Abstract
Coxsackievirus B3 (CVB3) infection of C57Bl/6 mice shows a sex bias with males developing more severe cardiac inflammation than females because males develop a Th1 inflammatory response, whereas females develop a Th2 response. Since their discovery, Toll-like receptors have been shown to play an important role in the development of the immune response against harmful pathogens. To assess the role of TLRs in coxsackievirus-induced myocarditis wild type and Toll-like Receptor 2 −/− male and female mice were infected and assessed for viral replication, myocarditis, helper T-cell generation, and regulatory T-cell generation. TLR2−/− mice show reduced Th1 expression compared to controls. Treatment of wild type mice with either Pam3CSK4 (TLR2) or LPS (TLR4) specific TLR agonists resulted in increased Th1 expression in male and female mice and a decrease in FoxP3+ regulatory T-cells in male mice. The suppression of Tregulatory cells by TLR signaling in males but not females correlates with the increased myocarditis susceptibility of the males.
Keywords: Coxsackievirus B3, Toll-like receptors, myocarditis
Introduction
Myocarditis is a form of inflammatory heart disease that affects men 2:1 over women [1]. In our model, disease results from antigenic mimicry when infection with coxsackievirus B3 (CVB3) results in an autoimmune disease where autoreactive T-cells recognizing the viral capsid proteins target infected myocytes, then react with epitopes of cardiac myosin leading to inflammation in the heart [2]. Male and female mice replicate virus in the heart to similar levels, however male mice develop myocarditis whereas female mice do not. Several factors contribute to this sex bias in myocarditis susceptibility including estrogen, Helper T-cell response, γδ+ T-cells, Regulatory T-cells and more recently, Toll-like receptors [3–8]. Previously studies from this laboratory have shown that male mice infected with CVB3 generate a predominantly Th1 response, whereas female develop a predominantly Th2 response. This effect can be reversed by the treatment of male mice with estradiol and female mice with testosterone [9].
In recent years, much attention has been paid to Toll-like receptors (TLR) and their role in disease. TLRs are a family of proteins that play an important role in the innate immune response. Unlike the specific nature of the adaptive immune response, TLRs are preformed receptors that recognize motifs common to microbial pathogens including lipids, nucleic acids and proteins known as pathogen associated molecular patterns (PAMPs). Currently, 11 mammalian TLRs have been identified, each of which responds to a specific class of PAMP [10]. TLRs have been shown to be involved in regulation of the T-helper cell response. Stimulation of TLR2 via PAM3CSK4 has been shown to promote a Th1 response in allergic airway inflammation [11]. In addition other studies have shown that TLR2 and TLR7 promote Th1 responses [12]. Contrary to the studies which provide a role for TLRs in the Th1 response, little evidence has been provided to show a role of TLRs in promoting a Th2 response. It has been shown that TLR4 deficient mice develop an attenuated Th2 response compared to wild-type strains [13]. In addition it has been shown that in allergic asthma models, low level of inhaled LPS results in Th2 signaling, whereas inhalation of higher levels of LPS with antigen results in a Th1 response [14].
The goal of this communication is to evaluate what effects TLR2 and 4 have on the development of the helper T-cell and regulatory T-cell response in CVB3-induced myocarditis. First, male and female C57Bl/6 mice were infected and evaluated for myocarditis development and for T-helper cell and regulatory T-cell development. In addition male and female C57Bl/6 mice were infected and treated with PAM3CSK4, a specific TLR2 ligand, Ultra-Pure LPS, a specific TLR4 ligand or PBS as a control. In both male and female mice, TLR2−/− strains show reduced Th1 cell (CD4+/IFNg+) expression compared to B6 controls. No difference was observed with Th2 cell expression. Additionally, treatment of male mice with both PAM and LPS resulted in an increase in the percentage of both Th1 and Th2 cells compared to control animals. Female mice treated with PAM and LPS resulted in an increase in Th1 cells, whereas only treatment with LPS resulted in an increase in Th2 cells compared to control animals. Treatment of male mice with PAM and LPS resulted in a decrease in the number of CD4+FoxP3+ Regulatory T-cells; however no effect was observed when female mice were treated with either of these two agonists.
Materials and Methods
Mice
Male and female C57Bl/6, and C57Bl/B6 TLR2−/− mice were purchased from the Jackson Laboratories, Bar Harbor Maine. Mice were housed at the University of Vermont in sterile ventilator cages. Adult mice ages 6–8 weeks were used in all experiments. Experiments consisted of groups of a minimum of 5 mice. All experiments were reviewed and approved by the University of Vermont Institutional Animal Care and Use Committee.
Virus
The H3 variant of CVB3 was derived from an infectious cDNA clone which has been described previously [15]. Mice were infected by intra-peritoneal (i.p.) injection of 0.5ml of phosphate-buffered saline (PBS) containing 102 plaque forming units (PFU) of the virus.
Organ Viral Titers
Hearts were aseptically removed, perfused with PBS, and weighed before being homogenized in RPMI-1640 media (Mediatech, Manassas, VA) containing 2% fetal bovine serum, antibiotic/mycotic, penicillin and streptomycin. Cellular debris was removed by centrifugation at 300×g for 10 minutes and the supernatants were subjected to a series of 10-fold serial dilutions in RPMI-1640-2%FBS and titers were determined by plaque-forming assay on HeLa cell monolayers as described previously [15].
Toll-Like Receptor Agonists
Both the TLR1/2 heterodimer complex ligand Pam3CSK4, a synthetic triacylated, lipopeptide and the TLR4 ligand Ultrapure LPS isolated from E.coli 0111.B4 were purchased from Invivogen San Diego, CA. Both ligands were resuspended in endotoxin free water and diluted in PBS for i.p. injection. PAM3CSK4 was injected at a concentration of 50ug/mouse [16, 17], and UP-LPS was injected at a concentration of 20mg/kg [18, 19].
Lymphocyte Preparation
Spleens were aseptically removed and processed through a fine-mesh screen to produce single-cell suspensions. Lymphocyte suspensions were centrifuged over Histopaque (Sigma Chemical Co., St. Louis, MO).
Antibodies
APC-Cy7 or PerCp-Cy5.5 conjugated anti-CD4 (clone RMA-5), Alexa 647 conjugated anti-IL4 (clone 11B11), and PE conjugated anti-IFNγ (clone XMG1.2) were purchased from BD Pharmagin, San Diego, CA. PE anti-FoxP3 (clone FJK-16s) was purchased from eBioscience, San Diego, CA. Antibodies were diluted 1:100 in PBS containing 1% Bovine Serum Albumen (BSA). Negative controls were anti-rat IgG2a conjugated with the same fluorochromes used with the antigen-specific antibodies. All antibody mixtures contained 1:100 rat anti-mouse CD16/CD32 (Fc Block; clone 2.4G2).
Flow Cytometry
All cells were analyzed using a BD LSR II flow cytometer using a single excitation wavelength (488nm) and band filters for PerCp-Cy5.5 (695/40nm), FITC (525nm), PE (575nm) and APC-Cy7 (633nm). The excitation wavelength for Alexa 647 is 643nm and a band filter of 660/20nm. The cell population was classified for cell size (forward scatter) and complexity (side scatter). A minimum of 10,000 cells were evaluated. Positive staining was determined based on isotype controls. Results were analyzed using FlowJo version 7.6.4, Tree Star Inc. Ashland, OR.
Intracellular Cytokine staining
1×105 spleen cells were cultured for 4 hours in RPMI-1640 medium containing 10% FBS, antibiotics, 10ug brefeldin A (BFA: Sigma), 50 ng/ml phorbol 12-myristate 13-acetate PMA: Sigma) and 500ng/ml ionomycin (Sigma). The cells were washed in PBS-1% bovine serum albumin (BSA: Sigma) containing BFA (BS-BSA-BFA), incubated on ice in PBS-BSA-BFA containing 1:100 dilution of FC Block, anti-CD4, and anti-CD8a. Cells were washed with PBS-BSA-BFA, fixed for 10 minutes in 2% paraformaldehyde (PFA) and resuspended in PBS-BSA containing 0.5% saponin containing 1:100 dilutions Fc Block, Normal Rat Serum, anti-IL4, and anti-IFNγ for 15 minutes on ice. Cells were washed with PBS-BSA-saponin followed PBS-BSA and resuspended in 2% PFA.
Regulatory T-cell staining
Staining was done using the FoxP3 staining kit from eBioscience (San Diego, CA) according to manufacturer's protocol. Lymphocytes were washed with 1XPBS-1%BSA and resuspended in PBS-BSA containing 1:100 dilution of Fc Block, PerCp-Cy5.5 anti-CD25 and Alexa647 anti-CD4, and incubated for 15 minutes on ice. Cells were washed with PBS-BSA and incubated at 4°C for 2 hours in eBioscience Fixation/Permeabilization buffer. Following incubation, cells were washed once with 1XPBS followed by two washes with eBioscience Permeabilization buffer and incubated in permeabilization buffer containing 1:100 dilution of Fc Block and normal rat serum on ice for 15 minutes as a blocking step. Cells were then stained with 1:100 dilution of Fc Block, PE anti-FoxP3 and normal rat serum in permeabilization buffer for 30 minutes on ice followed by 2 washes with permeabilization buffer and resuspended in 2%PFA.
Histology
Hearts were fixed in 10% formalin, sectioned and stained with hemotoxylin and eosin. Sections were blindly evaluated by an experienced member of the laboratory on a scale of 0 to 4 where 0 represents no inflammation, 1 represents 1 to 10 lesions per section, 2 represents 11–-20 lesions per sections, 3 represents 21 to 40 lesions per section, and 4 represents greater than 40 lesions per section. Mice with a score of 0 in the pancreas were assumed to be uninfected and removed from all data analysis.
Statistical Analysis
Wilcoxon ranked-score test was used to determine differences between individual mice for histology, organ viral titers and flow cytometry using SPSS PASW Statistics 18. Mortality was measured by the Mantel-Cox Log rank test using GraphPad Prism 5.
Results
Male mice have greater cardiac inflammation despite similar viral titers
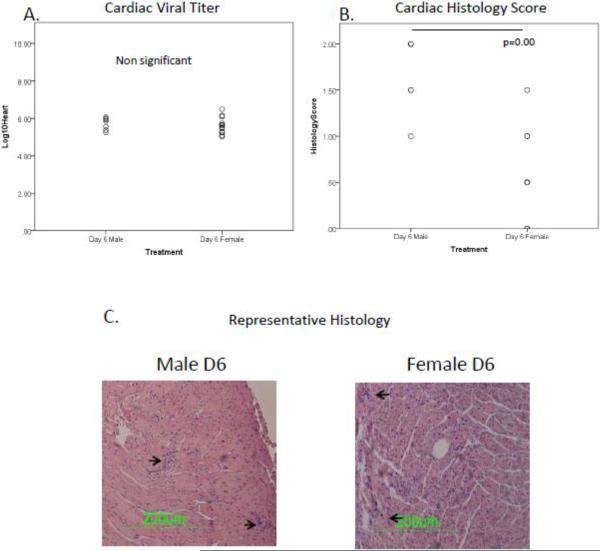
As can be seen in Figure 1, there is no significant difference in cardiac viral titers between male and female mice at Day 6p.i. (Figure 1a). Despite these similarities in viral replication, male mice have a greater myocarditis score than females at Day 6 (p=<0.001) (figure 1b). Figure 1c shows representative cardiac micrographs, arrows point to areas of inflammation. These results show that despite similar levels of viral replication in the hearts of male and female mice, male mice are more susceptible to developing myocarditis than female mice.
Figure 1.
Male mice have greater myocarditis than females despite similar levels of viral replication. Male and female C57Bl/6 mice have equivalent levels of viral replication in the heart (Figure 1a.), however male mice have a greater histology score compared to female mice (p=<0.001, Figure 1b.). Figure 1c shows a representative histology slide from both male and female mice.
No difference exists in titer or histology between wild type and TLR2−/− mice
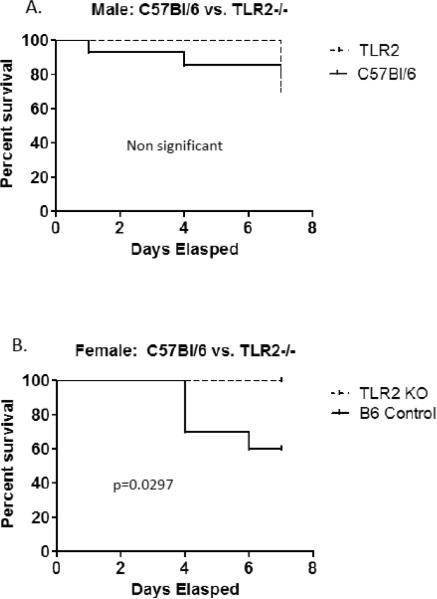
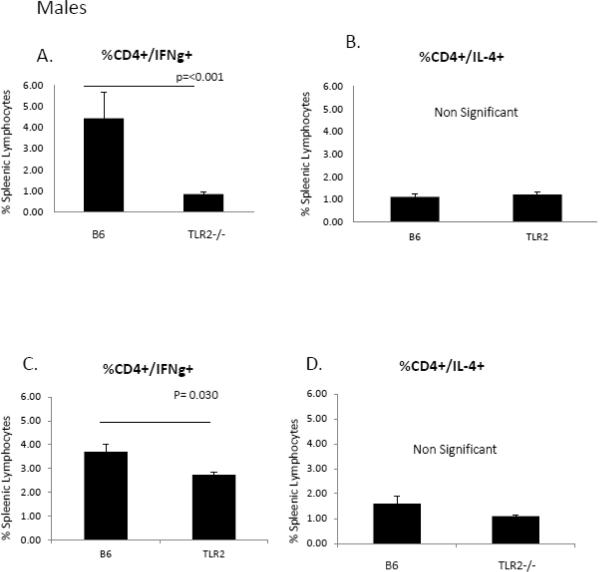
Signaling through TLR including TLR2 inhibits cardiomyocyte contractility [20], is responsible for myocardial ischemic/reperfusion injury [21] and promotes autoimmune perimyocarditis [22]. Prior studies of this laboratory demonstrated that female C57Bl/6 mice infected with CVB3 express increased TLR2 mRNA than similarly infected male mice (Roberts et al., manuscript in revision). To determine if TLR2 differences in expression determined sex bias in CVB3 myocarditis, male and female TLR2−/− mice were infected with CVB3. Survival curves are given in Figure 2 and show that mortality was decreased in both TLR2−/− male and female animals after infection. The trend did not reach significance in males but due to higher mortality in infected C57Bl/6 females, increased survival in TLR2−/− females was dramatic (p=0.0297). Hearts were evaluated for myocardial inflammation and virus titers (Figure 3). Despite increased survival, neither myocarditis or virus titers were significantly different between wild-type and TLR2−/− animals in either males or females. To evaluate whether TLR2 signaling impacts Th phenotype bias, spleen cells from male and female TLR2−/− mice 7 days after CVB3 infection were stimulated with PMA/ionomycin in the presence of brefeldin A and labeled with antibodies to CD4 and intracellularly with antibodies to IFNγ (Th1 cells) or IL-4 (Th2 cells)(Figure 4). Both female and male TLR2−/− mice have reduced Th1 cell expression (Figure 4a,c) compared to B6 controls of the same sex (p=<0.001), but no difference exists in Th2 cell expression (Figure 4b,d). Thus, as demonstrated by prior reports [11, 23], stimulating TLR2 directly influences the development of a Th1 response.
Figure 2.
Male TLR2−/− mice show no differences in titer or histology compared to control mice. B6 and TLR2−/− mice show equal levels of both viral replication (Figure 2a) and myocarditis (Figure 2b). Figure 2c shows representative histology from B6 and TLR2−/− mice.
Figure 3.
Female B6 and TLR2−/− mice have equal levels of viral replication and myocarditis. Similar to the results seen with male B6 and TLR2−/− mice, there is no difference in either viral titers (Figure 3a) or histology (Figure 3b) score seen between female B6 and TLR2−/− mice. Figure 3c shows representative histology from B6 and TLR2−/− mice.
Figure 4.
Toll-like receptor 2 contributes to the Th1 response in male and female mice. Male mice have a greater percentage of Th1 cells compared to TLR2−/− mice (4.44% vs. 0.86%, p=<0.001) (Figure 4a). Similar results were seen in female mice (3.70% vs. 2.73%, p=0.030) (Figure 4c). No difference was seen in Th2 expression between B6 and TLR2−/− mice in either male (Figure 4b) or female (Figure 4d) mice.
Treatment of mice with TLR agonist augments T-helper cell expression
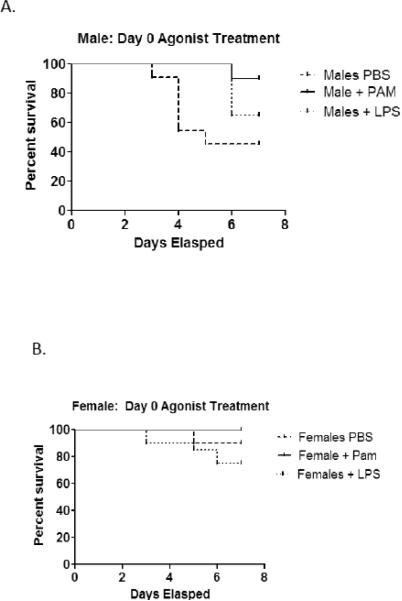
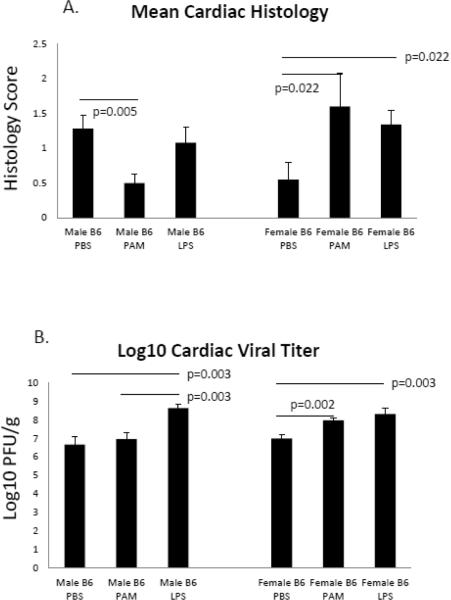
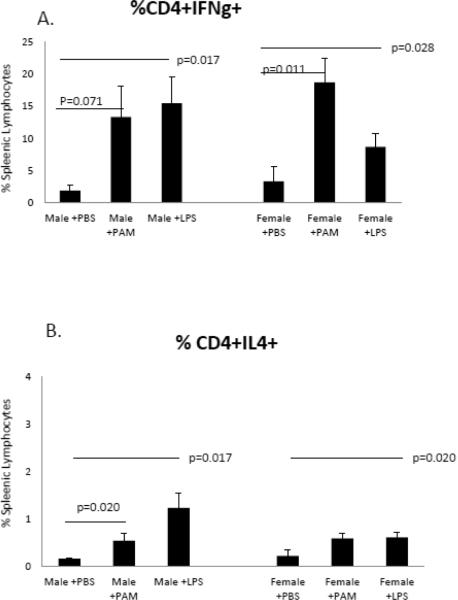
A separate approach to evaluating TLR in CVB3 myocarditis involved administering specific TLR2 (Pam3CSK4; PAM) or TLR4 (ultra-pure LPS) to wild-type C57Bl/6 male and female mice. Infected PBS control male mice had 55% mortality compared to only 10% seen in similarly treated female mice (Figure 5). PAM treatment of infected mice resulted in 10% mortality compared to control males (p=0.0261). Males treated with LPS delayed mortality but did not significantly alter mortality compared to control mice. TLR agonist treatment of female mice did not affect survival, not significant difference in mortality was observed in either PAM or LPS treated mice compared to PBS controls. Treatment of male mice with PAM, but not LPS resulted in reduced myocarditis compared to PBS controls (p=0.005) (Figure 6a). While there was no change in myocarditis when LPS was administered, male mice given LPS did have elevated viral titers compared to both PBS and PAM treatments groups (p=0.003). Both PAM and LPS treatment of female mice resulted in increased viral titers (p=0.002 and 0.003 respectively) (Figure 6b), however contrary to the results seen in male mice, treatment of females with either PAM or LPS resulted in elevated cardiac inflammation p=0.022) (Figure 6a). In both male and female mice, treatment with either PAM or LPS resulted in an increase in the percentage of Th1 cells compared to the PBS control group [(p=0.071 and 0.011 for male and female mice treated with PAM respectively) (p=0.017 and p=0.028 for male and female mice treated with LPS respectively] (Figure 7a). However, PAM treatment was significantly more effective in promoting Th1 bias in females compared to LPS (p=0.011 vs. 0.028 respectively). Both PAM and LPS slightly increased Th2 cell percentages in male mice (Figure 7b). Only LPS produced a small but significant increase in Th2 cells in females (Figure 7b).
Figure 5.
TLR2 agonist treatment effects mortality in male, but not female mice. Male mice treated with the TLR2-specific agonist, PAM3CSK4, resulted in 10% mortality compared to 55% mortality seen with PBS control mice (p=0.0261). LPS treatment had no effect or the survival of male mice. Neither PAM not LPS treatment had any effect on the survival of female mice compared to PBS controls.
Figure 6.
TLR agonist treatment effects myocarditis. Male mice treated with PAM, but not LPS caused reduced myocarditis compared to PBS control mice (p=0.005). Treating female mice with either PAM or LPS resulted in elevated levels of cardiac inflammation (p=0.022) (Figure 6a). LPS, but not PAM treatment of male mice caused increased levels of viral replication compared to both PBS and PAM treated males (p=0.003). Both PAM and LPS treatment of female mice caused elevated viral titers compared to PBS controls (p=0.002 and 0.003 respectively) (Figure 6b).
Figure 7.
TLR agonist treatment augments T-helper cell expression. Treatment of male mice with LPS (x=15.47) results in an increase in the percentage of Th1 cells over the PBS group (x=1.93) p=0.017. Both PAM (x=18.69) and LPS (x=8.71) result in an increase in the percentage in Th1 cells in female mice compared to the PBS group (x=3.38), p=0.011 and 0.028 respectively (Figure 7a). Treatment of male mice with either PAM (x=0.54) or LPS (x=1.23) results in an in an increase in the percentage of Th2 cells compared to the PBS group (x=0.16), p=0.020 and 0.017 respectively). Only when given LPS (x=0.61) do we see an increase in the percentage of Th2 in female mice when compared to the PBS group (x=.22), p=0.020 (Figure 7b).
Treatment with TLR agonist alters generation of Regulatory T-cells in male but not female mice
T regulatory cells (CD4+FoxP3+), in addition to Th1 cells, control CVB3 induced myocarditis susceptibility in CVB3 infected female mice [24]. To determine if TLR signaling alters Tregulatory response in male and female mice, spleen cells from mice treated with PAM or LPS were labeled with antibodies to CD4 and FoxP3, the transcription factors implicated in Tregulatory cell generation (Figure 7). Treating male mice with LPS significantly decreased the percentage (p=0.039) of Tregulatory cells. Neither PAM nor LPS treatment had any significant effect on T regulatory cells in female mice.
Discussion
The primary novel observation in the present communication is that while signaling through TLR4 both increases Th1 cell responses in male and female mice as reported by others previously in other disease model systems, there is a dramatic sex difference in the effects of TLR signaling in the Tregulatory cell response. Both male and female TLR2−/− mice have reduced Th1 cell expression, but not Th2 cell expression compared to wild-type controls. In addition, treatment of both male and female mice with either a TLR2 or TLR4-specific agonist results in an increase in the percentage of Th1 cells in the spleen compared to control animals. Studies from other laboratories have shown that such a relationship exists between TLR2 stimulation and Th1 responses. Patel et al [11] showed that administration of PAM3CSK4 to mice previously sensitized with OVA promoted a Th1 cell response and reduced the inflammatory cell infiltrate in bronchoalveolar lavage fluid during allergic airway inflammation. Imanishi et at all showed that stimulation of TLR2 with PAM3CSK4 resulted in the direct activation of Th1 cells in the absence of TCR stimulation [25]. Similarly, signaling through TLR4 is capable of promoting a Th1 response. Re and Strominger stimulated TLR2 and TLR4 on human dendritic cells with PAM and LPS respectively and found that when stimulated with LPS, hDCs produced IL-12, p70 and IP-10 which promote IFNg production by Th1 cells. However, in Re's study, PAM stimulation did not increase Th1 cell response [26]. Helmby and Grencis showed that both C3H/HeJ (which have a natural mutation in the TLR4 gene) and MyD88−/− mice are resistance to intestinal nematode infection and that these animals produce a predominantly Th2 response in contrast to wild-type mice which are susceptible and generate a Th1 response [27]. Why both of these TLRs can promote a Th1 response is likely due to the shared adaptor protein, MyD88. In addition to the studies by Helmby and Grencis, Schnare et al showed that MyD88−/− mice are defective in their ability to activate antigen-specific Th1 responses but not Th2 responses [28].
In addition our data show that treating infected male mice with either UP-LPS or PAM3CSK4 resulted in a decrease in the percentage of regulatory T-cells define by the CD4+/FoxP3+ phenotype in male mice, but not in infected female mice. These results are contrary to those obtained by other groups who saw an increase in Treg activity in response to TLR2 stimulation. Netea et al who treated female TLR2−/− mice with Candidia albicans observed a 50% decrease in Treg populations (defined by the CD4+CD25+ phenotype) in TLR2−/− mice compare to wild-type controls. They confirmed in vitro that survival of Tregs was induced by TLR2 agonists (peptidoglycan) [29]. Studies by Liu et al further confirmed that treatment with PAM3CSK4 and anti-CD3 resulted in the proliferation of CD4+/CD25+/FoxP3+ Tregs in vitro [30]. Why these differences exist is unclear, however the answer may be found in the differences between our methods. In our experiments, PAM3CSK4 was administered in vivo at the time of infection. Liu et al however incubated isolated Treg cells in vitro for three days with PAM3CSK4. Netea et al used similar methods to test the role of TLR2 agonists on Treg generation. Interestingly, Sutmuller et al showed that while Treg stimulation with PAM3CSK4 resulted in proliferation, the suppressive nature of Tregs is abolished indicating that TLR is important for Treg generation, but when stimulated on the cells themselves, they inhibit their function [31]. One possible explanation as to why we see a diminished Treg response could be due to the generation of T-cells displaying the T-cell receptor. While not evaluated in these experiments, previous data from our laboratory have shown that the H3 CVB3 variant induces γδ+ T-cells which prevent the generation of regulatory T-cells [32]. Male and female mice preferentially generate different subsets of γδ+ T-cells resulting in different disease outcomes, males generate Vγ4+ cells and females generate Vγ1+ cells which promote a Th1 or Th2 response respectively [33]. Some evidence has been provided to show that TLR2 signaling can affect γδ+ T-cell function. Deetz et al reported that TLR2 is present on Vγ2 Vδ2 cells in low amounts and that signaling through TLR2 specifically increases IFNγ release from this γδ+ cell subset [34].
Previous data from this lab has shown that female mice preferentially activate Tregs which help to confer a resistance to myocarditis whereas male mice do not. Data from our lab also shows that at Day 3 post infection male mice show an upregulation in TLR4 compared to similarly treated females (manuscript in revision). Treatment of female mice with either PAM or LPS does not affect Treg expression; however treatment with either ligand resulted in reduced Treg expression compared to control animals in males. Why both TLR2 and TLR4 treated male mice have reduced Treg expression is unclear, however the upregulation of TLR4 at the early infection time point in males may in part be the reason why these animals fail to develop the necessary protective response. While we have shown here that stimulation of male mice with either a TLR2 or a TLR4 ligand reduces the expression of regulatory T-cells, it should be noted that these same effects have not been evaluated in humans and one should take care when applying these results to a clinical setting.
Figure 8.
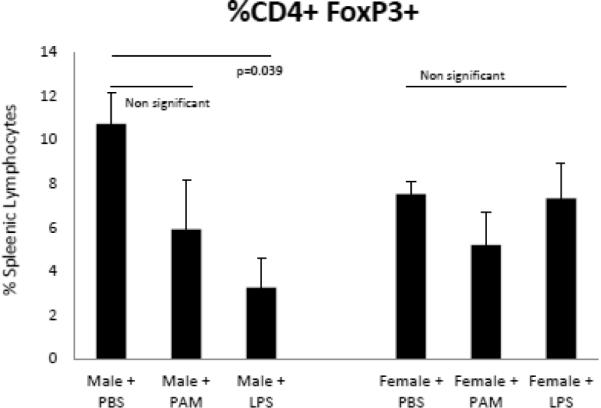
TLR4 agonist treatment suppresses Regulatory T-cell expression in male mice. Treatment of male B6 mice with UP-LPS (TLR4 specific agonist) results in the reduction of CD4+FoxP3+ Regulatory T-cells (10.76% vs., 3.45% respectively p=0.030). Treatment of male mice with PAM3CSK4, a TLR2-specific agonist had no effect on Treg expression. No effect was seen on female mice when treated with either UP-LPS or PAM3CSK4 compared to PBS controls.
Acknowledgements
The authors would like to thank the following people:
Colette Charland and Julie Wolfe of the Flow Cytometry Core Facility at the University of Vermont.
Alan Howard of the University of Vermont Statistics Counceling Center.
Pamela Burton for help in preparation of the manuscript.
The research was supported by HL108371 from the NHLBI, PO1 AI045666 from NIAID, and by T20-RR021905 from the NCRR.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References Cited
- 1.Woodruff J. Viral myocarditis. Am. J. Pathol. 1980;101:425–483. [PMC free article] [PubMed] [Google Scholar]
- 2.Huber S, Cunningham M. Streptococcal M protein peptide with similarity to myosin induces CD4+ T cell dependent myocarditis in MRL/++ mice and induces partial tolerance against coxsackiebiral myocarditis. J Immunol. 1996;156:3528–3534. [PubMed] [Google Scholar]
- 3.Huber SA, Born W, O'Brien R. Dual functions of murine gammadelta cells in inflammation and autoimmunity in coxsackievirus B3-induced myocarditis: role of Vgamma1+ and Vgamma4+ cells. Microbes Infect. 2005;7(3):537–43. doi: 10.1016/j.micinf.2004.12.011. [DOI] [PubMed] [Google Scholar]
- 4.Huber SA, Feldman AM, Sartini D. Coxsackievirus B3 induces T regulatory cells, which inhibit cardiomyopathy in tumor necrosis factor-alpha transgenic mice. Circ Res. 2006;99(10):1109–16. doi: 10.1161/01.RES.0000249405.13536.49. [DOI] [PubMed] [Google Scholar]
- 5.Huber S, et al. Cytokine Production by Vgamma+ T Cell Subsets is an Important Factor Determining CD4+ Th Cell Phenotype and Susceptibility of BALB/c mice to Coxsackievirus B3-Induced Myocarditis. J Virol. 2001;75(13):5860–5868. doi: 10.1128/JVI.75.13.5860-5869.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Huber S, Kupperman J, Newell M. Hormonal regulation of CD4+ T-cell responses in coxsackievirus B3-induced myocarditis in mice. J Virol. 1999;73:4689–4695. doi: 10.1128/jvi.73.6.4689-4695.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Huber S, Moraska A, Choate M. T cells expressing the gamma delta T-cell receptor potentiate coxsackievirus B3-induced myocarditis. J. Virol. 1992;66 doi: 10.1128/jvi.66.11.6541-6546.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frisancho-Kiss S, et al. Cutting edge: cross-regulation by TLR4 and T cell Ig mucin-3 determines sex differences in inflammatory heart disease. J Immunol. 2007;178(11):6710–4. doi: 10.4049/jimmunol.178.11.6710. [DOI] [PubMed] [Google Scholar]
- 9.Huber S, Pfaeffle B. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with Coxsackievirus Group B Type 3. J Virol. 1994;68:5126–5132. doi: 10.1128/jvi.68.8.5126-5132.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Underhill DM. Mini-review Toll-like receptors: networking for success. European Journal of Immunology. 2003;33(7):1767–1775. doi: 10.1002/eji.200324037. [DOI] [PubMed] [Google Scholar]
- 11.Patel M, et al. TLR2 Agonist Ameliorates Established Allergic Airway Inflammation by Promoting Th1 Response and Not via Regulatory T Cells. The Journal of Immunology. 2005;174(12):7558–7563. doi: 10.4049/jimmunol.174.12.7558. [DOI] [PubMed] [Google Scholar]
- 12.Dabbagh K, Lewis DB. Toll-like receptors and T-helper-1/T-helper-2 responses. Current Opinion in Infectious Diseases. 2003;16(3):199–204. doi: 10.1097/00001432-200306000-00003. [DOI] [PubMed] [Google Scholar]
- 13.Dabbagh K, et al. Toll-Like Receptor 4 Is Required for Optimal Development of Th2 Immune Responses: Role of Dendritic Cells. The Journal of Immunology. 2002;168(9):4524–4530. doi: 10.4049/jimmunol.168.9.4524. [DOI] [PubMed] [Google Scholar]
- 14.Eisenbarth SC, et al. Lipopolysaccharide-enhanced, Toll-like Receptor 4–dependent T Helper Cell Type 2 Responses to Inhaled Antigen. The Journal of Experimental Medicine. 2002;196(12):1645–1651. doi: 10.1084/jem.20021340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Knowlton KU, et al. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J Virol. 1996;70(11):7811–8. doi: 10.1128/jvi.70.11.7811-7818.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanchez PJ, et al. Combined TLR/CD40 Stimulation Mediates Potent Cellular Immunity by Regulating Dendritic Cell Expression of CD70 In Vivo. The Journal of Immunology. 2007;178(3):1564–1572. doi: 10.4049/jimmunol.178.3.1564. [DOI] [PubMed] [Google Scholar]
- 17.Longhi MP, et al. Dendritic cells require a systemic type I interferon response to mature and induce CD4+ Th1 immunity with poly IC as adjuvant. The Journal of Experimental Medicine. 2009;206(7):1589–1602. doi: 10.1084/jem.20090247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, et al. Regulation of Toll-like receptor–mediated inflammatory response by complement in vivo. Blood. 2007;110(1):228–236. doi: 10.1182/blood-2006-12-063636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saunders SP, et al. C-Type Lectin SIGN-R1 Has a Role in Experimental Colitis and Responsiveness to Lipopolysaccharide. The Journal of Immunology. 2010;184(5):2627–2637. doi: 10.4049/jimmunol.0901970. [DOI] [PubMed] [Google Scholar]
- 20.Boyd JH, et al. Toll-like receptor stimulation in cardiomyoctes decreases contractility and initiates an NF-kappaB dependent inflammatory response. Cardiovasc Res. 2006;72(3):384–93. doi: 10.1016/j.cardiores.2006.09.011. [DOI] [PubMed] [Google Scholar]
- 21.Arslan F, et al. Myocardial Ischemia/Reperfusion Injury Is Mediated by Leukocytic Toll-Like Receptor-2 and Reduced by Systemic Administration of a Novel Anti–Toll-Like Receptor-2 Antibody. Circulation. 2010;121(1):80–90. doi: 10.1161/CIRCULATIONAHA.109.880187. [DOI] [PubMed] [Google Scholar]
- 22.Popovic ZV, et al. The Proteoglycan Biglycan Enhances Antigen-Specific T Cell Activation Potentially via MyD88 and TRIF Pathways and Triggers Autoimmune Perimyocarditis. The Journal of Immunology. 2011;187(12):6217–6226. doi: 10.4049/jimmunol.1003478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Imanishi T, et al. Apoptosis of vascular smooth muscle cells is induced by Fas ligand derived from monocytes/macrophage. Atherosclerosis. 2002;161(1):143–51. doi: 10.1016/s0021-9150(01)00631-1. [DOI] [PubMed] [Google Scholar]
- 24.Huber SA. Coxsackievirus B3-induced myocarditis: Infection of females during the estrus phase of the ovarian cycle leads to activation of T regulatory cells. Virology. 2008 doi: 10.1016/j.virol.2008.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Imanishi T, et al. Cutting Edge: TLR2 Directly Triggers Th1 Effector Functions. The Journal of Immunology. 2007;178(11):6715–6719. doi: 10.4049/jimmunol.178.11.6715. [DOI] [PubMed] [Google Scholar]
- 26.Re F, Strominger JL. Toll-like Receptor 2 (TLR2) and TLR4 Differentially Activate Human Dendritic Cells. Journal of Biological Chemistry. 2001;276(40):37692–37699. doi: 10.1074/jbc.M105927200. [DOI] [PubMed] [Google Scholar]
- 27.Helmby H, Grencis RK. Essential role for TLR4 and MyD88 in the development of chronic intestinal nematode infection. European Journal of Immunology. 2003;33(11):2974–2979. doi: 10.1002/eji.200324264. [DOI] [PubMed] [Google Scholar]
- 28.Markus Schnare GB, Holt Agnieszka Czopik, Takeda Kiyoshi, Akiran Shizuo, Medzhitov Ruslan. Toll-Like Receptors control activation of adaptive immune responses. Nature Immunology. 2001;2:947–950. doi: 10.1038/ni712. [DOI] [PubMed] [Google Scholar]
- 29.Netea MG, et al. Toll-like receptor 2 suppresses immunity against Candida albicans through induction of IL-10 and regulatory T cells. J Immunol. 2004;172(6):3712–8. doi: 10.4049/jimmunol.172.6.3712. [DOI] [PubMed] [Google Scholar]
- 30.Liu H, et al. Toll-like receptor 2 signaling modulates the functions of CD4+CD25+ regulatory T cells. Proceedings of the National Academy of Sciences. 2006;103(18):7048–7053. doi: 10.1073/pnas.0601554103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sutmuller RPM, et al. Toll-like receptor 2 controls expansion and function of regulatory T cells. The Journal of Clinical Investigation. 2006;116(2):485–494. doi: 10.1172/JCI25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Huber SA. Depletion of gammadelta+ T cells increases CD4+ FoxP3 (T regulatory) cell response in coxsackievirus B3-induced myocarditis. Immunology. 2009;127(4):567–76. doi: 10.1111/j.1365-2567.2008.03034.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huber S, et al. Vgamma1+ T Cells Suppress and Vgamma4+ T Cells Promote Susceptibility to Coxsackievirus B3-Induced Myocarditis in Mice. J Immunol. 2000;165:4174–4181. doi: 10.4049/jimmunol.165.8.4174. [DOI] [PubMed] [Google Scholar]
- 34.Deetz CO, et al. Gamma Interferon Secretion by Human Vγ2Vδ2 T Cells after Stimulation with Antibody against the T-Cell Receptor plus the Toll-Like Receptor 2 Agonist Pam3Cys. Infection and Immunity. 2006;74(8):4505–4511. doi: 10.1128/IAI.00088-06. [DOI] [PMC free article] [PubMed] [Google Scholar]