INTRODUCTION
Erectile dysfunction (ED) is the most prevalent complication of prostate cancer treatment. Post-radical prostatectomy ED can occur in up to 70% of these patients even in centers of excellence that apply nerve sparing techniques [1–3], severely affecting the quality of life of patients, and posing a heavy burden on health care costs [4–6]. The fear of this complication is often a deterrent for the patient to opt for radical prostatectomy even when it is the treatment of choice.
In post-radical prostatectomy/radiotherapy, and possibly cryotherapy [7,8], cavernosal nerve damage causes first an acute neurogenic ED by interfering with the nitrergic neurotransmission originated from the brain, mediated by nitric oxide (NO) [9], and then a neuropraxia of the corpora cavernosa leading to fibrosis, apoptosis and loss of corporal smooth muscle cells (CSMC) [10]. This impairs the compliance of the tissue and its ability to retain blood during erection causing corporal veno-occlusive dysfunction (CVOD) [10–12]. This is a form of vasculogenic ED that is the prevalent type of ED [13]. The frequent resistance of post-radical prostatectomy CVOD to oral PDE 5 inhibitors (PDE5i), or intracorporeal injections applied “on demand” to induce erections [14], is because vasodilation by the oral PDE 5 inhibitors requires the integrity of both the cavernosal nerve to transmit the sexual stimulus and the CSMC to respond, and local vasodilators are ineffective when the corporal tissue is injured [8].
There is therefore a considerable clinical interest in finding new strategies for penile regeneration and this has spurred experimental studies in animal models of post-RP, essentially in rats and mice where the cavernosal nerve damage is induced by bilateral cavernosal nerve resection (BCNR) [11,12,15,16], transection (BCNT) [17], or controlled bilateral cavernosal nerve crush (BCNC) [18]. Using BCNR procedures in the rat, it has been demonstrated that long-term continuous administration of the three PDE 5 inhibitors (PDE 5i), sildenafil, tadalafil, and vardenafil, partially prevent CVOD by counteracting the underlying corporal histopathology by an antifibrotic mechanism [11,15,16,19]. This is different from the vasodilator mechanism that operates in the conventional “on demand” palliative interventions to facilitate penile erection upon sexual stimulation. However, since the daily doses of PDE5i in these BCNR studies were, when translated from rats to humans, about 2–3 fold higher than the usual ones used on demand, and so far only a few clinical studies with non-conclusive results have been performed on this modality with standard doses [20,21], further experimental studies with lower doses are needed to improve this approach.
In addition to this pharmacological therapy, alternative strategies, such as the use of stem cells, either by themselves or in combination with long-term continuous administration of PDE5i need to be examined. Despite the promising results in several studies with different stem cell types in animal models of ED associated with aging and diabetes [17,22–28], only a few reports are available for ED post-radical prostatectomy models, and they share some limitations [29]. This field of was opened in the BCNC model by applying embryonic stem cells [30], which suffer from the fact that their clinical use for non-life threatening conditions is unlikely. These cells failed to be detected at late stages, the correction of ED by EFS was partial, and no studies on corporal damage or CVOD were performed.
Some of these experimental concerns on stem cell therapy for ED apply to a study using skeletal muscle derived stem cells (MDSC) in a BCNT model [17], and more recently in a short report on the amelioration of CVOD in the BCNR model, compounded by questions on the “MDSC” population employed [31]. MDSC were effective correcting an impaired EFS response in aged rats and to convert to SMC and other differentiated cells in the corpora cavernosa and in the injured vagina [28,32]. To our knowledge, no combination of PDE5i and stem cell treatments or with NO donors has been tested for ED in animal models, even if this modality was studied for myocardial infarction with some inconclusive results [33].
In the present work we have investigated whether sildenafil given for 45 days to BCNR rats at 1/2 through 1/16 the doses previously tested, and the NO donor molsidomine, in combination or not with sildenafil, could prevent CVOD and improve the underlying corporal histopathology, and whether similar or better effects could be obtained with MDSC alone or in the presence of a chronic very low dose of sildenafil.
MATERIALS AND METHODS
MDSC isolation and culture
MDSC were prepared from the hind limb muscles from the mouse [34–36], using the preplating procedure, a validated standardized method for MDSC isolation [37], as in our previous reports [28,32,38]. Tissues were dissociated using sequentially collagenase XI, dispase II and trypsin, and after filtration through 60 nylon mesh and pelleting, the cells were suspended in Dulbecco’s Modified Eagle’s Medium (DMEM) with 20% fetal bovine serum. Cells were plated onto collagen I-coated flasks for 1 hr (preplate 1 or pP1), and 2 hrs (preplate 2 for pP2), followed by sequential daily transfers of non-adherent cells and re-platings for 2 to 6 days, until preplate 6 (pP6). The latter is the cell population containing MDSC. Cells were then selected using magnetic beads coated with the Sca 1 antibody. Cells were replicated on regular culture flasks (no coating) and used in the 5th-10th passage, since the mouse counterparts have been maintained in our laboratory for at least 40 passages with the same, or even increasing, growth rate. Flow cytometry was performed to show that these cultures were Sca 1+/CD34+/CD44+ cells and expressed the key stem cell gene Oct 4 [38].
BCNR procedure
Five month-old male Fisher 344 rats (Charles River, Wilmington, MA) were subjected to BCNR as previously described [12,15,16,19]. All animal experiments were approved by the IACUC at our institution. Essentially, animals were operated under aseptic conditions and isoflurane anesthesia. In supine position, a midline incision was done, the pelvic cavity was opened, and the bladder and prostate were located. Under an operating microscope, the major pelvic ganglion and its inflow and outflow nerve fibers were identified after removing the fascia and fat on the dorsolateral lobe of the prostate. The main branch of the cavernosal nerve is the largest efferent nerve which runs along the surface of the prostatic wall. Above the main branch there are another four to six small efferent fibers which also run towards the membranous urethra, considered as ancillary branches of the CN. In order to recognize the main cavernosal nerve, stimulation with an electrode to induce penile erection was applied. In the sham-operated group both cavernosal nerves were identified but not resected. In BCNR, the main cavernosal nerves and ancillary branches were resected by removing a 5-mm segment. This procedure mainly eliminates the nitrergic NANC stimulation to the corporal smooth muscle that elicits its relaxation during penile erection, while also interrupting some vasoconstrictor neuro-transmission through coalescent adrenergic fibers in the cavernosal nerve.
Animal treatments
Animals (total: 80 rats; n=8/group) were treated as follows: 1) SH: sham-operated, untreated; Series I: 2) LS: BCNR, low dose sildenafil, in water: 2.5 mg/kg/day, 1/8 of the dose previously used [15]; 3) LS(RL): BCNR, low dose sildenafil, retrolingual; 4) MS: BCNR, medium dose sildenafil, in water: 10 mg/kg/day; 5) M: BCNR, molsidomine, IP, 10 mg/kg/day [29]; 6) M+LS: BCNR, molsidomine IP, with low dose sildenafil in water; 7) M+MS: BCNR, molsidomine IP, with medium dose sildenafil in water; Series II: 8) SC: MDSC (106 cells) injected in 0.05 ml Hanks, intracorporal [28]; the MDSC were labeled with the nuclear fluorescent stain 4′,6-diamidino-2-phenylindole (DAPI) and implanted aseptically into two different sites in the mid-part of the shaft in anaesthetized rats; tacrolimus was given daily (1 mg/kg, s.c.) to avoid immuno-rejection of the mouse stem cells; 9) SC+VLS: MDSC (106 cells) injected, with tacrolimus, as in #8, and supplemented with very low dose sildenafil, in water: 1.25 mg/kg/day, 1/16 of the dose previously used [15]; and 10) VLS: very low dose sildenafil, in water.
Treatments were interrupted at 42 days, and the experiment was finalized 3 days later (washout). The drinking volumes were determined daily, and body weights were recorded weekly. The daily sildenafil doses given to these animals were as stated above. They are estimated at approximately equivalent to doses 10-fold higher in men when expressed in mg/day (i.e., 1.25 mg/kg/day in rats equivalent to 12.5 mg/day in men), based on differences in total body surface area between the rat and the human [40,41].
Dynamic Infusion Cavernosometry (DIC)
Cavernosometry was performed as previously described [12,15,16,19]. Briefly, basal intracavernosal pressure (ICP) was recorded, and 0.1ml papaverine (20 mg/ml) was administered through a cannula into the corpora cavernosa. The ICP during tumescence was recorded as “ICP after papaverine”. Saline was then infused through another cannula, increasing infusion rate by 0.05 ml/min every 10 seconds, until the ICP reached 100 mmHg (“infusion rate”). The “drop rate” was determined by recording the fall in ICP within the next 1 minute after the infusion was stopped.
Determinations in tissue sections
After cavernosometry, animals were sacrificed and the skin-denuded penile shafts were fixed overnight in 10% buffered formalin, washed, and stored in alcohol (70%) at 4°C until processed for paraffin embedded tissue sections (6–8 μm). Adjacent tissue sections were used for [12,15,16,19]: a) Masson trichrome staining for collagen (blue) and SMC (red); and b) immunodetection with monoclonal antibodies against α-smooth muscle actin (ASMA) as a SMC marker (Sigma kit, Sigma Diagnostics, St Louis, MO). For immunodetection sections were then incubated with biotinylated anti-Mouse IgG, followed by ABC complex (Vector labs, Temecula, CA) and 3,3′diaminobenzidine (Sigma) (PCNA and iNOS). Sections were counterstained with hematoxylin. Negative controls in the immunohistochemical detections were done by replacing the first antibody with IgG isotype.
Quantitative image analysis (QIA) was performed by computerized densitometry using the ImagePro 4.01 program (Media Cybernetics, Silver Spring, MD), coupled to an Olympus BHS microscope equipped with an Olympus digital camera [12,15,16,19]. For Masson staining, 40× magnification pictures of the penis comprising half of the corpora cavernosa were analyzed for SMC (stained in red) and collagen (stained in blue), and expressed as SMC/collagen ratio. For ASMA, only the corpora cavernosa were analyzed in a computerized grid and expressed as % of positive area vs. total area of the corpora cavernosa. In all cases, two fields at 40× (both sides of the corpora cavernosa) or 8 fields at 400×, were analyzed per tissue section, with at least 4 matched sections per animal and 8 animals per group.
Qualitative dual immunofluorescence determinations were also performed in frozen tissue sections (8–10 μm) for neurofilament 70 (NF70) with an antibody clone DA2, mouse monoclonal (Millipore, Billerica, MA), and for neuronal nitric oxide synthase (nNOS) using a rabbit monoclonal (ABCAM, Cambridge, MA).
Determinations in tissue homogenates
Penile tissue homogenates (80–100 mg tissue) were obtained in T-PER (PIERCE, Rockford, IL) and protease inhibitors (3 μM leupeptin, 1 μM pepstatin A, 1mM phenyl methyl sulfonyl fluoride), and centrifuged at 10,000 g for 5 min. Supernatant proteins (30–50 μg) were subjected to western blot analyses [12,15,16,19] by 7–10 % Tris-HCl polyacrylamide gel electrophoresis (PAGE) (Bio-Rad, Hercules, CA) in running buffer (Tris/Glycine/SDS). Proteins were transferred overnight at 4°C to nitrocellulose membranes in transfer buffer (Tris/glycine/methanol) and the next day, the non-specific binding was blocked by immersing the membranes into 5% non-fat dried milk, 0.1% (v/v) Tween 20 in PBS for 1hour at room temperature. After several washes with washing buffer (PBS Tween 0.1%), the membranes were incubated with the primary antibodies for 1 hour at room temperature. Monoclonal antibodies were as follows: a) calponin 1 (Calp 1) mouse monoclonal (Santa Cruz Biotechnology, Inc. Santa Cruz, CA); b) Src homology region 2-containing protein tyrosine phosphatase (SHP-2) rabbit polyclonal (Santa Cruz Biotechnology, Inc); c) BAX rabbit polyclonal (Santa Cruz Biotechnology, Inc); d) NF70, as for immunofluorescence; e) nNOS, as for immunofluorescence; f) brain-derived neurotrophic factor (BDNF), rabbit monoclonal ABCAM, Cambridge, MA; g) PDE 5, rabbit polyclonal (Santa Cruz), and g) mouse glyceraldehyde 3-phosphate dehydrogenase (GAPDH) mouse monoclonal (Millipore, Billerica, MA), as a reference housekeeping protein.
The washed membranes were incubated for 1 hour at room temperature with 1/3,000 dilution (anti-mouse), followed by a secondary antibody linked to horseradish peroxidase. After several washes, the immunoreactive bands were visualized using the ECL plus western blotting chemiluminescence detection system (Amersham Biosciences, Piscataway, NJ). The densitometric analyses of the bands were performed with Image J (NIH, Bethesda, MD). A positive control was run throughout all gels for each antibody to standardize for variations in exposures and staining intensities. Negative controls were performed omitting the primary antibody. Band intensities were determined by densitometry and corrected by the respective intensities for GAPDH, upon reprobing.
Collagen was estimated by the picrosirius red procedure [42], using an aliquot of the tissue homogenates prepared for western lotting, mixing it with Sirius Red saturated in picric acid incubated for 30 min, and centrifuged at 15,000 × g for 5 min, to pellet the collagen. This pellet is rinsed once with 0.1M HCl to remove excess dye, centrifuged again, and the pellet is extracted in 0.5M NaOH, clarified, and measured spectrophotometrically at 550 nm. The standard curve is Type I Collagen, acid soluble, (Sigma Chemical Corp) from 0–80μg. Values are expressed as micrograms of collagen per milligram of tissue.
Statistical analysis
Values were expressed as mean ± SEM. For Series I, the normality distribution of the data was established using the Wilk-Shapiro test. Multiple comparisons were analyzed by a two factor (time and treatment) analysis of variance (two way ANOVA), followed by post-hoc comparisons with Tukey post test, according to the GraphPad Prism v 5.1. For Series II, comparisons between a single group and the respective control group (as indicated in each figure) were done with the unpaired t-test. Differences were considered significant at p < 0.05.
RESULTS
A sustained administration of sildenafil at medium and low doses prevents CVOD after BCNR and reduces collagen deposition in the corpora cavernosa, but combination with molsidomine is not more effective than the drugs alone
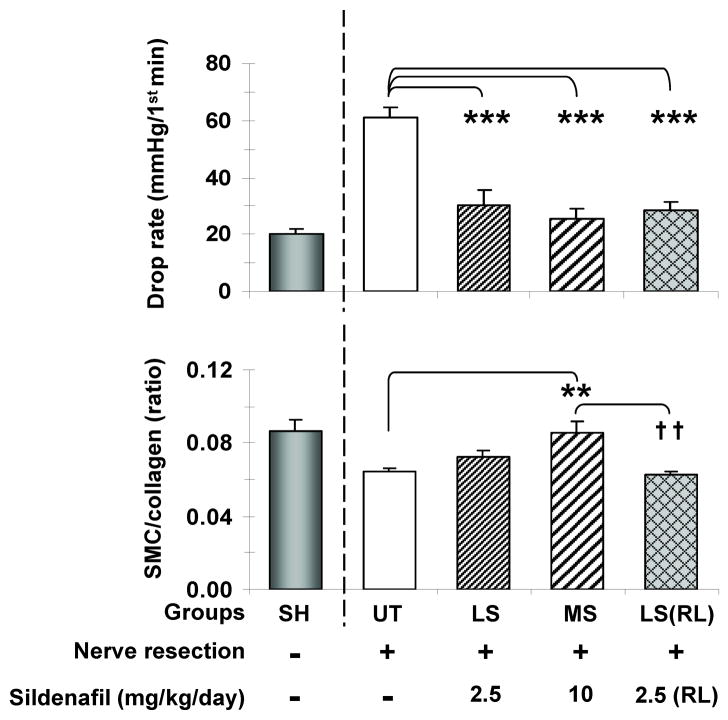
In order to determine whether long-term continuous sildenafil given to BCNR rats in the drinking water could prevent CVOD at doses lower than the previously used high dose of 20 mg/kg/day [15], rats were treated with 1/2 (MS) and 1/8 (LS) of this dose for 45 days. Fig. 1 top shows that the very high drop rate measured by cavernosometry in the UT rats, an indication of CVOD, was reduced by MS and even by LS to a normal level, as compared to our standard values in sham operated animals. The same effect was achieved by LS(RL) a once daily retrolingual administration of low dose sildenafil, to mimic clinical administration. However, unexpectedly, the LS and LS(RL) virtually did not modify the corporal SMC/collagen ratio measured histochemically by Masson trichrome, in contrast to the MS dose that did increase it and normalized the value (bottom).
Fig. 1. Effects of sustained low and medium dose sildenafil on CVOD and the corporal SMC/collagen ratio after BCNR.
Sildenafil was given continuously for 45 days in the drinking water, except as stated. Top: drop rate values during cavernosometry, as a measure of CVOD; Bottom: corporal SMC/collagen ratios as a measure of tissue fibrosis. UT: BCNR rats, untreated; LS: BCNR rats with low dose sildenafil, 2.5 mg/kg/day; MS: BCNR rats with medium dose sildenafil, 10 mg/kg/day LS(RL): as LS, but once a day, retrolingual. SH: sham animals are taken from another series of animals and used as reference, but not for the statistical comparisons. *: p<0.05; **: p<0.01; ***: p<0.001 as compared to UT; ††: p<0.01 as compared to MS.
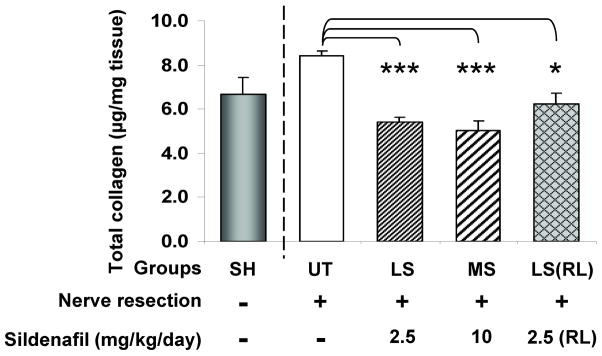
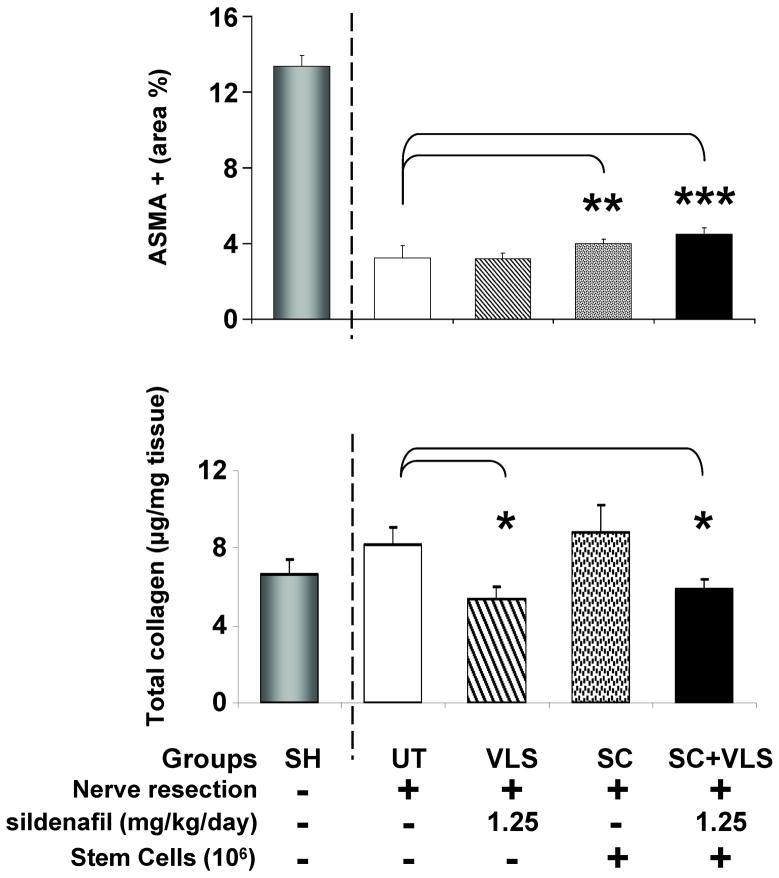
The effects of all treatments on the penile shaft collagen content, measured by a colorimetric Sirius red procedure (Fig. 2) were the expected ones, since it was reduced even below the sham rats value used as reference.
Fig. 2. Effects of sustained low and medium dose sildenafil on the corporal collagen content after BCNR.
Sildenafil was given as in Fig. 1. Penile shaft collagen content estimated from penile shaft homogenates by a spectrophotometric picrosirius red elution method. UT: BCNR rats, untreated; LS: BCNR rats with low dose sildenafil, 2.5 mg/kg/day; MS: BCNR rats with medium dose sildenafil, 10 mg/kg/day LS(RL): as LS, but once a day, retrolingual. SH: sham animals are taken from another series of animals and used as reference, but not for the statistical comparisons. *: p<0.05; **: p<0.01; ***: p<0.001.
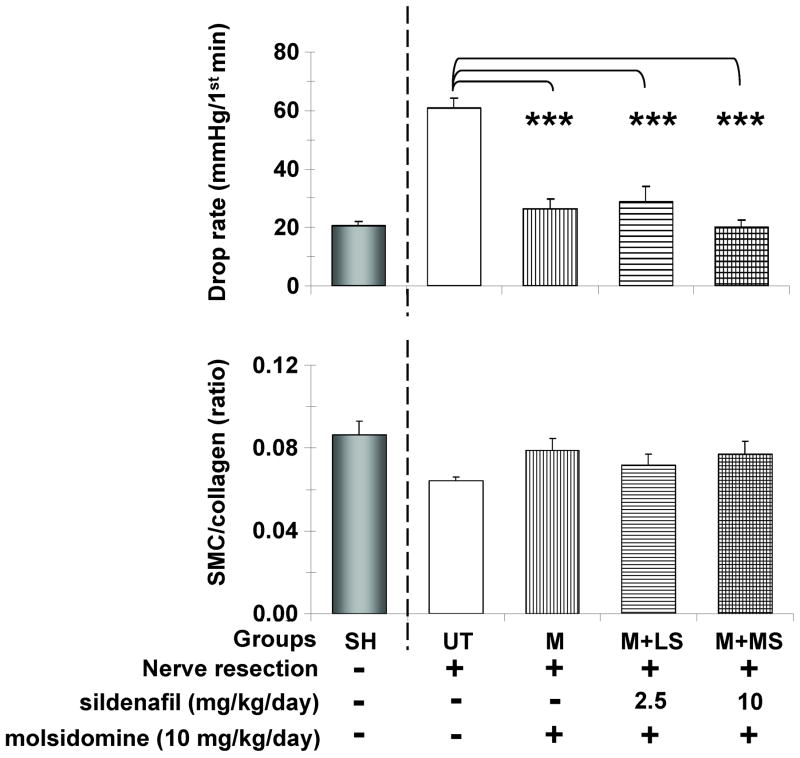
The increase in cGMP levels induced by long-term daily IP molsidomine was sufficient to also normalize the drop rate in the cavernosometry (Fig. 3 top), and therefore the supplementation with LS or MS was in fact unnecessary and both treatments acted similarly. However, the small increase in the Masson trichrome estimate of the corporal SMC/collagen by M and M+MS is non significant and much lower than expected (bottom), despite MS by itself did improve this ratio as shown on Fig. 1.
Fig. 3. Effects of sustained molsidomine and sildenafil combination on CVOD and the corporal SMC/collagen ratio after BCNR.
Molsidomine was given daily IP and continuous sildenafil supplementation was done in the drinking water for 45 days. Top: drop rate values during cavernosometry, as a measure of CVOD; Bottom: corporal SMC/collagen ratios as a measure of tissue fibrosis; UT: BCNR rats, untreated; M: BCNR rats, molsidomine 10 mg/kg/day; M+LS: as M plus low dose sildenafil 2.5 mg/kg/day; M+MS: as M plus medium dose sildenafil 10 mg/kg/day. SH: sham animals are taken from another series of animals and used as reference, but not for the statistical comparisons. *: p<0.05; **: p<0.01; ***: p<0.001.
Implantation of MDSC also prevents CVOD and reduces collagen, but supplementation with very low dose sildenafil does not substantially enhance these effects
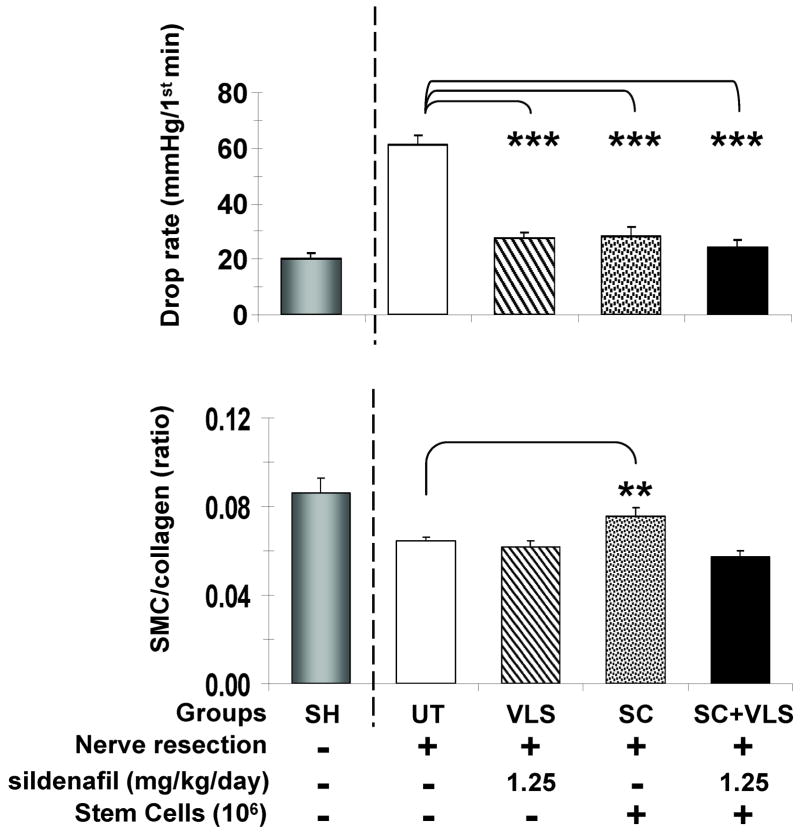
Since MDSC counteracted ED measured by EFS in a rat model of aging [28], we examined whether these stem cells can prevent CVOD measured by cavernosometry in the BCNR rat model, in order to investigate whether the effects can be enhanced by sildenafil supplementation at even lower doses than above. Fig. 4 top shows that MDSC did normalize the drop rate in the cavernosometry determination and that this was also achieved by a very low dose of sildenafil, 1.25 mg/kg/day (VLS), which is 1/16 of the dose previously used [15]. Contrary to our expectations of pharmacological stimulation of the MDSC, the combination of both treatments, SC+VLS, was not different from the individual treatments. However, only the MDSC increased significantly the low SMC/collagen ratio by Masson trichrome seen in the UT rats (bottom).
Fig. 4. Effects of MDSC implantation and sildenafil supplementation on CVOD and the corporal SMC/collagen ratio after BCNR.
MDSC were implanted intracorporally and sildenafil supplementation was done continuously in the water for 45 days. Top: drop rate values during cavernosometry, as a measure of CVOD; Bottom: corporal SMC/collagen ratios as a measure of tissue fibrosis; UT: BCNR rats, untreated; VLS: BCNR rats, very low sildenafil, 1.25 mg/kg/day; SC: BCNR rats, stem cells (106) alone; SC+VLS: as SC plus very low sildenafil 1.25 mg/kg/day. SH: sham animals are taken from another series of animals and used as reference, but not for the statistical comparisons. *: p<0.05; **: p<0.01; ***: p<0.001.
The corporal SMC content identified by immunohistochemistry for the ASMA marker in the trabecular region was also marginally increased by the MDSC, alone or in combination with VLS, but not by VLS alone (Fig. 5 top). In the case of collagen, estimated by Sirius red in the whole penile corporal shaft, its content was reduced by VLS alone or in combination with MDSC, but MDSC failed to change the levels seen in the UT rats (bottom).
Fig. 5. Effects of MDSC implantation and sildenafil supplementation on the corporal SMC and collagen contents after BCNR.
MDSC and sildenafil were given as in Fig. 4. Top: corporal SMC content estimated by immunohistochemistry for ASMA; Bottom: penile shaft collagen content estimated by from penile shaft homogenates by a spectrophotometric picrosirius red elution method. UT: BCNR rats, untreated; VLS: BCNR rats, very low sildenafil, 1.25 mg/kg/day; SC: BCNR rats, stem cells (106) alone; SC+VLS: as SC plus very low sildenafil 1.25 mg/kg/day. SH: sham animals are taken from another series of animals and used as reference, but not for the statistical comparisons. *: p<0.05; **: p<0.01; ***: p<0.001.
MDSC preserve some markers of the SMC relaxation/contraction phenotype and of the nitrergic nerve content, but combination with a very low dose of sildenafil does not enhance the effects
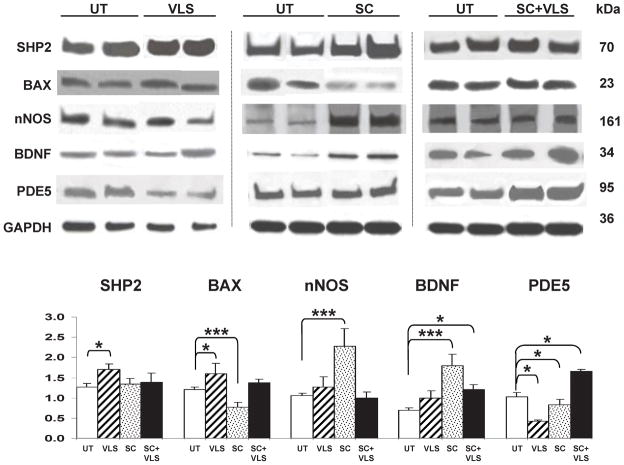
The previous results show that despite the clear effect of the separate MDSC and low or very low sildenafil (or molsidomine at normal doses) treatments in improving the CVOD, these agents only moderately prevented the underlying corporal fibrosis that sildenafil counteracted at higher doses [15]. Therefore, ancillary mechanisms are likely to cooperate with these mild beneficial effects on the histopathology by acting on other targets that help to maintain corporal compliance and thus counteract CVOD. One of them would be the enhancement of SMC calponin I, and of SHP-2, a protein that inactivates the anti-SMC relaxation Rho-A kinase by dephosphorylating Vav [43]. Fig. 6 presents the quantitative western blot determination in the penile shaft homogenates, showing only those markers for which significant changes were observed. MDSC, but not sildenafil, increased calponin in penile shaft homogenates, albeit non-significantly, and reduced the proaptotic Bax, in agreement to the effects on ASMA, but there were no changes in SHP-2 expression. The latter was stimulated by VLS, as had been seen with higher doses of sildenafil [15]. An unexpected result was the upregulation of Bax by VLS
Fig. 6. Effects of intracorporal MDSC implantation and sildenafil supplementation on the expression of some key proteins in the corpora cavernosa after BCNR.
MDSC and sildenafil were given as in Figs. 4 and 5. Top: Representative immunoblots for each group from n=8/group and for each antibody, with the respective GAPDH. UT: BCNR rats, untreated; VLS: BCNR rats, very low sildenafil 1.25 mg/kg/day; SC: BCNR rats, stem cells (106) alone; SC+VLS; as SC plus very low sildenafil 1.25 mg/kg/day; Bottom: densitometric ratios of each antibody value corrected by GAPDH. Each treatment group was run against the UT specimens, and then the statistical comparison of each group was performed separately against the normalized UT. *: p<0.05; **: p<0.01; ***: p<0.001.
The well known neurotrophic effects of MDSC alone [44] and sildenafil alone [46,45] may lead to an amelioration of neural damage and hence of the impact of the neuropraxia on the corporal histology and erectile function. However, neither VLS nor MDSC or their combination increased the expression measured by western blot of the neural marker NF-70, but interestingly, MDSC was the only treatment that did upregulate nNOS and BDNF, thus suggesting a neural trophic effect. The overall failure of the MDSC + VLS combination to improve any of the effects seen by MDSC alone could be due to upregulation of PDE 5 expression by this treatment. Indeed this was the case, particularly in comparison to each treatment alone.
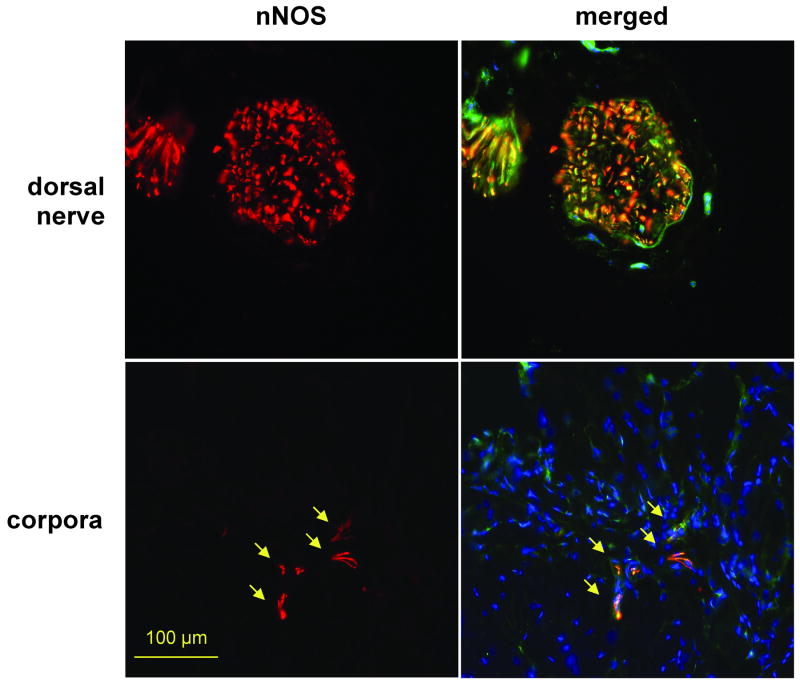
At least part of the nNOS expression detected by western blot occurs in the nerves, specifically in cavernosal nerve terminals, in addition to the dorsal nerve, as shown by dual fluorescence for both proteins (Fig. 7). This suggests that some improvement in nitrergic nerves and hence nitrergic neurotransmission with NO release impacts both the overall erectile response per se and specifically the contractile machinery of the corporal smooth muscle.
Fig. 7. Immunofluorescent identification of nitrergic nerve generation stimulated by stem cell implantation.
Fresh tissue sections were subjected to dual immunofluorescence and nuclei stained with DAPI. Pictures were taken at 200X at the dorsal nerve and corporal areas of the penile shaft. Left: DAPI staining (blue) and nNOS (red); right: merge of left panel with NF-70 (green). SC: BCNR rats, stem cells (106) alone; SC+VLS: as SC with VLS
To facilitate an overall comparison of the different groups the general results are schematically summarized in Tables 1 and 2.
Table 1.
Comparative summary of effects of sildenafil and molsidomine on ED and the underlying histopathology in BCNR rats.
| sildenafil | molsidomine | ||||||
|---|---|---|---|---|---|---|---|
| Effects on | Endpoint | LS | MS | LS(RL) | M | M+LS | M+MS |
| Function | Drop rate | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ | ↓↓↓ |
| Corporal | SMC/collagen | - | ↑ | - | ↑ | - | - |
| Shaft | collagen | ↓↓↓ | ↓↓↓ | ↓ | ND | ND | ND |
Table 2.
Comparative summary of effects of sildenafil and MDSC on ED and the underlying histopathology in BCNR rats.
| Effects on | Endpoint | VLS | sc | SC+VLS |
|---|---|---|---|---|
|
| ||||
| Function | Drop rate | ↓↓↓ | ↓↓↓ | ↓↓↓ |
|
| ||||
| Corporal | SMC/collagen | - | ↑ | - |
| ASMA | - | ↑ | ↑ | |
|
| ||||
| Shaft | collagen | ↓ | - | ↓ |
| Calponin 1 | - | ↑NS | - | |
| SHP-2 | ↑ | - | - | |
| BAX | ↑ | ↓↓↓ | - | |
| NF 70 | - | - | - | |
| nNOS | - | ↑↑↑ | - | |
| BDNF | - | ↑↑↑ | - | |
| PDE5 | ↓ | - | ↑ | |
DISCUSSION
In this work we have investigated whether reducing the dose of sildenafil in the long-term continuous treatment of ED after BCNR from the 20 mg/kg/day previously used [15], which is roughly equivalent to 200 mg daily in men based on rat/human conversion factors (40,41), that prevented the CVOD and the corporal fibrosis and loss of SMC, would preserve efficacy at 1/2, 1/8 and 1/16 lower doses. We also investigated whether the intraperitoneal injection of molsidomine, or the intracorporeal injection of MDSC would also able to prevent the development of CVOD and the impairment of smooth muscle compliance caused by the neuropraxia [12]. The BCNC model [18] had basically confirmed our previous results with sildenafil, and on the other hand it is known that chronic PDE5i protects selectively endothelium-dependent relaxations of strips of corpus cavernosum in vitro [46]. Long-term sildenafil reversed CVOD and improved the underlying corporal histology in a rat model of aging [47]. No studies are available on whether the same effects can be elicited by long-term NO donors or soluble guanyl cyclase stimulators, through the elevation of cGMP levels.
The model that we selected, the BCNR (12,16) mimicks a severe injury that may occur under standard surgical conditions, whereas the (BCNC) [18] resembles the milder damage induced by bilateral nerve sparing techniques. Although BCNR is the most severe form of damage that may exceed the one induced in men in nerve sparing procedures, it has two advantages over BCNC, i.e., absolute reproducibility of the extent of injury, and the fact that interventions ameliorating its impact on both the nerves and the corpora cavernosa will obviously be more successful in repairing the milder BCNC damage.
Our results show that in terms of the functional effect, the expectation was proven, and that even a single daily retrolingual low dose sildenafil, mimicking the clinical administration as tablets, was also effective. So were the combinations of molsidomine or MDSC with sildenafil, but contrary to expectations no additive effect was observed, possibly because the drop rates estimated by cavernosometry were already normalized by the individual treatments alone. However, although these treatments exerted some antifibrotic and SMC-protective effects in the corpora, they were surprisingly much less effective than with the previous 20 mg/kg dose [15], and the sildenafil/molsidomine or sildenafil/MDSC combinations failed to improve them. At least for the single treatments, it is likely that their observed beneficial effects on the SMC relaxation phenotype and nitrergic nerves may contribute to the antifibrotic effects. Of all treatments, the MDSC intracorporal implantation, was the one with more coherent effects on the corporal histopathology, except for the lack of reduction in collagen content in the penile shaft that is unexplained.
Irrespective of the mechanism, it is clear that the beneficial effects of long-term continuous PDE5i on erectile function were not strictly dependent on sustained high cGMP levels induced by the high doses previously applied, so that the lower continuous doses used here are more clinically relevant and may give a window in men from perhaps daily 15 to 50 mg sildenafil. Obviously, considering the long-acting features of tadalafil [16], its doses and frequency may be even lower. In contrast, since molsidomine at the safe but relatively high dose used alone or in combination (about daily 100 mg), was not better than sildenafil alone, it is unlikely that it may have any advantage over PDE5i alone, confirming its only modest performance in the diabetic mouse [39]. However, the potential of NO stimulation of cGMP levels through other longer lasting NO donors [48] should be tested, as well as guanyl cyclase stimulators [49], perhaps in combination with PDE5i at low doses.
In the case of the MDSC intracorporal implantation, the current work has shown that they can prevent CVOD after cavernosal nerve damage, a condition that was not studied in a previous BCNT report using MDSC [17], where only the EFS response was tested and no effects on the corpora SMC were studied. The fast recovery of the EFS to about 60 mmHg at 2 weeks after MDSC (pP6 fraction in the preplating procedure), and the subsequent decay of this response at 4 weeks is difficult to reconcile with a successful BCNT that initially should interrupt completely nitrergic neurotransmission and hence the EFS response, so this may need reassessment. A clarification regarding the nature of the MDSC that in a recent short communication normalized the drop rate and stimulated the papaverine response 4 weeks after BCNR is also required, since here the pP4 fraction was employed, a fraction enriched in satellite cells but not MDSC [31]. Satellite cells are committed to skeletal myofiber formation and are not strictly stem cells. In any case, in the absence of Sca 1 selection, flow cytometry, stem cell markers, and assessment of differentiation capacity, this cell fraction is undefined. As no evaluation of the impact on corporal SMC and fibrosis was performed, in contrast to the current and other reports with MDSC [28,32,33,35] it is not possible to draw a conclusion as to why the CVOD was ameliorated by the non-stem cell, presumably muscle-committed, pP4 fraction.
The upregulation of corporal nNOS by MDSC is promising, may be derived from the neurogenic potential of these stem cells [44], and may help to counteract the neuropraxia, and release NO that would also assist in overcoming the CVOD. The increase in BDNF, also by the MDSC, is a logical candidate for triggering some type of neural repair, since it is widely recognized as a key factor in this process and specifically in the corpora after cavernosal nerve damage [50]. The evaluation of how effective this process may be in the BCNR model, where the nerves are resected, would then require not just the measurement of erectile function by cavernosometry, but also by EFS, similarly to what is done in the much milder damage of the BCNC models. Contrary to our expectations a concurrent very low dose sildenafil administration at 1.25 mg/kg/day obliterated these beneficial effects, similarly to what we have reported for the very low dose sildenafil/MDSC combination in the fibrotic process of myocardial infarction [33]. In both cases PDE 5 was up-regulated by the combination (but not by MDSC or sildenafil alone), either in the MDSC themselves (since they express PDE 5: ref 33) and/or the corporal tissue, probably resulting from a sildenafil effect on MDSC differentiation.
In summary, although both low and very low dose sildenafil, MDSC, and their combination ameliorate CVOD post-cavernosal nerve damage, more work is needed to define the optimal drug dose and cell input as well as their optimal proportion, that may require higher sildenafil doses to compensate for the PDE 5 upregulation that may not be dose-dependent, and thus saturate the enzyme activity to inhibit it. Also, with MDSC, as well as with the other stem cells, several main hurdles affecting stem cell therapy in ED need to be resolved in experimental models, such as poor differentiation efficacy and uncontrolled lineage commitment, spontaneous senescence of the stem cells, deleterious environment of the corporal tissue affected by the neuropraxia, and the inefficient repair of cavernosal nerves. Although a phase 1 clinical trial for post-radical prostatectomy ED has started in France [51] with bone marrow stem cells, it is advisable to go more in depth pre-clinically into the biology of stem cells under the paracrine hues of the corporal tissue, and the optimization of their pharmacological modulation, before expecting an effective clinical application.
Acknowledgments
This work was supported by DOD W81XWH-07-1-0181 and W81XWH-07-1-0129 grants, and partially by NIH R21DK-070003 grant, to NGC
Footnotes
Conflict of Interest: None
References
- 1.Mazzola C, Mulhall JP. Penile rehabilitation after prostate cancer treatment: outcomes and practical algorithm. Urol Clin North Am. 2011;38:105–18. doi: 10.1016/j.ucl.2011.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Magheli A, Burnett AL. Medscape. Erectile dysfunction following prostatectomy: prevention and treatment. Nat Rev Urol. 2009;6:415–27. doi: 10.1038/nrurol.2009.126. [DOI] [PubMed] [Google Scholar]
- 3.Tal R, Valenzuela R, Aviv N, Parker M, Waters WB, et al. Persistent erectile dysfunction following radical prostatectomy: the association between nerve-sparing status and the prevalence and chronology of venous leak. J Sex Med. 2009;6:2813–9. doi: 10.1111/j.1743-6109.2009.01437.x. [DOI] [PubMed] [Google Scholar]
- 4.Miller DC, Saigal CS, Litwin MS. The demographic burden of urologic diseases in America. Urol Clin North Am. 2009;36:11–27. doi: 10.1016/j.ucl.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wilke DR, Krahn M, Tomlinson G, Bezjak A, Rutledge R, Warde P. Sex or survival: short-term versus long-term androgen deprivation in patients with locally advanced prostate cancer treated with radiotherapy. Cancer. 2010;116:1909–17. doi: 10.1002/cncr.24905. [DOI] [PubMed] [Google Scholar]
- 6.Giannakopoulos X, Charalabopoulos K, Charalabopoulos A, Golias C, Peschos D, Sofikitis N. Quality of life survey in patients with advanced prostate cancer. Exp Oncol. 2005;27:13–7. [PubMed] [Google Scholar]
- 7.Mendenhall WM, Henderson RH, Indelicato DJ, Keole SR, Mendenhall NP. Erectile dysfunction after radiotherapy for prostate cancer. Am J Clin Oncol. 2009;32:443–7. doi: 10.1097/COC.0b013e318173a563. [DOI] [PubMed] [Google Scholar]
- 8.Kimura M, Yan H, Rabbani Z, Satoh T, Baba S, Yin FF, Polascik TJ, Donatucci CF, Vujaskovic Z, Koontz BF. Radiation-induced erectile dysfunction using prostate-confined modern radiotherapy in a rat model. J Sex Med. 2011;8:2215–26. doi: 10.1111/j.1743-6109.2011.02351.x. [DOI] [PubMed] [Google Scholar]
- 9.Nangle MR, Keast JR. Reduced efficacy of nitrergic neurotransmission exacerbates erectile dysfunction after penile nerve injury despite axonal regeneration. Exp Neurol. 2007;207:30–41. doi: 10.1016/j.expneurol.2007.05.011. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Cadavid NF. Mechanisms of penile fibrosis. J Sex Med. 2009;(Suppl 3):353–62. doi: 10.1111/j.1743-6109.2008.01195.x. [DOI] [PubMed] [Google Scholar]
- 11.Rambhatla A, Kovanecz I, Ferrini M, Gonzalez-Cadavid NF, Rajfer J. Rationale for phosphodiesterase 5 inhibitor use post-radical prostatectomy: experimental and clinical review. Int J Impot Res. 2008;20:30–4. doi: 10.1038/sj.ijir.3901588. [DOI] [PubMed] [Google Scholar]
- 12.Ferrini MG, Kovanecz I, Sanchez S, Umeh C, Rajfer J, Gonzalez-Cadavid NF. Fibrosis and loss of smooth muscle in the corpora cavernosa precede corporal veno-occlusive dysfunction (CVOD) induced by experimental cavernosal nerve damage in the rat. J Sex Med. 2009;6:415–28. doi: 10.1111/j.1743-6109.2008.01105.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kendirci M, Trost L, Sikka SC, Hellstrom WJ. The effect of vascular risk factors on penile vascular status in men with erectile dysfunction. J Urol. 2007;178:2516–20. doi: 10.1016/j.juro.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 14.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 15.Kovanecz I, Rambhatla A, Ferrini M, Vernet D, Sanchez S, et al. Long-term continuous sildenafil treatment ameliorates corporal veno-occlusive dysfunction (CVOD) induced by cavernosal nerve resection in rats. Int J Impot Res. 2008;20:202–12. doi: 10.1038/sj.ijir.3901612. [DOI] [PubMed] [Google Scholar]
- 16.Kovanecz I, Rambhatla A, Ferrini MG, Vernet D, Sanchez S, et al. Chronic daily tadalafil prevents the corporal fibrosis and veno-occlusive dysfunction that occurs after cavernosal nerve resection. BJU Int. 2008;101:203–10. doi: 10.1111/j.1464-410X.2007.07223.x. [DOI] [PubMed] [Google Scholar]
- 17.Kim Y, de Miguel F, Usiene I, Kwon D, Yoshimura N, Huard J, Chancellor MB. Injection of skeletal muscle-derived cells into the penis improves erectile function. Int J Impot Res. 2006;18:329–34. doi: 10.1038/sj.ijir.3901434. [DOI] [PubMed] [Google Scholar]
- 18.Müller A, Tal R, Donohue JF, Akin-Olugbade Y, Kobylarz K, Paduch D, Cutter SC, Mehrara BJ, Scardino PT, Mulhall JP. The effect of hyperbaric oxygen therapy on erectile function recovery in a rat cavernous nerve injury model. J Sex Med. 2008;5:562–70. doi: 10.1111/j.1743-6109.2007.00727.x. [DOI] [PubMed] [Google Scholar]
- 19.Ferrini MG, Davila HH, Kovanecz I, Sanchez SP, Gonzalez-Cadavid NF, Rajfer J. Vardenafil prevents fibrosis and loss of corporal smooth muscle that occurs after bilateral cavernosal nerve resection in the rat. Urology. 2006;68:429–35. doi: 10.1016/j.urology.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 20.Hatzimouratidis K, Burnett AL, Hatzichristou D, McCullough AR, Montorsi F, Mulhall JP. Phosphodiesterase type 5 inhibitors in postprostatectomy erectile dysfunction: a critical analysis of the basic science rationale and clinical application. Eur Urol. 2009;55:334–47. doi: 10.1016/j.eururo.2008.10.028. [DOI] [PubMed] [Google Scholar]
- 21.Fusco F, Razzoli E, Imbimbo C, Rossi A, Verze P, Mirone V. A new era in the treatment of erectile dysfunction: chronic phosphodiesterase type 5 inhibition. BJU Int. 2010;105:1634–9. doi: 10.1111/j.1464-410X.2010.09244.x. [DOI] [PubMed] [Google Scholar]
- 22.Huang YC, Ning H, Shindel AW, Fandel TM, Lin G, Harraz AM, Lue TF, Lin CS. The Effect of Intracavernous Injection of Adipose Tissue-Derived Stem Cells on Hyperlipidemia-Associated Erectile Dysfunction in a Rat Model. J Sex Med. 2010;7:1391–400. doi: 10.1111/j.1743-6109.2009.01697.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Song YS, Lee HJ, Park IH, Kim WK, Ku JH, Kim SU. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19:378–85. doi: 10.1038/sj.ijir.3901539. [DOI] [PubMed] [Google Scholar]
- 24.Bivalacqua TJ, Deng W, Kendirci M, Usta MF, Robinson C, Taylor BK, Murthy SN, Champion HC, Hellstrom WJ, Kadowitz PJ. Mesenchymal stem cells alone or ex vivo gene modified with endothelial nitric oxide synthase reverse age-associated erectile dysfunction. Am J Physiol Heart Circ Physiol. 2007;292:H1278–90. doi: 10.1152/ajpheart.00685.2006. [DOI] [PubMed] [Google Scholar]
- 25.Qiu X, Sun C, Yu W, Lin H, Sun Z, Chen Y, Wang R, Dai Y. Combined Strategy of Mesenchymal Stem Cells Injection with VEGF Gene Therapy for the Treatment of Diabetes Associated Erectile Dysfunction. J Androl. 2012;33:37–44. doi: 10.2164/jandrol.110.012666. [DOI] [PubMed] [Google Scholar]
- 26.Qiu X, Lin H, Wang Y, Yu W, Chen Y, Wang R, Dai Y. Intracavernous transplantation of bone marrow-derived mesenchymal stem cells restores erectile function of streptozocin-induced diabetic rats. J Sex Med. 2011;8:427–36. doi: 10.1111/j.1743-6109.2010.02118.x. [DOI] [PubMed] [Google Scholar]
- 27.Deng W, Bivalacqua TJ, Champion HC, Hellstrom WJ, Murthy SN, Kadowitz PJ. Superoxide dismutase - a target for gene therapeutic approach to reduce oxidative stress in erectile dysfunction. Methods Mol Biol. 2010;610:213–27. doi: 10.1007/978-1-60327-029-8_13. [DOI] [PubMed] [Google Scholar]
- 28.Nolazco G, Kovanecz I, Vernet D, Ferrini M, Gelfand B, et al. Effect of muscle derived stem cells on the restoration of corpora cavernosa smooth muscle and erectile function in the aged rat. BJU Int. 2008;101:1156–64. doi: 10.1111/j.1464-410X.2008.07507.x. [DOI] [PubMed] [Google Scholar]
- 29.Albersen M, Kendirci M, Van der Aa F, Hellstrom WJ, Lue TF, Spees JL. Multipotent stromal cell therapy for cavernous nerve injury-induced erectile dysfunction. J Sex Med. 2012;9:385–403. doi: 10.1111/j.1743-6109.2011.02556.x. [DOI] [PubMed] [Google Scholar]
- 30.Bochinski D, Lin GT, Nunes L, Carrion R, Rahman N, et al. The effect of neural embryonic stem cell therapy in a rat model of cavernosal nerve injury. BJU Int. 2004;94:904–9. doi: 10.1111/j.1464-410X.2003.05057.x. [DOI] [PubMed] [Google Scholar]
- 31.Woo JC, Bae WJ, Kim SJ, Kim SD, Sohn DW, Hong SH, Lee JY, Hwang TK, Sung YC, Kim SW. Transplantation of muscle-derived stem cells into the corpus cavernosum restores erectile function in a rat model of cavernous nerve injury. Korean J Urol. 2011;52:359–63. doi: 10.4111/kju.2011.52.5.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ho MH, Heydarkhan S, Vernet D, Kovanecz I, Ferrini MG, Bhatia NN, Gonzalez-Cadavid NF. Stimulating vaginal repair in rats through skeletal muscle-derived stem cells seeded on small intestinal submucosal scaffolds. Obstet Gynecol. 2009;114:300–9. doi: 10.1097/AOG.0b013e3181af6abd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang JS-C, Kovanecz I, Vernet D, Nolazco G, Kopchok GE, Chow SL, White RA, Gonzalez-Cadavid NF. Effects of sildenafil and/or muscle derived stem cells on myocardial infarction. J Transl Med. 2012 doi: 10.1186/1479-5876-10-159. preliminary acceptance. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oshima H, Payne TR, Urish KL, Sakai T, Ling Y, Gharaibeh B, Tobita K, Keller BB, Cummins JH, Huard J. Differential myocardial infarct repair with muscle stem cells compared to myoblasts. Mol Ther. 2005;12:1130–41. doi: 10.1016/j.ymthe.2005.07.686. [DOI] [PubMed] [Google Scholar]
- 35.Payne TR, Oshima H, Okada M, Momoi N, Tobita K, Keller BB, Peng H, Huard J. A relationship between vascular endothelial growth factor, angiogenesis, and cardiac repair after muscle stem cell transplantation into ischemic hearts. J Am Coll Cardiol. 2007;50:1677–84. doi: 10.1016/j.jacc.2007.04.100. [DOI] [PubMed] [Google Scholar]
- 36.Okada M, Payne TR, Zheng B, Oshima H, Momoi N, Tobita K, Keller BB, Phillippi JA, Péault B, Huard J. Myogenic endothelial cells purified from human skeletal muscle improve cardiac function after transplantation into infarcted myocardium. J Am Coll Cardiol. 2008;52:1869–80. doi: 10.1016/j.jacc.2008.07.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gharaibeh B, Lu A, Tebbets J, Zheng B, Feduska J, Crisan M, Péault B, Cummins J, Huard J. Isolation of a slowly adhering cell fraction containing stem cells from murine skeletal muscle by the preplate technique. Nat Protoc. 2008;3:1501–9. doi: 10.1038/nprot.2008.142. [DOI] [PubMed] [Google Scholar]
- 38.Tsao J, Vernet D, Gelfand R, Kovanecz I, Nolazco G, Bruhn KW, Gonzalez-Cadavid NF. Myostatin genetic inactivation inhibits myogenesis by muscle derived stem cells in vitro but not when implanted in the mdx mouse muscle. Stem Cells Res Ther. 2012 doi: 10.1186/scrt152. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ferrini MG, Moon J, Rivera S, Rajfer J, Gonzalez-Cadavid NF. Amelioration of diabetes-induced fibrosis by antioxidant and anti-TGFβ1 therapies in the penile corpora cavernosa in the absence of iNOS expression. Br J Urol. 2012;109:586–93. doi: 10.1111/j.1464-410X.2011.10397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freireich EJ, Gehan EA, Rall DP, Schmidt LH, Skipper HE. Quantitative comparison of toxicity of anticancer agents in mouse, rat, hamster, dog, monkey, and man. Cancer Chemother Rep. 1966;50:219–44. [PubMed] [Google Scholar]
- 41.Valente EG, Vernet D, Ferrini MG, Qian A, Rajfer J, Gonzalez-Cadavid NF. L-arginine and phosphodiesterase (PDE) inhibitors counteract fibrosis in the Peyronie’s fibrotic plaque and related fibroblast cultures. Nitric Oxide. 2003;9:229–44. doi: 10.1016/j.niox.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 42.Keira SM, Ferreira LM, Gragnani A, Duarte IS, Barbosa J. Experimental model for collagen estimation in cell culture. Acta Cir Bras. 2004;19S1:17–22. [Google Scholar]
- 43.Kovanecz I, Ferrini MG, Davila HH, Rajfer J, Gonzalez-Cadavid NF. Ageing related corpora veno-occlusive dysfunction in the rat is ameliorated by pioglitazone. BJU Int. 2007;2007(100):867–74. doi: 10.1111/j.1464-410X.2007.07070.x. [DOI] [PubMed] [Google Scholar]
- 44.Ii M, Nishimura H, Sekiguchi H, Kamei N, Yokoyama A, Horii M, Asahara T. Concurrent vasculogenesis and neurogenesis from adult neural stem cells. Circ Res. 2009;105:860–8. doi: 10.1161/CIRCRESAHA.109.199299. [DOI] [PubMed] [Google Scholar]
- 45.Gómez-Pinedo U, Rodrigo R, Cauli O, Herraiz S, Garcia-Verdugo JM, Pellicer B, Pellicer A, Felipo V. cGMP modulates stem cells differentiation to neurons in brain in vivo. Neuroscience. 2010;165:1275–83. doi: 10.1016/j.neuroscience.2009.11.046. [DOI] [PubMed] [Google Scholar]
- 46.Behr-Roussel D, Gorny D, Mevel K, Caisey S, Bernabé J, Burgess G, Wayman C, Alexandre L, Giuliano F. Chronic sildenafil improves erectile function and endothelium-dependent cavernosal relaxations in rats: lack of tachyphylaxis. Eur Urol. 2005;47:87–91. doi: 10.1016/j.eururo.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 47.Ferrini MG, Kovanecz I, Sanchez S, Vernet D, Davila HH, Rajfer J, Gonzalez-Cadavid NF. Long-term continuous treatment with sildenafil ameliorates aging-related erectile dysfunction and the underlying corporal fibrosis in the rat. Biol Reprod. 2007;76:915–23. doi: 10.1095/biolreprod.106.059642. [DOI] [PubMed] [Google Scholar]
- 48.Hemmrich K, Gummersbach C, Paul NE, Goy D, Suschek CV, Kröncke KD, Pallua N. Nitric oxide and downstream second messenger cGMP and cAMP enhance adipogenesis in primary human preadipocytes. Cytotherapy. 2010;12:547–53. doi: 10.3109/14653241003695042. [DOI] [PubMed] [Google Scholar]
- 49.Mujoo K, Sharin VG, Bryan NS, Krumenacker JS, Sloan C, Parveen S, Nikonoff LE, Kots AY, Murad F. Role of nitric oxide signaling components in differentiation of embryonic stem cells into myocardial cells. Proc Natl Acad Sci U S A. 2008;105:18924–9. doi: 10.1073/pnas.0810230105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang HY, Jin XB, Lue TF. Three important components in the regeneration of the cavernous nerve: brain-derived neurotrophic factor, vascular endothelial growth factor and the JAK/STAT signaling pathway. Asian J Androl. 2011;13:231–5. doi: 10.1038/aja.2010.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Clinical trial NCT01089387. Intracavernous Bone Marrow Stem-cell Injection for Post Prostatectomy Erectile Dysfunction. http://clinicaltrials.gov.