Abstract
The production of anti-donor antibodies to HLA class I and class II antigens following transplantation is associated with development of transplant vasculopathy and graft loss. Antibodies against HLA class I (HLA-I) molecules are thought to contribute to transplant vasculopathy by triggering signals that elicit the activation and proliferation of endothelial cells. The proximal molecular events that regulate HLA-I dependent signal transduction are not well understood. We demonstrated a mutual dependency between HLA-I and integrin β4 to stimulate signal transduction and cell proliferation. Similarly, we found that integrin β4-mediated cell migration was dependent upon its interactions with HLA-I molecules. Since integrin β4 has been implicated in angiogenesis and tumor formation, associations between integrin β4 and HLA-I may play an important role in cancer. Further characterization of interactions between HLA-I and integrin β4 may lead to the development of therapeutic strategies for the treatment and prevention of chronic allograft rejection and cancer.
Keywords: Antibody Mediated Rejection, Endothelial Cells, HLA class I, Integrin β4, Signal Transduction
1. Introduction
Improvements in immunosuppression and patient management have significantly reduced cell mediated rejection, but antibody mediated rejection remains a main obstacle to long-term survival of solid organ transplants [1, 2]. Numerous studies have shown that patients producing post-transplant anti-donor antibodies to HLA are at a higher risk of chronic rejection and graft loss [3-5]. The contribution of HLA-I antibodies to the development of transplant vasculopathy has also been demonstrated in several experimental murine allograft models. Passive transfer of anti-donor MHC-I antibodies in immunodeficient RAG1 knockout mice leads to the development of characteristic features of antibody mediated rejection and transplant vasculopathy [6-9]. Chronic rejection manifests as transplant vasculopathy which is characterized by intimal proliferation of the vessels of the allograft [10-13]. Histologically, the vessels of the graft show perivascular fibrosis, smooth muscle cell proliferation accompanied by subendothelial lymphocytes and macrophages [14, 15]. Proliferation is a central feature of transplant vasculopathy lesions and grafts show increased expression of mitogenic factors, such as PDGF, TGFα and TGFβ, and vascular endothelial growth factor, an essential soluble factor which regulates angiogenesis [16-18].
The endothelium is located at the interface between the allograft and recipient blood and is directly targeted by HLA-I antibodies. HLA-I antibodies can cause endothelial injury by activating complement [19]. Antibodies to HLA can also lead to recruitment and activation of neutrophils through P-selectin, NK cells and macrophages via Fc receptors [8, 20]. Activation of NK cells and macrophages leads to the secretion of cytokines which can mediate neointimal thickness and transplant vasculopathy [8, 21]. While Fc and complement interactions are important contributors to acute antibody-mediated rejection, transplant vasculopathy can occur in the absence of complement deposition [19]. Studies from our group and others have shown that the signaling events elicited by crosslinking HLA-I with antibodies in endothelial cells and smooth muscle cells contribute to the process of transplant vasculopathy [10, 22]. The proximal signaling events that trigger the HLA-I dependent signaling cascades are not well understood. HLA-I does not have intrinsic kinase activity, suggesting it must associate with other molecules that have the capacity to transduce intracellular signals. The mechanisms underlying how HLA-I molecules transduce signals in endothelial and smooth muscle cells will be the focus of this review.
2. HLA Antibodies induce survival and proliferation signaling pathways in endothelial cells
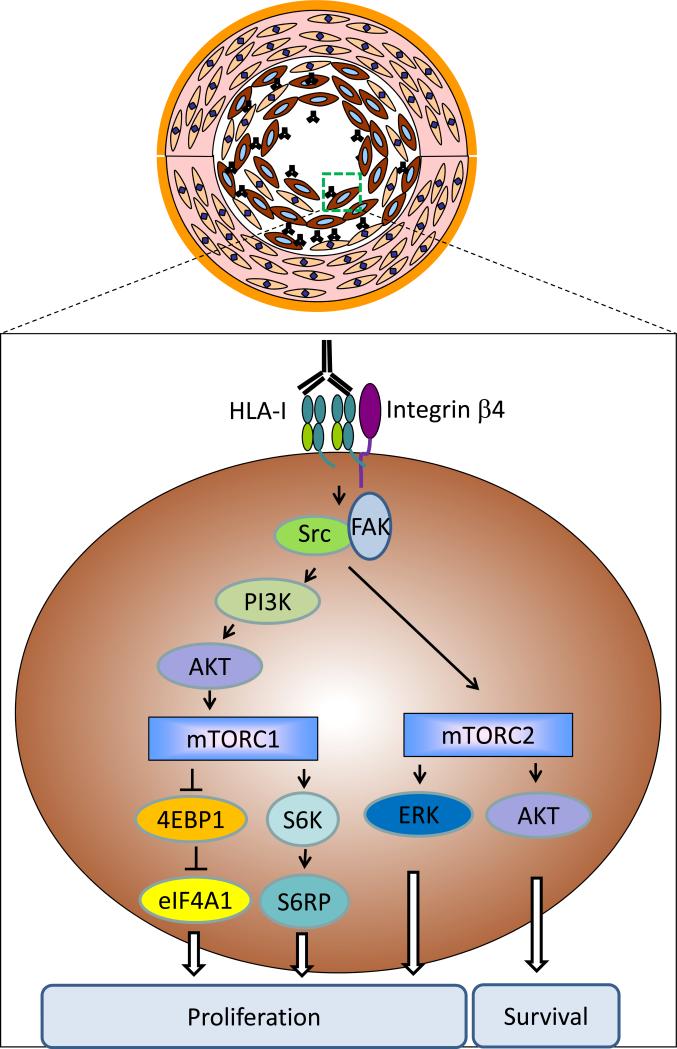
Studies by our group and others have shown that ligation of HLA-I on cultured endothelial cells with antibodies stimulates cell proliferation and stress fiber formation through RhoA, Src and focal adhesion kinase (FAK) signaling in endothelial cells [10, 23, 24]. Phosphorylation of Src and FAK leads to the activation of the PI3K/Akt pathway which initiates cell survival and proliferation pathways via mTOR signaling. mTOR exists in two distinct molecular complexes: mTOR complex 1 (mTOR1) and mTOR complex 2 (mTOR2). HLA-I-mediated activation of mTORC1 causes the phosphorylation of several downstream targets including S6 kinase (S6K) and 4E-BP1 which in turn increase protein synthesis and cell proliferation via S6 ribosome protein (S6RP) and eukaryotic translation initiation factor 4A1 (EIF4A1) [24, 25]. Activation of mTORC2 stimulates cell proliferation and survival via the ERK and Akt signaling pathways [25, 26]. These in vitro findings are supported by in vivo studies performed in a murine heart allograft model in which phosphorylation of the signaling molecules involved in the MHC-I mediated proliferation and survival pathways are switched on in the endothelium of MHC-I antibody treated mice [27].
We investigated the molecular basis of HLA-I to stimulate survival and proliferation of endothelial cells. Integrins are cell adhesion molecules that mediate attachment between the cell and the extracellular matrix. Integrins also have capacity to transduce signals that regulate cell proliferation, survival and migration. Upon ligand binding, integrins activate various kinases, including FAK, Src, PI3K, and ERK [28]. Integrin β4 is mainly expressed in epithelial cells and endothelial cells. Integrin β4 pairs with integrin α6 to form a functional dimer to bind its ligand extracellular matrix laminin. Upon binding to laminin, integrin β4 facilitates cell attachment to the extracellular matrix through an adhesion structure called the hemidesmosome. Integrin β4 differs from other integrins by a long cytoplasmic tail that has been shown to interact with FAK and Src to elicit cell survival and proliferation signals [29, 30]. Given the similarity between the integrin β4 and HLA-I signaling pathways, we questioned whether HLA-I partnered with integrin β4 to tranduce signals. We found that ligation of HLA-I with antibodies induced complex formation between integrin β4 and HLA-I [31]. Furthermore, we demonstrated that depletion of integrin β4 inhibited the phosphorylation of Src, ERK and AKT, and cell proliferation induced by HLA-I antibodies. Our findings suggest anti-donor HLA-I antibodies cause transplant vasculopathy by stimulating endothelial cell proliferation and migration via integrin β4 signaling.
The role of integrin β4 in transplant vasculopathy is supported by previous studies showing increased expression of integrin β4 in the endothelium of thoracic aorta with atherosclerosis [32].
Previously we showed that the capacity of HLA-I to transduce signals is dependent upon the degree of molecular aggregation of the HLA-I molecules which is dependent upon the level of HLA-I expression and HLA antibody titer [33]. Ligation of HLA-I molecules with high titer antibodies stimulates intracellular signals that synergize with FGF receptors to stimulate endothelial cell proliferation via the MAPK signaling pathway [33]. On the other hand, ligation of HLA-I molecules with low titer antibodies activates the PI3K/Akt and mTORC2 pathways and upregulates cell survival proteins on the endothelium including Bcl-2 and Bcl-xL [33]. Expression of cell survival proteins protects the endothelium from cytotoxic T cell-mediated and complement-mediated damage [34]. It is plausible that intravenous immunoglobulin and/or plasmapheresis that are widely used in desensitization protocols to deplete donor specific HLA antibodies, can skew the HLA-I signaling pathway towards activation of the PI3K/Akt pathway promoting endothelial cell survival [35]. Additionally, regimens directly targeting B-cells, such as the proteasome inhibitor Bortezomib and the CD20 antibody Rituximab may also decrease antibody production, which may favor activation of cell survival signaling pathways [36-39]. However, we poster that long-term exposure of the endothelium to low levels of HLA-I antibodies will ultimately result in transplant vasculopathy because low levels of HLA-I antibody may simultaneously activate the mTORC1 signaling pathway, protein synthesis and proliferation. This idea is supported by data in a murine model of antibody-mediated rejection where long-term (>15 days), but not short-term (<15 days) exposure to anti-MHC antibodies promoted development of transplant vasculopathy [6].
Our demonstration of a mutual dependency between integrin β4 and HLA-I raises the question whether integrin β4 is also recruited during the crosslinking of HLA-I by the T cell receptor on lymphocytes or by the killer cell immunoglobulin like receptor (KIR) on natural killer cells. This concept is consistent with recent data showing that engagement of MHC-I in neurons by the T cell receptors on CD8+ T cells alters the electrophysiological properties of neurons by increasing the frequency of grouped action potentials or bursts over the neuronal network [40]. This suggests that similar to crosslinking of HLA-I with antibodies, ligation of HLA-I with the T cell receptor elicits intracellular signals. Ligation of HLA-I with KIR expressed on NK cells will likely have similar effect on triggering HLA-I signaling.
To further dissect the molecular basis of HLA-I and integrin β4 interactions, we generated deletion mutants of HLA-I and demonstrated that the cytoplasmic domain of the HLA-I heavy chain was required for the association with integrin β4 [31]. Deletion of the cytoplasmic domain abolished HLA-I antibody stimulated signaling and cell proliferation. We are currently investigating the domains of integrin β4 which are required for association with HLA-I. Integrin β4 has two primary functions: 1) mediating cell adhesion to the extracellular matrix through structures called hemidesmosomes and 2) transducing signals that regulate cell shape, motility and proliferation. The distal region of the integrin β4 tail appears dispensable for cell adhesion function in vivo because mice lacking the C-terminal portion of the integrin β4 tail, downstream of amino acid 1355, have a structurally well-defined hemidesmosome and live a normal lifespan [41]. However this portion of the integrin β4 tail is required for signal transduction and is referred as the signaling domain of integrin β4. Deletion of the signaling domain specifically inhibits integrin β4 signaling that is required for endothelial cell invasion at the beginning of the invasive phase of angiogenesis. Mice carrying a targeted deletion of this integrin β4 signaling domain exhibit defective neoangiogenesis in response to tumor xenografts. [41]. These findings suggest that the signaling function of integrin β4 can be teased apart from the cell adhesion function. It is suggested that agents specifically targeting integrin β4 signaling, but not affecting adhesion function, will not have significant toxicity in patients [30]. Development of novel approaches to block interactions between integrin β4 and HLA-I may lead to development of therapeutic strategies to prevent HLA-I antibody induced transplant vasculopathy.
3. Role for HLA-I and integrin β4 molecules in tumor progression
Integrin β4 was first identified as a tumor antigen as its expression was increased in several types of tumors and correlated with tumor motility [42, 43]. Expression of integrin β4 facilitates colorectal carcinoma cell invasion in Matrigel [44]. Using in vivo models it was shown that blocking integrin β4 function by specific antibodies produced a significant reduction in cancer cell extravasation and migration [45]. Integrin β4 stimulates tumor cell migration and invasion through the Akt and/or ERK signaling pathways. In addition, previous studies showed that integrin β4 signaling can stimulate tumor invasion via matrix metalloproteinase 2 (MMP-2), which is critical for the degradation of basement membrane [46]. Recent studies have shown that HLA-I antibodies are also mitogenic for smooth muscle cells through a signaling mechanism involving matrix metalloproteinases (MMPs) (membrane type 1 MMP and MMP-2). Silencing of MMP-2 blocks this mitogenic signaling and subsequent DNA synthesis. Furthermore, pharmacological inhibitors of MMPs reduced intimal thickening and transplant vasculopathy induced by HLA-I antibodies in vivo [47]. The role of MMPs in transplant vasculopathy was confirmed in an independent study where the use of the MMP inhibitor (ONO-4817) prevented neointimal proliferation in a rat cardiac transplant model [48] .
Integrin β4 signaling not only advances tumor progression but also promotes tumor angiogenesis. Inhibition of integrin β4 signaling in endothelial cells prevents cell migration, invasion and neoangiogenesis in vivo [41]. It has been shown that integrin β4 stimulates tumor angiogenesis via the ERK signaling pathway [41]. Our data demonstrate that HLA-I plays a critical role in angiogenesis. Knockdown of HLA-I or β2 microglobulin in endothelial cells was accompanied by a loss in the capacity of laminin-5 to induce the phosphorylation of ERK. In contrast, siRNA inhibition of HLA-I had no effect on integrin β1 capacity to induce ERK phosphorylation upon binding to collagen [31]. In addition, depletion of HLA-I reduced cell migration in response to laminin-5. These findings implicate HLA-I in the process of angiogenesis. It is interesting to note that integrin β4 can act in concert with a discrete set of proteins to facilitate the aggressive behavior of certain breast tumors [49] and non-small cell lung carcinomas [50]. Given the role of integrin β4 in migration, invasion and metastasis of tumor cells, increased expression of HLA-I may promote angiogenesis by augmenting integrin β4 signaling. In fact, increased expression of HLA-I is associated with tumor progression in gastric cancer [51] in which the expression of integrin β4 is also up-regulated [52]. Furthermore, tumor progression is often associated with inflammation [53]. The contribution of inflammation to the progression of gastric cancer has long been appreciated [54, 55]. Up-regulation of HLA-I in the inflammatory microenvironment, such as in gastric cancer, may further enhance integrin β4 signaling and promote tumor progression. On the other hand, since integrin β4 needs HLA-I to activate ERK signaling and to promote cell migration, reduced expression of HLA-I may limit the ability of integrin β4 to promote tumor growth. Reduced HLAI expression in non-small cell lung cancer and breast cancer was reported to be associated with improved survival [56, 57].
4. Role for HLA-I and integrin β4 in neuronal development
A key question that remains is what is the physiologic role for HLA-I:integrin β4 interactions? Recent data suggest that HLA-I and integrin β4 are important in neuronal development. In addition to epithelial cells and endothelial cells, integrin β4 is also expressed in neuronal cells including astrocytes and Schwann cells [58-60]. Integrin β4 is implicated in the migration of neural precursors [61-63], suggesting that integrin β4 plays a critical role in brain development [64].
Historically, it was believed that the brain was immunologically privileged and neurons did not express HLA-I. However recent studies clearly demonstrate that HLA-I molecules are important for neuronal differentiation, synaptic plasticity, ionotropic receptor traffic and brain development [65, 66]. It appears that HLA-I interacts with similar proteins found in the immune system to mediate its function in neurons. CD3ζ, a signaling component of T-cell receptors, Ly49, a member of KIR family and paired-immunoglobulin-like receptor B (PIR-B) are putative receptors for MHC-I in neurons [67, 68]. MHC-I enhances regeneration of neurons in mice [67] and the absence of surface expression of MHC-I in TAP1 (transporter associated with antigen processing–1) or β2 microglobulin knockout mice impairs the ability of neurons to regenerate axons [69]. The mouse strain A/J in which the expression of MHC-I is increased dramatically after axotomy, shows a strong axonal re-growth potential [70]. On the other hand, the C57BL/6J mouse strain in which the expression of MHC-I is lower than the A/J mouse displays a comparatively poorer regenerative potential [70]. Interestingly, conditional deletion of the integrin β4 in Schwann cells also delays regeneration of axons [71], indicating MHC-I and integrin β4 have the similar functions in axon regeneration. Furthermore, the differentiation of neuronal stem cells induced by erythropoietin is linked with increased expression of HLA-I, suggesting HLA-I is involved in neuron stem cell differentiation [72]. Integrin β4 signaling can promote neuronal stem cell differentiation in vitro as well [73]. Taken together, these findings suggest that HLA-I:integrin β4 interactions play critical roles in neuronal development. Our studies provide a potential mechanistic explanation for these findings. Specifically, it is plausible that the integrin β4 and HLA-I form a signaling complex that mediates axonal growth in neurons and/or promotes differentiation of neuron stem cells.
5. Challenges and future directions
It is conceivable that increased expression of HLA-I and/or integrin β4 can augment signal transduction. The expression of HLA-A, HLA-B and HLA-C is regulated in a tissue specific manner. For example, HLA-A and HLA-B are expressed at higher level than HLA-C in human umbilical vein endothelial cells, whereas HLA-A and HLA-C are expressed more than HLA-B in cervical cancer Hela cells [74]. This difference may be caused by distinct cis elements in promoter regions of HLA-A, HLA-B and HLA-C, and transcription factors available in specific types of cells. Whether HLA-A, HLA-B and HLA-C differ in their capacity to transduce signals in distinct tissues is not clear yet. Polymorphisms outside of the promoter region can also affect the expression of HLA-I. An extreme example is HLA-C*04:09N. This allele has a point deletion in exon 7 which shifts the open reading frame, and causes elongation of the cytoplasmic domain and failed surface expression [75]. Polymorphisms in the HLA-I molecule may also affect its association with integrin β4. Recent studies by Jin et. al. indicate a differential capacity of specific HLA alleles to form a molecular complex with integrin β4 [76]. These results may explain different outcomes in transplant recipients producing antibodies to different HLA alleles.
The integrin β4 gene shows a limited degree of polymorphism which may have an impact on protein function. For example, a single-nucleotide polymorphism (SNP) in the 3’ untranslated region of integrin β4 is associated with breast cancer metastasis [77]. It is suggested that this SNP disrupts microRNA miR-34a binding site and enhances the expression of integrin β4 and thus increases tumor cell growth and invasion [77]. Hirano et. al. found 9 SNPs in the integrin β4 gene, including two SNPs located in the region required for signal transduction [78]. It will be interesting to determine if the integrin β4 alleles have differing capacities to partner with HLA-I to transduce signals, and promote tumor progression and transplant vasculopathy. Taken together, certain polymorphisms in HLA-I or integrin β4 may predispose the patient to increased risk of transplant vasculopathy or tumor progression. Studies on interactions between different HLA-I:integrin β4 alleles is needed to advance our understanding of how HLA-I molecules promote transplant vasculopathy, tumor progression and neural development.
6. Concluding remarks
We have demonstrated a mutual dependency of integrin β4 and HLA-I to transduce signals in endothelial cells which may be important in various disease states including transplant vasculopathy and cancer. Crosslinking HLA-I with antibodies or laminin-5 stimulation of integrin β4 increases complex formation between integrin β4 and HLA-I, and augments downstream signaling. In the situation of antibody-mediated rejection, HLA-I commandeers the integrin β4 molecule to transduce intracellular signals that stimulate cell survival and proliferation, which may contribute to neointimal proliferation of endothelial cells and smooth muscle cells and transplant vasculopathy (Figure 1). Efforts to reduce HLA and integrin β4 interactions in the cells of the graft could be of therapeutic benefit. In the setting of tumor angiogenesis and tumor progression, HLA-I is required for laminin-5-mediated integrin β4 signaling. Depletion of HLA-I inhibits endothelial cell migration induced by laminin-5, suggesting that HLA-I can regulate the integrin β4 mediated tumor progression. The physiological significance of integrin β4's association with HLA-I may lie in neuron development. Further dissection of the interactions between integrin β4 and HLA-I should lead to the development of novel therapeutic strategies to prevent antibody-mediated transplant rejection and tumor progression.
Figure 1.
HLA-I antibodies stimulate intracellular signals via integrin β4 in endothelial cells and smooth muscle cells in the setting of transplant atherosclerosis. Crosslinking of HLA-I with antibodies increases its association with integrin β4, which in turn activates the Src/FAK signaling pathways. Phosphorylation of Src/FAK causes the activation of PI3K/Akt pathway which stimulates the formation of mTORC1. mTORC1 initiates protein synthesis and cell proliferation via 4EBP1 and S6K. Phosphorylation of Src/FAK also stimulates mTORC2 signaling. Activation of mTORC2 promotes cell survival or proliferation though the AKT and ERK signaling pathways, respectively.
Acknowledgements
This work was supported by the National Institute of Allergy and Infectious Diseases Grant RO1 AI 042819 (E. F. R.), NIH U01AI077821 (E. F. R.), the National Heart Lung and Blood Institute Grant RO1 HL 090995 (E. F. R.).
Abbreviations
- KIR
the killer cell immunoglobulin like receptor
- S6K
S6 kinase
- S6RP
S6 ribosome protein
- EIF4A1
eukaryotic translation initiation factor 4A1
- MMP
matrix metalloproteinase
- SNP
single-nucleotide polymorphism
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Reed EF, Demetris AJ, Hammond E, Itescu S, Kobashigawa JA, Reinsmoen NL, et al. Acute antibody-mediated rejection of cardiac transplants. J Heart Lung Transplant. 2006;25:153. doi: 10.1016/j.healun.2005.09.003. [DOI] [PubMed] [Google Scholar]
- 2.Singh N, Pirsch J, Samaniego M. Antibody-mediated rejection: treatment alternatives and outcomes. Transplant Rev (Orlando) 2009;23:34. doi: 10.1016/j.trre.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 3.Leffell MS, Zachary AA. Antiallograft antibodies: relevance, detection, and monitoring. Curr Opin Organ Transplant. 2010;15:2. doi: 10.1097/MOT.0b013e3283342798. [DOI] [PubMed] [Google Scholar]
- 4.Clatworthy MR, Espeli M, Torpey N, Smith KG. The generation and maintenance of serum alloantibody. Curr Opin Immunol. 2010;22:669. doi: 10.1016/j.coi.2010.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Q, Cecka JM, Gjertson DW, Ge P, Rose ML, Patel JK, et al. HLA and MICA: targets of antibody-mediated rejection in heart transplantation. Transplantation. 2011;91:1153. doi: 10.1097/TP.0b013e3182157d60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Uehara S, Chase CM, Cornell LD, Madsen JC, Russell PS, Colvin RB. Chronic cardiac transplant arteriopathy in mice: relationship of alloantibody, C4d deposition and neointimal fibrosis. Am J Transplant. 2007;7:57. doi: 10.1111/j.1600-6143.2006.01599.x. [DOI] [PubMed] [Google Scholar]
- 7.Hirohashi T, Uehara S, Chase CM, DellaPelle P, Madsen JC, Russell PS, et al. Complement independent antibody-mediated endarteritis and transplant arteriopathy in mice. Am J Transplant. 2010;10:510. doi: 10.1111/j.1600-6143.2009.02958.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirohashi T, Chase CM, Della Pelle P, Sebastian D, Alessandrini A, Madsen JC, et al. A novel pathway of chronic allograft rejection mediated by NK cells and alloantibody. Am J Transplant. 2012;12:313. doi: 10.1111/j.1600-6143.2011.03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galvani S, Auge N, Calise D, Thiers JC, Canivet C, Kamar N, et al. HLA class I antibodies provoke graft arteriosclerosis in human arteries transplanted into SCID/beige mice. Am J Transplant. 2009;9:2607. doi: 10.1111/j.1600-6143.2009.02804.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Reed EF. Effect of Antibodies on Endothelium. Am J Transplant. 2009;9:2459. doi: 10.1111/j.1600-6143.2009.02819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leffell MS, Zachary AA. Anti-allograft antibodies: some are harmful, some can be overcome, and some may be beneficial. Discov Med. 2010;9:478. [PubMed] [Google Scholar]
- 12.Colvin RB. Pathology of chronic humoral rejection. Contrib Nephrol. 2009;162:75. doi: 10.1159/000170814. [DOI] [PubMed] [Google Scholar]
- 13.Stegall MD, Gloor JM. Deciphering antibody-mediated rejection: new insights into mechanisms and treatment. Curr Opin Organ Transplant. 2010;15:8. doi: 10.1097/MOT.0b013e3283342712. [DOI] [PubMed] [Google Scholar]
- 14.Mehra MR, Crespo-Leiro MG, Dipchand A, Ensminger SM, Hiemann NE, Kobashigawa JA, et al. International Society for Heart and Lung Transplantation working formulation of a standardized nomenclature for cardiac allograft vasculopathy-2010. J Heart Lung Transplant. 2010;29:717. doi: 10.1016/j.healun.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 15.Mitchell RN, Libby P. Vascular remodeling in transplant vasculopathy. Circ Res. 2007;100:967. doi: 10.1161/01.RES.0000261982.76892.09. [DOI] [PubMed] [Google Scholar]
- 16.Hosenpud JD, Morris TE, Shipley GD, Mauck KA, Wagner CR. Cardiac allograft vasculopathy. Preferential regulation of endothelial cell-derived mesenchymal growth factors in response to a donor-specific cell-mediated allogeneic response. Transplantation. 1996;61:939. doi: 10.1097/00007890-199603270-00017. [DOI] [PubMed] [Google Scholar]
- 17.Bayliss J, Bailey M, Leet A, Stein AN, Thomson NM, McLean CA. Late onset antibody-mediated rejection and endothelial localization of vascular endothelial growth factor are associated with development of cardiac allograft vasculopathy. Transplantation. 2008;86:991. doi: 10.1097/TP.0b013e318186d734. [DOI] [PubMed] [Google Scholar]
- 18.Aziz T, Hasleton P, Hann AW, Yonan N, Deiraniya A, Hutchinson IV. Transforming growth factor beta in relation to cardiac allograft vasculopathy after heart transplantation. J Thorac Cardiovasc Surg. 2000;119:700. doi: 10.1016/s0022-5223(00)70004-3. [DOI] [PubMed] [Google Scholar]
- 19.Colvin RB, Smith RN. Antibody-mediated organ-allograft rejection. Nat Rev Immunol. 2005;5:807. doi: 10.1038/nri1702. [DOI] [PubMed] [Google Scholar]
- 20.Yamakuchi M, Kirkiles-Smith NC, Ferlito M, Cameron SJ, Bao C, Fox-Talbot K, et al. Antibody to human leukocyte antigen triggers endothelial exocytosis. Proc Natl Acad Sci U S A. 2007;104:1301. doi: 10.1073/pnas.0602035104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kitchens WH, Chase CM, Uehara S, Cornell LD, Colvin RB, Russell PS, et al. Macrophage depletion suppresses cardiac allograft vasculopathy in mice. Am J Transplant. 2007;7:2675. doi: 10.1111/j.1600-6143.2007.01997.x. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Zhang X, Jin YP, Mulder A, Reed EF. Antibody ligation of human leukocyte antigen class I molecules stimulates migration and proliferation of smooth muscle cells in a focal adhesion kinase-dependent manner. Hum Immunol. 2011;72:1150. doi: 10.1016/j.humimm.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jin YP, Korin Y, Zhang X, Jindra PT, Rozengurt E, Reed EF. RNA interference elucidates the role of focal adhesion kinase in HLA class I-mediated focal adhesion complex formation and proliferation in human endothelial cells. J Immunol. 2007;178:7911. doi: 10.4049/jimmunol.178.12.7911. [DOI] [PubMed] [Google Scholar]
- 24.Ziegler ME, Souda P, Jin YP, Whitelegge JP, Reed EF. Characterization of the endothelial cell cytoskeleton following HLA class I ligation. PLoS One. 2012;7:e29472. doi: 10.1371/journal.pone.0029472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jindra PT, Jin YP, Rozengurt E, Reed EF. HLA class I antibody-mediated endothelial cell proliferation via the mTOR pathway. J Immunol. 2008;180:2357. doi: 10.4049/jimmunol.180.4.2357. [DOI] [PubMed] [Google Scholar]
- 26.Jindra PT, Jin YP, Jacamo R, Rozengurt E, Reed EF. MHC class I and integrin ligation induce ERK activation via an mTORC2-dependent pathway. Biochem Biophys Res Commun. 2008;369:781. doi: 10.1016/j.bbrc.2008.02.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jindra PT, Hsueh A, Hong L, Gjertson D, Shen XD, Gao F, et al. Anti-MHC class I antibody activation of proliferation and survival signaling in murine cardiac allografts. J Immunol. 2008;180:2214. doi: 10.4049/jimmunol.180.4.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- 29.Abdel-Ghany M, Cheng H-C, Elble RC, Pauli BU. Focal Adhesion Kinase Activated by beta 4 Integrin Ligation to mCLCA1 Mediates Early Metastatic Growth. J. Biol. Chem. 2002;277:34391. doi: 10.1074/jbc.M205307200. [DOI] [PubMed] [Google Scholar]
- 30.Giancotti FG. Targeting integrin beta4 for cancer and anti-angiogenic therapy. Trends Pharmacol Sci. 2007;28:506. doi: 10.1016/j.tips.2007.08.004. [DOI] [PubMed] [Google Scholar]
- 31.Zhang X, Rozengurt E, Reed EF. HLA class I molecules partner with integrin beta4 to stimulate endothelial cell proliferation and migration. Sci Signal. 2010;3:ra85. doi: 10.1126/scisignal.2001158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sun C, Liu X, Qi L, Xu J, Zhao J, Zhang Y, et al. Modulation of vascular endothelial cell senescence by integrin beta4. J Cell Physiol. 2010;225:673. doi: 10.1002/jcp.22262. [DOI] [PubMed] [Google Scholar]
- 33.Jindra PT, Zhang X, Mulder A, Claas F, Veale J, Jin YP, et al. Anti-HLA antibodies can induce endothelial cell survival or proliferation depending on their concentration. Transplantation. 2006;82:S33. doi: 10.1097/01.tp.0000231447.34240.3c. [DOI] [PubMed] [Google Scholar]
- 34.Salama AD, Delikouras A, Pusey CD, Cook HT, Bhangal G, Lechler RI, et al. Transplant accommodation in highly sensitized patients: a potential role for Bcl-xL and alloantibody. Am J Transplant. 2001;1:260. doi: 10.1034/j.1600-6143.2001.001003260.x. [DOI] [PubMed] [Google Scholar]
- 35.Montgomery RA, Lonze BE, King KE, Kraus ES, Kucirka LM, Locke JE, et al. Desensitization in HLA-incompatible kidney recipients and survival. N Engl J Med. 2011;365:318. doi: 10.1056/NEJMoa1012376. [DOI] [PubMed] [Google Scholar]
- 36.Hachem RR, Yusen RD, Meyers BF, Aloush AA, Mohanakumar T, Patterson GA, et al. Anti-human leukocyte antigen antibodies and preemptive antibody-directed therapy after lung transplantation. J Heart Lung Transplant. 2010;29:973. doi: 10.1016/j.healun.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jordan SC, Kahwaji J, Toyoda M, Vo A. B-cell immunotherapeutics: emerging roles in solid organ transplantation. Curr Opin Organ Transplant. 2011;16:416. doi: 10.1097/MOT.0b013e32834874f7. [DOI] [PubMed] [Google Scholar]
- 38.Walsh RC, Alloway RR, Girnita AL, Woodle ES. Proteasome inhibitor-based therapy for antibody-mediated rejection. Kidney Int. 2012;81:1067. doi: 10.1038/ki.2011.502. [DOI] [PubMed] [Google Scholar]
- 39.Guthoff M, Schmid-Horch B, Weisel KC, Haring HU, Konigsrainer A, Heyne N. Proteasome inhibition by bortezomib: Effect on HLA-antibody levels and specificity in sensitized patients awaiting renal allograft transplantation. Transpl Immunol. 2012;26:171. doi: 10.1016/j.trim.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 40.Chevalier G, Suberbielle E, Monnet C, Duplan V, Martin-Blondel G, Farrugia F, et al. Neurons are MHC class I-dependent targets for CD8 T cells upon neurotropic viral infection. PLoS Pathog. 2011;7:e1002393. doi: 10.1371/journal.ppat.1002393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 42.Previtali S, Quattrini A, Nemni R, Truci G, Ducati A, Wrabetz L, et al. Alpha6 beta4 and alpha6 beta1 integrins in astrocytomas and other CNS tumors. J Neuropathol Exp Neurol. 1996;55:456. doi: 10.1097/00005072-199604000-00008. [DOI] [PubMed] [Google Scholar]
- 43.Ni H, Dydensborg AB, Herring FE, Basora N, Gagne D, Vachon PH, et al. Upregulation of a functional form of the beta4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene. 2005;24:6820. doi: 10.1038/sj.onc.1208848. [DOI] [PubMed] [Google Scholar]
- 44.Chao C, Lotz MM, Clarke AC, Mercurio AM. A function for the integrin alpha6beta4 in the invasive properties of colorectal carcinoma cells. Cancer Res. 1996;56:4811. [PubMed] [Google Scholar]
- 45.Robertson JH, Yang SY, Winslet MC, Seifalian AM. Functional blocking of specific integrins inhibit colonic cancer migration. Clin Exp Metastasis. 2009;26:769. doi: 10.1007/s10585-009-9276-5. [DOI] [PubMed] [Google Scholar]
- 46.Daemi N, Thomasset N, Lissitzky JC, Dumortier J, Jacquier MF, Pourreyron C, et al. Anti-beta4 integrin antibodies enhance migratory and invasive abilities of human colon adenocarcinoma cells and their MMP-2 expression. Int J Cancer. 2000;85:850. doi: 10.1002/(sici)1097-0215(20000315)85:6<850::aid-ijc19>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 47.Galvani S, Trayssac M, Auge N, Thiers JC, Calise D, Krell HW, et al. A key role for matrix metalloproteinases and neutral sphingomyelinase-2 in transplant vasculopathy triggered by anti-HLA antibody. Circulation. 2011;124:2725. doi: 10.1161/CIRCULATIONAHA.111.021790. [DOI] [PubMed] [Google Scholar]
- 48.Hariya A, Takazawa K, Yamamoto T, Amano A. ONO-4817, a novel matrix metalloproteinase inhibitor, attenuates allograft vasculopathy in a rat cardiac transplant. The Journal of Heart and Lung Transplantation. 2004;23:1163. doi: 10.1016/j.healun.2003.08.022. [DOI] [PubMed] [Google Scholar]
- 49.Lu S, Simin K, Khan A, Mercurio AM. Analysis of integrin beta4 expression in human breast cancer: association with basal-like tumors and prognostic significance. Clin Cancer Res. 2008;14:1050. doi: 10.1158/1078-0432.CCR-07-4116. [DOI] [PubMed] [Google Scholar]
- 50.Boelens MC, van den Berg A, Vogelzang I, Wesseling J, Postma DS, Timens W, et al. Differential expression and distribution of epithelial adhesion molecules in non-small cell lung cancer and normal bronchus. J Clin Pathol. 2007;60:608. doi: 10.1136/jcp.2005.031443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ueda Y, Ishikawa K, Shiraishi N, Yokoyama S, Kitano S. Clinical significance of HLA class I heavy chain expression in patients with gastric cancer. J Surg Oncol. 2008;97:451. doi: 10.1002/jso.20985. [DOI] [PubMed] [Google Scholar]
- 52.Holmes K, Egan B, Swan N, O'Morain C. Genetic Mechanisms and Aberrant Gene Expression during the Development of Gastric Intestinal Metaplasia and Adenocarcinoma. Curr Genomics. 2007;8:379. doi: 10.2174/138920207783406460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rakoff-Nahoum S. Why cancer and inflammation? Yale J Biol Med. 2006;79:123. [PMC free article] [PubMed] [Google Scholar]
- 54.Ferrone C, Dranoff G. Dual roles for immunity in gastrointestinal cancers. J Clin Oncol. 2010;28:4045. doi: 10.1200/JCO.2010.27.9992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsujimoto H, Ono S, Ichikura T, Matsumoto Y, Yamamoto J, Hase K. Roles of inflammatory cytokines in the progression of gastric cancer: friends or foes? Gastric Cancer. 2010;13:212. doi: 10.1007/s10120-010-0568-x. [DOI] [PubMed] [Google Scholar]
- 56.Ramnath N, Tan D, Li Q, Hylander BL, Bogner P, Ryes L, et al. Is downregulation of MHC class I antigen expression in human non-small cell lung cancer associated with prolonged survival? Cancer Immunol Immunother. 2006;55:891. doi: 10.1007/s00262-005-0085-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Madjd Z, Spendlove I, Pinder SE, Ellis IO, Durrant LG. Total loss of MHC class I is an independent indicator of good prognosis in breast cancer. Int J Cancer. 2005;117:248. doi: 10.1002/ijc.21163. [DOI] [PubMed] [Google Scholar]
- 58.Jaakkola S, Savunen O, Halme T, Uitto J, Peltonen J. Basement membranes during development of human nerve: Schwann cells and perineurial cells display marked changes in their expression profiles for laminin subunits and beta 1 and beta 4 integrins. J Neurocytol. 1993;22:215. doi: 10.1007/BF01246360. [DOI] [PubMed] [Google Scholar]
- 59.Su L, Zhao B, Lv X, Wang N, Zhao J, Zhang S, et al. Safrole oxide induces neuronal apoptosis through inhibition of integrin beta4/SOD activity and elevation of ROS/NADPH oxidase activity. Life Sci. 2007;80:999. doi: 10.1016/j.lfs.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 60.Milner R, Campbell IL. Increased expression of the beta4 and alpha5 integrin subunits in cerebral blood vessels of transgenic mice chronically producing the pro-inflammatory cytokines IL-6 or IFN-alpha in the central nervous system. Mol Cell Neurosci. 2006;33:429. doi: 10.1016/j.mcn.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jacques TS, Relvas JB, Nishimura S, Pytela R, Edwards GM, Streuli CH, et al. Neural precursor cell chain migration and division are regulated through different beta1 integrins. Development. 1998;125:3167. doi: 10.1242/dev.125.16.3167. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Z, Galileo DS. Retroviral transfer of antisense integrin alpha6 or alpha8 sequences results in laminar redistribution or clonal cell death in developing brain. J Neurosci. 1998;18:3336928. doi: 10.1523/JNEUROSCI.18-17-06928.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Whitley M, Treloar H, De Arcangelis A, Georges Labouesse E, Greer CA. The alpha6 integrin subunit in the developing mouse olfactory bulb. J Neurocytol. 2005;34:81. doi: 10.1007/s11068-005-5049-5. [DOI] [PubMed] [Google Scholar]
- 64.Su L, Lv X, Miao J. Integrin beta 4 in neural cells. Neuromolecular Med. 2008;10:316. doi: 10.1007/s12017-008-8042-1. [DOI] [PubMed] [Google Scholar]
- 65.Boulanger LM, Shatz CJ. Immune signalling in neural development, synaptic plasticity and disease. Nat Rev Neurosci. 2004;5:521. doi: 10.1038/nrn1428. [DOI] [PubMed] [Google Scholar]
- 66.Fourgeaud L, Davenport CM, Tyler CM, Cheng TT, Spencer MB, Boulanger LM. MHC class I modulates NMDA receptor function and AMPA receptor trafficking. Proc Natl Acad Sci U S A. 2010;107:22278. doi: 10.1073/pnas.0914064107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Thams S, Oliveira A, Cullheim S. MHC class I expression and synaptic plasticity after nerve lesion. Brain Res Rev. 2008;57:265. doi: 10.1016/j.brainresrev.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 68.Zohar O, Reiter Y, Bennink JR, Lev A, Cavallaro S, Paratore S, et al. Cutting edge: MHC class I-Ly49 interaction regulates neuronal function. J Immunol. 2008;180:6447. doi: 10.4049/jimmunol.180.10.6447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Oliveira AL, Thams S, Lidman O, Piehl F, Hokfelt T, Karre K, et al. A role for MHC class I molecules in synaptic plasticity and regeneration of neurons after axotomy. Proc Natl Acad Sci U S A. 2004;101:17843. doi: 10.1073/pnas.0408154101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sabha M, Jr., Emirandetti A, Cullheim S, De Oliveira AL. MHC I expression and synaptic plasticity in different mice strains after axotomy. Synapse. 2008;62:137. doi: 10.1002/syn.20475. [DOI] [PubMed] [Google Scholar]
- 71.Van der Zee CE, Kreft M, Beckers G, Kuipers A, Sonnenberg A. Conditional deletion of the Itgb4 integrin gene in Schwann cells leads to delayed peripheral nerve regeneration. J Neurosci. 2008;28:11292. doi: 10.1523/JNEUROSCI.3068-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Fu ZQ, Shao QL, Shen JL, Zhang YJ, Zhao XX, Yao L. Effect of carbamylated erythropoietin on major histocompatibility complex expression and neural differentiation of human neural stem cells. J Neuroimmunol. 2010;221:15. doi: 10.1016/j.jneuroim.2010.01.016. [DOI] [PubMed] [Google Scholar]
- 73.Su L, Lv X, Xu J, Yin D, Zhang H, Li Y, et al. Neural stem cell differentiation is mediated by integrin beta4 in vitro. Int J Biochem Cell Biol. 2009;41:916. doi: 10.1016/j.biocel.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 74.Johnson DR. Locus-specific constitutive and cytokine-induced HLA class I gene expression. J Immunol. 2003;170:1894. doi: 10.4049/jimmunol.170.4.1894. [DOI] [PubMed] [Google Scholar]
- 75.Balas A, Santos S, Aviles MJ, Garcia-Sanchez F, Lillo R, Alvarez A, et al. Elongation of the cytoplasmic domain, due to a point deletion at exon 7, results in an HLA-C null allele, Cw*0409 N. Tissue Antigens. 2002;59:95. doi: 10.1034/j.1399-0039.2002.590204.x. [DOI] [PubMed] [Google Scholar]
- 76.Jin YP, Zhang XH, Mulder A, Claas FHJ, Lee JH, Reed EF. NOT ALL CLASS I MOLECULES ASSOCIATE WITH INTEGRIN BETA4 TO TRANSDUCE SIGNALS: MECHANISMS AND CLINICAL IMPLICATIONS. Hum Immunol. 2011;72:S178. [Google Scholar]
- 77.Brendle A, Lei H, Brandt A, Johansson R, Enquist K, Henriksson R, et al. Polymorphisms in predicted microRNA-binding sites in integrin genes and breast cancer: ITGB4 as prognostic marker. Carcinogenesis. 2008;29:1394. doi: 10.1093/carcin/bgn126. [DOI] [PubMed] [Google Scholar]
- 78.Hirano A, Nagai H, Harada H, Terada Y, Haga S, Kajiwara T, et al. Nine novel single-nucleotide polymorphisms in the integrin beta4 (ITGB4) gene in the Japanese population. J Hum Genet. 2001;46:35. doi: 10.1007/s100380170122. [DOI] [PubMed] [Google Scholar]