ruiz DI, Meijler MM, Zorrilla EP, Janda KD. Proc Natl Acad Sci U S A. 2008;105:17487–17492. doi: 10.1073/pnas.0711808105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.An initial report of product-based inhibitors has been described in ref. 19a. Yang J, Zhao TJ, Goldstein JL, Brown MS. Proc Natl Acad Sci U S A. 2008;105:10750–10755. doi: 10.1073/pnas.0805353105.Ohgusu H, Shirouzu K, Nakamura Y, Nakashima Y, Ida T, Sato T, Kojima M. Biochem Biophys Res Commun. 2009;386:153–158. doi: 10.1016/j.bbrc.2009.06.001.
- 20.a) Bednarek MA, Feighner SD, Pong SS, McKee KK, Hreniuk DL, Silva MV, Warren VA, Howard AD, Van der Ploeg LHY, Heck JV. J Med Chem. 2000;43:4370–4376. doi: 10.1021/jm0001727. [DOI] [PubMed] [Google Scholar]; b) Matsumoto M, Hosoda H, Kitajima Y, Morozumi N, Minamitake Y, Tanaka S, Matsuo H, Kojima M, Hayashi Y, Kangawa K. Biochem Biophys Res Commun. 2001;287:142–146. doi: 10.1006/bbrc.2001.5553. [DOI] [PubMed] [Google Scholar]
- 21.Chan TR, Hilgraf R, Sharpless KB, Fokin VV. Org Lett. 2004;6:2853–2855. doi: 10.1021/ol0493094. [DOI] [PubMed] [Google Scholar]
- 22.Hong V, Presolski SI, Ma C, Finn MG. Angew Chem. 2009;121:10063–10067. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem Int Ed. 2009;48:9879–9883. doi: 10.1002/anie.200905087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang J-H, Chung TDY, Oldenburg KR. J Biomol Screen. 1999;4:67–73. doi: 10.1177/108705719900400206. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


