Abstract
AIM
The voluntary termination of exercise has been hypothesized to occur at a sensory tolerance limit which is significantly determined by feedback from group III and IV muscle afferents, and is associated with a specific level of peripheral quadriceps fatigue during whole body cycling. Therefore, the purpose of this study was to reduce the amount of muscle mass engaged during dynamic leg exercise to constrain the source of muscle afferent feedback to the central nervous system (CNS), and examine the effect on peripheral quadriceps fatigue.
METHOD
Eight young males performed exhaustive large (cycling – BIKE) and small (knee extensor – KE) muscle mass dynamic exercise at 85% of the modality-specific maximal workload. Pre- vs post-exercise maximal voluntary contractions (MVC) and supramaximal magnetic femoral nerve stimulation (Qtw,pot) were used to quantify peripheral quadriceps fatigue.
RESULT
Significant quadriceps fatigue was evident following both exercise trials; however, the exercise-induced changes in MVC (−28±1% vs −16±2%) and Qtw,pot (−53±2% vs −34±2%) were far greater following KE compared to BIKE exercise, respectively. The greater degree of quadriceps fatigue following KE exercise was in proportion to the greater exercise time (9.1±0.4 vs. 6.3±0.5 minutes, p<0.05), suggestive of a similar rate of peripheral fatigue development.
CONCLUSION
These data suggest that when the source of skeletal muscle afferent feedback is confined to a small muscle mass, the CNS tolerates a greater magnitude of peripheral fatigue, and likely a greater intramuscular metabolic disturbance. An important implication of this finding is that the adoption of small muscle mass exercise may facilitate greater exercise-induced muscular adaptation.
Keywords: dynamic exercise, locomotor muscle, knee-extension
INTRODUCTION
During high-intensity cycle exercise (BIKE), a centrally mediated sensory tolerance limit (Gandevia, 2001) associated with a set level of peripheral locomotor muscle fatigue has been documented (Amann and Dempsey, 2008, Amann et al., 2006a, Amann et al., 2006b, Amann, 2011). This level of peripheral fatigue appears to be consistent across exercise bouts in which variations in arterial oxygenation alter the rate of development of peripheral fatigue and exercise time (Amann et al., 2006b), but at the point of task failure, end-exercise locomotor muscle fatigue appears to be quite similar (Amann et al., 2006a, Amann et al., 2006b, Amann et al., 2007). In line with this observation, utilizing plantar flexion (Hogan et al., 1999) or single leg knee-extension (Vanhatalo et al., 2010), which recruits a larger muscle mass, exercise and nuclear magnetic resonance spectroscopy, the metabolic disturbance at exhaustion has been documented to be invariant despite variations in arterial oxygenation, or work rate, altering exercise time to exhaustion. Therefore, it seems that it is possible to alter the rate of peripheral fatigue development and endurance time, but at exhaustion following short duration, high intensity exercise a similar intramuscular metabolic disturbance and level of peripheral locomotor muscle fatigue is achieved (Amann and Dempsey, 2008, Amann et al., 2006a, Amann et al., 2006b, Hogan et al., 1999, Vanhatalo et al., 2010).
Group III and IV muscle afferents are active during dynamic exercise (Adreani et al., 1997, Amann et al., 2011b, Amann et al., 2010) and provide input to the central nervous system (CNS) regarding mechanical deformation and the metabolic milieu within the working skeletal muscle (Mitchell et al., 1983, Rotto and Kaufman, 1988). Pharmacological blockade of these thin fiber afferents from the lower limbs during high intensity cycling exercise has highlighted the role of afferent feedback in limiting the development of peripheral fatigue (Amann et al., 2011a, Amann et al., 2009). Indeed, in the absence of group III/IV muscle afferents, subjects accumulated a significantly greater amount of peripheral fatigue such that atypical ambulatory difficulties were observed at exercise cessation (Amann et al., 2011a, Amann et al., 2009). The authors of this work postulated that group III/IV afferent feedback from the working muscles modifies central motor drive to the locomotor muscle to ensure that the overall homeostasis of the organism is not threatened (Amann, 2011). Thus, the voluntary termination of exercise during high-intensity constant load endurance exercise occurs once a sensory tolerance limit (Gandevia, 2001) is reached that is substantially dependant, amongst other factors, on the ensemble afferent input from the active locomotor muscles (Amann and Dempsey, 2008, Amann et al., 2006a, Amann et al., 2009, Amann et al., 2006b, Amann, 2011). The corresponding level of peripheral fatigue presumably depends on the exercise task, and may vary with the amount of muscle mass recruited.
A relationship between the magnitude of afferent feedback and muscle mass has long been established (Freund et al., 1978, Iwamoto and Botterman, 1985). Freund et al. (Freund et al., 1978) demonstrated that post-bicycle exercise occlusion of blood flow to both legs maintained mean arterial pressure at a higher level than occlusion of one leg alone. In addition, time to task failure has been documented to be shorter, with less end-exercise quadriceps fatigue, for bilateral compared with unilateral sustained maximal voluntary contractions (MVC) of the knee extensor muscle group (Matkowski et al., 2011). In combination, these observations reveal that increasing active muscle mass augments the ensemble feedback to the CNS from the periphery and these changes in ensemble feedback may alter the level of end exercise fatigue and endurance time. However, the link between exercising muscle mass, the associated changes in afferent feedback and the degree of peripheral muscle fatigue following dynamic exercise has not been elucidated.
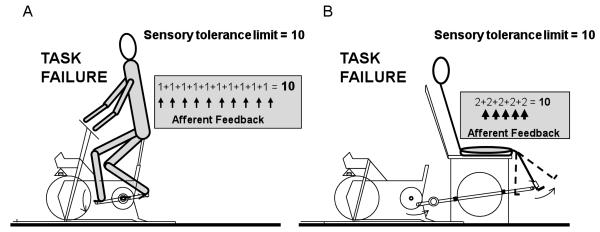
Therefore, the purpose of this study was to reduce the amount of active muscle mass during dynamic exercise to confine group III/IV afferent feedback to one muscle group. Thus, the sensory tolerance limit associated with task failure and a critical amount of ensemble afferent input, would eventually be reached by a strong local afferent signal from the isolated muscle group. This would contrast with the sum of the more diffuse weaker signals, with an equal ensemble magnitude, associated with whole body exercise (Figure 1). Specifically, we tested the hypothesis that exhaustive high intensity constant-load dynamic knee extensor (KE) exercise, utilizing ~2.5 kg of muscle (Andersen et al., 1985, Mortensen et al., 2005), would result in a greater degree of end-exercise quadriceps fatigue compared to the equivalent challenge utilizing a far larger muscle mass (BIKE, ~15 kg of muscle (Mortensen et al., 2005)).
Figure 1. Conceptual schematic illustrating the equal magnitude of ensemble group III/IV skeletal muscle afferent feedback at task failure in both large (cycling - BIKE) and small (knee extensor - KE) muscle mass exercise.
Group III/IV afferent feedback from active skeletal muscle is represented by the grey area. Accordingly, the sensory tolerance limit (10) influenced by the magnitude of ensemble afferent feedback and obtained by the sum of many diffuse signals during BIKE exercise (thin arrows; 1×10=10) (A) is reached with a focused, but very strong, local signal elicited by a greater intramuscular metabolic disturbance in the quadriceps (thick arrows; 5×2=10) at task failure during knee extensor exercise (B).
METHODS
Subjects
Eight young, healthy males (24 ± 1 years, 83 ± 6 kg, 178 ± 4 cm) volunteered to participate in this study. Written, informed consent was obtained from participants prior to their inclusion and the Institutional Review Boards of the University of Utah and the Salt Lake City VA Medical Center approved the study. All testing was performed in a thermoneutral environment (22°C).
Protocol
Prior to data collection, subjects were familiarized with BIKE and KE exercise as well as the neuromuscular function assessment procedure during preliminary visits to the laboratory. On subsequent visits, separated by at least 48 hours, time to exhaustion (Tlim) during constant load exercise trials at 85% of maximal workload, for each exercise modality were used to induce quadriceps fatigue. This workload (85%) was selected because, during pilot testing, this intensity of effort elicited task failure in all subjects in ~5 to ~12 minutes in both exercise modalities. Throughout each trial, ventilation, gas exchange, heart rate (HR), and rating of perceived exertion (RPE) were assessed. Prior to each exercise bout, 2 minutes of resting data were collected and subjects were allowed a 3-minute warm-up period (unloaded KE exercise and BIKE exercise at ~85 W). To quantify peripheral fatigue before exercise and 2 minutes following task failure, neuromuscular function tests were performed on the same leg used for both modalities. Task failure was defined as a drop of 10 rpm for both the KE and BIKE. The experimental limb was randomized and balanced between dominant and non-dominant legs (Oldfield, 1971).
BIKE and KE exercise
Dynamic, small muscle mass exercise was performed on a cycle erogmeter (Monark, Sweden) modified to allow KE exercise (Richardson et al., 1998). Briefly, this exercise modality recruits the quadriceps muscle group for active leg extension from 90 to ~170 degrees before a lever arm attached to a flywheel passively returns the leg to 90 degrees. Subjects were instructed to maintain a rate of 60 contractions per minute during KE exercise. For BIKE exercise, a cycle ergometer was employed (Excalibur, Lode, The Netherlands) and a constant pedaling rate was self-selected by all subjects (~75 rpm). For both exercise modalities, subjects performed one-minute stage, incremental exercise tests to exhaustion to determine peak workload (10 W + 5 W min−1 for KE and 20 W + 25 W min−1 for BIKE (Amann et al., 2004)).
Ventilation, gas exchange and heart rate
Ventilation and pulmonary gas exchange were measured at rest and during exercise with a metabolic cart (ParvoMedics, Sandy, UT). HR was determined from the R-R interval of a three-lead electrocardiogram (ECG) acquired at 200 Hz using a data acquisition system (AcqKnowledge; Biopac Systems, Goleta, CA). RPE was taken every minute during the TLim trials using Borg’s modified CR10 scale (Borg, 1998).
Neuromuscular function
The magnitude of peripheral quadriceps fatigue was quantified by supramaximal magnetic stimulation of the femoral nerve (Polkey et al., 1996, Kufel et al., 2002, Amann and Dempsey, 2008): the exercise induced reduction in potentiated quadriceps twitch force (Qtw,pot) assessed before exercise and again 2 minutes after both Tlim trials. This time delay was necessary to transfer the subjects from either exercise ergometer (BIKE or KE) to the neuromuscular function assessment apparatus, and was thus standardized for both exercise modalities. For the neuromuscular function test procedure, while subjects were semi-recumbent in a separate KE chair, with a knee joint angle of 90 degrees, a magnetic stimulator (Magstim 200, The Magstim Company Ltd, Wales) connected to a double 70 mm coil was used to stimulate the femoral nerve. The evoked twitch force was obtained from a calibrated load cell (Transducer Techniques, Temecula, CA) connected to a non-compliant strap placed around the subject’s ankle. To record magnetically evoked compound action potentials (M-waves) and evaluate changes in membrane excitability, quadriceps EMG was recorded from the vastus lateralis (VL) muscle (Amann and Dempsey, 2008). Electrodes were placed in a bipolar configuration over the middle of the muscle belly, with the active electrodes placed over the motor point of the muscle and reference electrode placed in an electrically neutral site. During a separate visit, supramaximality of stimulation was determined by serial, single unpotentiated twitch (Qtw) forces obtained every 30 seconds at 50, 60, 70, 80, 85, 90, 95, and 100% of maximal stimulator output.
As potentiated, compared to unpotentiated, twitch force has been documented to be a more sensitive indicator of fatigue (Kufel et al., 2002), Qtw,pot was assessed following a 5 second MVC of the quadriceps muscle. A series of 6 MVCs and Qtw,pot maneuvers were performed with 30 seconds between each MVC, such that the entire procedure lasted 2.5 minutes. In addition, to quantify activation of the quadriceps during the MVCs, a superimposed twitch technique was employed (Merton, 1954, Amann and Dempsey, 2008). Briefly, the additional force generated by a single twitch superimposed on the MVC was compared with the force produced by the potentiated twitch immediately following the MVC to determine the percent voluntary muscle activation (%VMA). Peak force, maximal rate of force development (MRFD), and maximal relaxation rate (MRR) were analyzed for all Qtw,pot values (Lepers et al., 2002).
Statistical Analysis
Two-way repeated measure analysis of variance were used to compare the effect of exercise modality on physiological parameters during exercise, with the Tukey’s honestly significant difference test used for post-hoc analysis if a significant main or interaction effect was found. Student’s paired t tests were used to compare the effect of exercise modality on end-exercise physiological parameters and the magnitude of peripheral fatigue. Statistical significance was set at α = 0.05. Results are expressed as means ± S.E.M.
RESULTS
Exercise responses
The maximal workload for the incremental exercise test was 280±9 W for BIKE and 60±4 W for KE exercise. Peak oxygen uptake was significantly higher for BIKE compared to KE exercise (3.2±0.1 vs 1.6±0.2 L/min). Tlim trial data are documented in Table 1. Cardiovascular and respiratory responses to the BIKE and KE TLim differed, with ventilation (VE), oxygen consumption (VO2), carbon dioxide production (VCO2), and HR all being higher during BIKE exercise (p<0.05). Tlim time was longer, by 31±5% (range 46 seconds to 283 seconds), for KE compared with BIKE exercise (p<0.05). RPE was lower for KE exercise at minutes 4 and 5 (p<0.05), but was not different at exhaustion (Table 1).
Table 1.
Physiological responses to constant workload trials at exhaustion during large (BIKE) and small (KE) muscle mass leg exercise.
| BIKE (85% of peak workload) |
KE (85% of peak workload) |
|
|---|---|---|
| Time to Exhaustion (s) | 378 ± 30 | 547 ± 22.7* |
| Workload (W) | 238 ± 8 | 52 ± 4* |
| Oxygen Consumption (L/min) | 3.1 ± 0.1 | 1.8 ± 0.2* |
| Carbon Dioxide Production (L/min) | 3.4 ± 0.2 | 2 ± 0.2* |
| Ventilation (L/min) | 116 ± 9 | 84 ± 6* |
| VE/VCO2 | 34.9 ± 1.9 | 42.6 ± 0.9* |
| Heart Rate (bpm) | 166 ± 4 | 130 ± 5* |
| RPE | 10 ± 0 | 10 ± 0 |
Values expressed as mean ± S.E.M. VE/VCO2, ventilation relative to carbon dioxide production. RPE, rating of perceived exertion.
Significant difference between KE and BIKE exercise.
Neuromuscular function
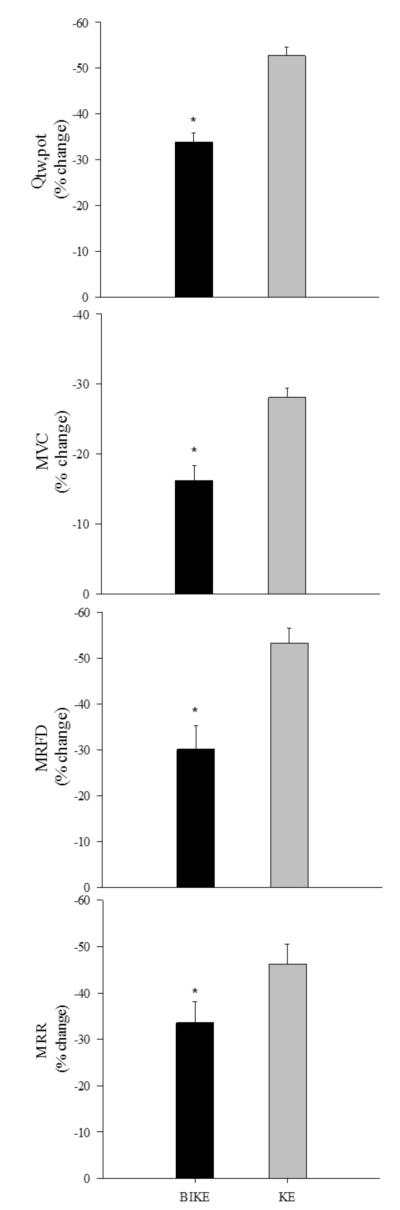
A plateau in Qtw and M-wave amplitudes with increasing stimulus intensity documented maximal depolarization of the femoral nerve in all subjects. Membrane excitability was maintained from pre- to post-exercise in all trials, as indicated by unchanged M-wave characteristics, indicating that the observed changes in Qtw,pot were predominantly due to changes within the quadriceps. Qtw,pot measured after exercise was significantly reduced from pre-exercise values for both exercise modalities, with a fall of 52±2% for KE and 34±2% for BIKE exercise, with no difference in the %VMA from pre- to post-exercise or between modalities. The fall in Qtw,pot was significantly greater for KE compared to BIKE exercise, by 36±4%, and in proportion to the greater exercise time (Figure 2). Other indices of fatigue (MVC, MRFD and MRR) were significantly reduced from pre-exercise values, and also revealed greater peripheral fatigue following KE exercise (Figure 2).
Figure 2. Change in quadriceps muscle function following constant load large (cycle-BIKE) and small (knee extensor - KE) exercise to exhaustion.
Data are represented as mean ± S.E.M. and values represent the percent change from pre- to post-exercise. MVC, maximal voluntary contraction; Qtw,pot, potentiated twitch force; MRFD, maximal rate of force development; MRR, maximal rate of relaxation. *Significant difference between KE and BIKE exercise.
DISCUSSION
The magnitude of group III/IV mediated afferent feedback from the active limbs substantially influences the voluntary termination of exercise, which has been suggested to occur once a sensory tolerance limit is reached (Amann, 2011, Gandevia, 2001). By reducing the amount of active muscle mass during dynamic exercise, we sought to confine the source of afferent feedback to one muscle group in contrast to the ensemble feedback from many muscles during whole body exercise. Thus, a greater local metabolic disturbance at task failure with small muscle mass exercise would be required to elicit an afferent signal of equal magnitude to that achieved during whole body exercise (Figure 1). As the degree of metabolic disturbance significantly influences the magnitude of peripheral fatigue assessed immediately after exercise, we hypothesized that small muscle mass exercise to exhaustion would result in a greater amount of peripheral fatigue than large muscle mass exercise. This was, indeed, the case, with KE exercise inducing significantly greater peripheral fatigue than BIKE exercise. These findings suggest that the CNS tolerates a greater degree of peripheral fatigue when the amount of active muscle mass is reduced. As exercise-induced peripheral adaptation responds to the degree of local perturbation, this study has implications for optimizing exercise training-induced muscle adaptations.
Peripheral fatigue and large muscle mass (BIKE) exercise
Previous work quantifying exercise-induced locomotor muscle fatigue following constant load, large muscle mass cycle exercise has identified a consistent level of peripheral fatigue in a variety of populations (Amann, 2011). In a group of young, endurance trained individuals exercising at a high-intensity constant workload, Amann et al. (Amann et al., 2006a) identified a ~34% decline in Qtw,pot from pre-exercise at exhaustion; altering arterial oxygenation affected endurance time, but not the observed threshold level of peripheral fatigue (Amann et al., 2006a). In older, sedentary individuals, work by Mador et al. (Mador et al., 2000) documented a similar ~36% fall in Qtw,pot following exhaustive constant-load cycling. Interestingly, even moderate to severe chronic obstructive pulmonary disease patients have been documented to exhibit a ~35% decline in potentiated twitch force following cycle exercise (Saey et al., 2005, Mador et al., 2001).
In the current study, BIKE exercise to exhaustion elicited quadriceps fatigue in all subjects, as exemplified by a consistent pre- to post-exercise decline in MVC force (16%) as well as a 34% decline in Qtw,pot (Figure 2). Our intra-twitch indices, MRFD and MRR, which reflect reductions in the rate of calcium reuptake by the sarcoplasmic reticulum as well as cross-bridge dissociation (Sandiford et al., 2005), were similarly attenuated (~30% and ~33%, respectively). Of note, the mean decline in Qtw,pot was in agreement with values observed in the aforementioned studies. These similarities suggest that while factors such as oxygen availability, fitness level, age, and disease state influence the rate of development of peripheral fatigue, an apparently similar level of peripheral fatigue coincides with task failure during large muscle mass exercise.
Peripheral fatigue and small muscle mass (KE) exercise
The knee extensor ergometer model provides a paradigm in which dynamic exercise can be limited to the quadriceps muscles (Richardson et al., 1998). During KE exercise, Vanhatalo et al. (Vanhatalo et al., 2010) has documented a similar increase in inorganic phosphates and ADP, and fall in phosphocreatine and intramuscular pH assessed by nuclear magnetic resonance spectroscopy, at task failure across work rates in both normoxia and hyperoxia. In addition, Fulco et al. (Fulco et al., 1996) documented an equivalent fall in MVC force following exhaustive KE exercise in both hypobaria and normoxia. Utilizing evoked, potentiated twitch forces, Burnley et al. (Burnley, 2009) documented a ~52% decline in Qtw,pot with electrical femoral nerve stimulation following exhaustive intermittent isometric contractions of the quadriceps, and Polkey et al. (Polkey et al., 1996) found an equivalent reduction (~55%), following a similar exercise bout, with magnetic stimulation of the femoral nerve.
In the current study, the quantification of exercise-induced quadriceps fatigue revealed a 53% reduction in Qtw,pot and ~28% fall in MVC force following KE exercise, while MRFD and MRR were reduced by ~53 and 46%, respectively (Figure 2). Interestingly, the magnitude of the exercise-induced decrease in the evoked twitch force in this study (~53%) was very similar to the values observed in previous work (~52% and 55%) utilizing the Qtw,pot maneuver following quadriceps exercise to task failure (Polkey et al., 1996, Burnley, 2009). Taken together, these studies, and our results, are suggestive of a similar level of end exercise peripheral quadriceps fatigue following small muscle mass exercise to task failure. Interestingly, the magnitude of peripheral fatigue when the exercise is confined to a small muscle mass may be greater then the obtained at exhaustion with whole body exercise.
Muscle mass, afferent feedback and fatigue
This study sought to compare the magnitude of peripheral fatigue following the voluntary termination of KE and BIKE exercise. Despite the matching of exercise intensity (85% of peak workload), KE exercise time was longer, by ~31%, and the magnitude of peripheral fatigue assessed following task failure was ~36% greater in comparison to BIKE exercise. The proportionality between the increased time to fatigue and magnitude of fatigue, although speculative, suggests a similar rate of peripheral fatigue development, with the ability to achieve a greater degree of end-exercise peripheral fatigue potentially contributing to the greater exercise time. These results imply that at any given time point, achieved in both BIKE and KE trials, the magnitude of peripheral quadriceps fatigue was equivalent for both exercise modalities. During KE exercise with a reduced active muscle mass limiting the source of muscle afferent signal to the quadriceps, a similar magnitude of peripheral fatigue was likely associated with decreased ensemble input to the CNS. Indeed, during BIKE exercise, peripheral fatigue in both legs substantially augmented afferent feedback. Thus, subjects continued to drive the quadriceps muscle and delve deeper into its functional capacity during KE exercise, eventually reaching a greater level of quadriceps fatigue and presumably end-exercise metabolic disturbance, ultimately eliciting a muscle afferent signal of equal ensemble magnitude to that obtained with whole body BIKE exercise at task failure.
A greater magnitude of peripheral fatigue was evident in all measured indices following KE exercise (Figure 2). With this reduction in active muscle mass, subjects surpassed the ~34% decline in Qtw,pot observed following BIKE exercise, and continued until quadriceps contractile function was reduced by ~53%. This difference was also reflected in all other indicators of peripheral fatigue with an approximate two-fold greater decrease in MVC force, despite equal %VMA and therefore similar levels of central fatigue, and attenuated intra-twitch indices (Figure 2) following KE exercise. It is likely that when the source of afferent feedback to the CNS is constrained to the ~2.5 kg of muscle engaged during KE exercise, in contrast to the sum of multiple, more diffuse, inputs from the ~15 kg of muscle utilized during BIKE exercise, the CNS tolerates a greater local accumulation of metabolic byproducts in the quadriceps, which impact peripheral fatigue (Allen et al., 2008b, Allen et al., 2008a, Westerblad and Allen, 2003). Thus, the sensory tolerance limit associated with exercise cessation and significantly influenced by, amongst other factors, the magnitude of ensemble afferent feedback, was likely reached with a greater degree of local skeletal muscle homeostatic disturbance during small muscle mass exercise (Figure 1).
Active muscle mass and the potential for adaptation
The utility of reducing active muscle mass during endurance training bouts to enhance peripheral muscle adaptation has been previously documented (Abbiss et al., 2011, Magnusson et al., 1996, Richardson et al., 2000). Magnussen et al. (Magnusson et al., 1996) trained one set of quadriceps at a time with dynamic KE exercise and elicited large peripheral skeletal muscle adaptations (increased oxidative enzyme activity and capillarity) in chronic heart failure patients. Richardson et al. (Richardson et al., 2000) documented a ~35% increase in quadriceps VO2peak in young, healthy subjects following an eight week KE training program, which outstrips the typical 6-20% gains following whole body training. In addition, Abbiss et al. (Abbiss et al., 2011) documented increased cytochrome c oxidase subunits II and IV following single leg, compared with double leg, cycle training. As exercise training induces an adaptive response to a homeostatic disturbance, a greater degree of peripheral fatigue during small muscle mass exercise may provide a greater impetus for skeletal muscle adaptation. Thus, when the active muscle mass is kept small during dynamic exercise, an enhanced adaptation in response to a greater homeostatic disturbance may therefore be possible. In essence, the adoption of such a small muscle mass approach to exercise training could mean that the sum of the parts will be greater than the whole, which has significant implications for exercise training in both healthy and diseased populations.
Experimental Considerations
It is important to acknowledge that dissimilarities in the cardiovascular and respiratory responses to KE and BIKE exercise were evident which could raise concerns about the role of oxygen supply and utilization (Table 1). However, experimental manipulations of oxygen availability by achieved by altering arterial oxygen content (Amann and Calbet, 2008) during exercise performance tests have been proposed to act via oxygen delivery to the working muscle and not the end-exercise level of peripheral fatigue (Amann and Calbet, 2008). In addition, although fatiguing respiratory muscle work may increase afferent activity (Hill, 2000), the alleviation of diaphragm fatigue with proportional assist ventilation does not alter the end-exercise level of peripheral fatigue during BIKE exercise (Amann et al., 2007). Therefore, in the current study, as in previous work (Gagnon et al., 2009, Amann, 2011), peripheral fatigue likely played a significant, autonomous role in curtailing exercise performance.
Additionally, while our conceptual schematic (Figure 1) paints a uniform picture of increased afferent feedback with increasing muscle recruitment, we acknowledge that the characteristics of group III/IV afferents are more complicated. Certainly, Some pathophysiological conditions are associated with alterations in group III/IV afferent sensitivity or their elicited reflexes (Murphy et al., 2011). In addition, the role of these fibers can vary across skeletal muscle fiber type and muscle group (Iwamoto and Botterman, 1985, Martin et al., 2006), and thus the picture is not actually so simple. Indeed, the increase in afferent feedback achieved by the increase in active muscle mass in our study from KE to BIKE was likely not directly proportional to the change in muscle mass. However, BIKE exercise included the addition of the contralateral quadriceps performing the same action (knee extension from ~90 to ~180 degrees), with added afferent feedback from other active muscles, which, although of unknown magnitude, would probably have greatly exaggerated the difference in ensemble afferent feedback.
Alternative explanations for the differences in peripheral fatigue following BIKE and KE exercise are also possible. Task specificity is an important facet in the etiology of fatigue (Barry and Enoka, 2007) and while BIKE and KE exercise are similar, differences in neural activation strategies could have affected our results. Of importance, the relative contributions of peripheral and central fatigue could have varied between the two modalities. Although we attempted to measure central fatigue by the superimposed twitch technique and %VMA, and while we did not observe any differences from pre to post exercise, or between BIKE and KE exercise, the time delay (2 minutes) between task failure and our fatigue measurements was probably too great to validly detect central fatigue. In addition, this time delay potentially could have led to an underestimation of the degree of peripheral fatigue at the moment of task failure, due to the extremely fast kinetics of phosphocreatine recovery. However, this time delay was logistically unavoidable to standardize the fatigue assessment protocol for both KE and BIKE exercise, so these effects, if present, were presumably constant between both trials.
The ability of the magnetic stimulation technique to achieve supramaximility of stimulation following exercise is another potential concern. Specifically, varying degrees of activity-dependant hyperpolarization (Vagg et al., 1998) of the quadriceps motor neurons following exercise may have led to a change in motor unit recruitment elicited by the supramaximal stimulation intensity prior to exercise. However, a recent comparison by Verges et al. (Verges et al., 2009) of the magnetic stimulation technique with electrical stimulation revealed no difference between techniques in the magnitude of measured fatigue following exercise. Thus, we contend that the twitch data do, in fact, represent changes within the quadriceps muscle, but a change in axon excitability, and thus axon recruitment following exercise can not be completely ruled out.
Finally, our conclusions are largely based on the assumption that afferent feedback was different between exercise modalities and in proportion to the metabolic disturbance in the quadriceps, neither of which were directly measured. Indeed, this study would have benefited greatly from a valid and feasible technique for the measurement of group III/IV afferent activity during dynamic exercise in humans, but currently this does not exist. Our group has previously utilized a partial afferent block to demonstrate the obligatory role of these afferents in the cardiovascular and respiratory response to both BIKE (Amann et al., 2010) and KE (Amann et al., 2011b) exercise, but in the current study, it was not deemed that this approach would actually have better elucidated the role of afferent feedback. In regard to differences in the end exercise metabolic disturbance, we have previously documented intramuscular pH values following KE exercise of ~6.5 (Richardson et al., 1995), a value considerably lower than that typically reported following maximal BIKE exercise (Jeneson and Bruggeman, 2004). Therefore, in the context of previous research, and not without limitations, we contend that we were able to vary afferent feedback, which enabled subjects to accumulate a greater degree of peripheral fatigue during small muscle mass exercise.
Conclusion
Confining group III/IV afferent feedback to a small muscle mass during dynamic KE exercise, in contrast to the multiple sources during whole body cycling, resulted in a greater degree of peripheral fatigue following constant load exercise to exhaustion. This finding further highlights the role of afferent feedback in limiting the development of peripheral fatigue. Additionally, this study reveals that much greater local skeletal muscle fatigue can be achieved by utilizing small muscle mass exercise and thus may promote enhanced exercise-induced adaptation, with implications for the application of exercise training in both health and disease.
Acknowledgements
The authors would like to thank the subjects for their gracious participation and are thankful for financial support by the National Institutes of Health grant PO1 HL 09830.
References
- Abbiss CR, Karagounis LG, Laursen PB, Peiffer JJ, Martin DT, Hawley JA, Fatehee NN, Martin JC. Single-leg cycle training is superior to double-leg cycling in improving the oxidative potential and metabolic profile of trained skeletal muscle. J Appl Physiol. 2011;110:1248–55. doi: 10.1152/japplphysiol.01247.2010. [DOI] [PubMed] [Google Scholar]
- Adreani CM, Hill JM, Kaufman MP. Responses of group III and IV muscle afferents to dynamic exercise. J Appl Physiol. 1997;82:1811–7. doi: 10.1152/jappl.1997.82.6.1811. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Impaired calcium release during fatigue. J Appl Physiol. 2008a;104:296–305. doi: 10.1152/japplphysiol.00908.2007. [DOI] [PubMed] [Google Scholar]
- Allen DG, Lamb GD, Westerblad H. Skeletal muscle fatigue: cellular mechanisms. Physiol Rev. 2008b;88:287–332. doi: 10.1152/physrev.00015.2007. [DOI] [PubMed] [Google Scholar]
- Amann M. Central and peripheral fatigue: interaction during cycling exercise in humans. Med Sci Sports Exerc. 2011;43:2039–45. doi: 10.1249/MSS.0b013e31821f59ab. [DOI] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Group III and IV muscle afferents contribute to ventilatory and cardiovascular response to rhythmic exercise in humans. J Appl Physiol. 2010;109:966–76. doi: 10.1152/japplphysiol.00462.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Blain GM, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Implications of Group Iii and Iv Muscle Afferents for High Intensity Endurance Exercise Performance in Humans. J Physiol. 2011a doi: 10.1113/jphysiol.2011.213769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Calbet JA. Convective oxygen transport and fatigue. J Appl Physiol. 2008;104:861–70. doi: 10.1152/japplphysiol.01008.2007. [DOI] [PubMed] [Google Scholar]
- Amann M, Dempsey JA. Locomotor muscle fatigue modifies central motor drive in healthy humans and imposes a limitation to exercise performance. J Physiol. 2008;586:161–73. doi: 10.1113/jphysiol.2007.141838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Eldridge MW, Lovering AT, Stickland MK, Pegelow DF, Dempsey JA. Arterial oxygenation influences central motor output and exercise performance via effects on peripheral locomotor muscle fatigue in humans. J Physiol. 2006a;575:937–52. doi: 10.1113/jphysiol.2006.113936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Proctor LT, Sebranek JJ, Pegelow DF, Dempsey JA. Opioid-mediated muscle afferents inhibit central motor drive and limit peripheral muscle fatigue development in humans. J Physiol. 2009;587:271–83. doi: 10.1113/jphysiol.2008.163303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Romer LM, Pegelow DF, Jacques AJ, Hess CJ, Dempsey JA. Effects of arterial oxygen content on peripheral locomotor muscle fatigue. J Appl Physiol. 2006b;101:119–27. doi: 10.1152/japplphysiol.01596.2005. [DOI] [PubMed] [Google Scholar]
- Amann M, Romer LM, Subudhi AW, Pegelow DF, Dempsey JA. Severity of arterial hypoxaemia affects the relative contributions of peripheral muscle fatigue to exercise performance in healthy humans. J Physiol. 2007;581:389–403. doi: 10.1113/jphysiol.2007.129700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Runnels S, Morgan DE, Trinity J, Fjeldstad A, Wray DW, Reese VR, Richardson RS. On the Contribution of Group Iii and Iv Muscle Afferents to the Circulatory Response to Rhythmic Exercise in Humans. J Physiol. 2011b doi: 10.1113/jphysiol.2011.209353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amann M, Subudhi A, Foster C. Influence of testing protocol on ventilatory thresholds and cycling performance. Med Sci Sports Exerc. 2004;36:613–22. doi: 10.1249/01.mss.0000122076.21804.10. [DOI] [PubMed] [Google Scholar]
- Andersen P, Adams RP, Sjogaard G, Thorboe A, Saltin B. Dynamic knee extension as model for study of isolated exercising muscle in humans. J Appl Physiol. 1985;59:1647–53. doi: 10.1152/jappl.1985.59.5.1647. [DOI] [PubMed] [Google Scholar]
- Barry BK, Enoka RM. The neurobiology of muscle fatigue: 15 years later. Integr Comp Biol. 2007;47:465–73. doi: 10.1093/icb/icm047. [DOI] [PubMed] [Google Scholar]
- Borg G. Borg’s Perceived Exertion and Pain Scales. Human Kinetics; Champaign, IL: 1998. [Google Scholar]
- Burnley M. Estimation of critical torque using intermittent isometric maximal voluntary contractions of the quadriceps in humans. J Appl Physiol. 2009;106:975–83. doi: 10.1152/japplphysiol.91474.2008. [DOI] [PubMed] [Google Scholar]
- Freund PR, Hobbs SF, Rowell LB. Cardiovascular responses to muscle ischemia in man--dependency on muscle mass. J Appl Physiol. 1978;45:762–7. doi: 10.1152/jappl.1978.45.5.762. [DOI] [PubMed] [Google Scholar]
- Fulco CS, Lewis SF, Frykman PN, Boushel R, Smith S, Harman EA, Cymerman A, Pandolf KB. Muscle fatigue and exhaustion during dynamic leg exercise in normoxia and hypobaric hypoxia. J Appl Physiol. 1996;81:1891–900. doi: 10.1152/jappl.1996.81.5.1891. [DOI] [PubMed] [Google Scholar]
- Gagnon P, Saey D, Vivodtzev I, Laviolette L, Mainguy V, Milot J, Provencher S, Maltais F. Impact of preinduced quadriceps fatigue on exercise response in chronic obstructive pulmonary disease and healthy subjects. J Appl Physiol. 2009;107:832–40. doi: 10.1152/japplphysiol.91546.2008. [DOI] [PubMed] [Google Scholar]
- Gandevia SC. Spinal and supraspinal factors in human muscle fatigue. Physiol Rev. 2001;81:1725–89. doi: 10.1152/physrev.2001.81.4.1725. [DOI] [PubMed] [Google Scholar]
- Hill JM. Discharge of group IV phrenic afferent fibers increases during diaphragmatic fatigue. Brain Res. 2000;856:240–4. doi: 10.1016/s0006-8993(99)02366-5. [DOI] [PubMed] [Google Scholar]
- Hogan MC, Richardson RS, Haseler LJ. Human muscle performance and PCr hydrolysis with varied inspired oxygen fractions: a 31P-MRS study. J Appl Physiol. 1999;86:1367–73. doi: 10.1152/jappl.1999.86.4.1367. [DOI] [PubMed] [Google Scholar]
- Iwamoto GA, Botterman BR. Peripheral factors influencing expression of pressor reflex evoked by muscular contraction. J Appl Physiol. 1985;58:1676–82. doi: 10.1152/jappl.1985.58.5.1676. [DOI] [PubMed] [Google Scholar]
- Jeneson JA, Bruggeman FJ. Robust homeostatic control of quadriceps pH during natural locomotor activity in man. FASEB J. 2004;18:1010–2. doi: 10.1096/fj.03-0762fje. [DOI] [PubMed] [Google Scholar]
- Kufel TJ, Pineda LA, Mador MJ. Comparison of potentiated and unpotentiated twitches as an index of muscle fatigue. Muscle Nerve. 2002;25:438–44. doi: 10.1002/mus.10047. [DOI] [PubMed] [Google Scholar]
- Lepers R, Maffiuletti NA, Rochette L, Brugniaux J, Millet GY. Neuromuscular fatigue during a long-duration cycling exercise. J Appl Physiol. 2002;92:1487–93. doi: 10.1152/japplphysiol.00880.2001. [DOI] [PubMed] [Google Scholar]
- Mador MJ, Kufel TJ, Pineda LA. Quadriceps and diaphragmatic function after exhaustive cycle exercise in the healthy elderly. Am J Respir Crit Care Med. 2000;162:1760–6. doi: 10.1164/ajrccm.162.5.2001005. [DOI] [PubMed] [Google Scholar]
- Mador MJ, Kufel TJ, Pineda LA, Steinwald A, Aggarwal A, Upadhyay AM, Khan MA. Effect of pulmonary rehabilitation on quadriceps fatiguability during exercise. Am J Respir Crit Care Med. 2001;163:930–5. doi: 10.1164/ajrccm.163.4.2006125. [DOI] [PubMed] [Google Scholar]
- Magnusson G, Gordon A, Kaijser L, Sylven C, Isberg B, Karpakka J, Saltin B. High intensity knee extensor training, in patients with chronic heart failure. Major skeletal muscle improvement. Eur Heart J. 1996;17:1048–55. doi: 10.1093/oxfordjournals.eurheartj.a015001. [DOI] [PubMed] [Google Scholar]
- Martin PG, Smith JL, Butler JE, Gandevia SC, Taylor JL. Fatigue-sensitive afferents inhibit extensor but not flexor motoneurons in humans. J Neurosci. 2006;26:4796–802. doi: 10.1523/JNEUROSCI.5487-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matkowski B, Place N, Martin A, Lepers R. Neuromuscular fatigue differs following unilateral vs bilateral sustained submaximal contractions. Scand J Med Sci Sports. 2011;21:268–76. doi: 10.1111/j.1600-0838.2009.01040.x. [DOI] [PubMed] [Google Scholar]
- Merton PA. Voluntary strength and fatigue. J Physiol. 1954;123:553–64. doi: 10.1113/jphysiol.1954.sp005070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex: its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol. 1983;45:229–42. doi: 10.1146/annurev.ph.45.030183.001305. [DOI] [PubMed] [Google Scholar]
- Mortensen SP, Dawson EA, Yoshiga CC, Dalsgaard MK, Damsgaard R, Secher NH, Gonzalez-Alonso J. Limitations to systemic and locomotor limb muscle oxygen delivery and uptake during maximal exercise in humans. J Physiol. 2005;566:273–85. doi: 10.1113/jphysiol.2005.086025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy MN, Mizuno M, Mitchell JH, Smith SA. Cardiovascular regulation by skeletal muscle reflexes in health and disease. Am J Physiol Heart Circ Physiol. 2011;301:H1191–204. doi: 10.1152/ajpheart.00208.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Polkey MI, Kyroussis D, Hamnegard CH, Mills GH, Green M, Moxham J. Quadriceps strength and fatigue assessed by magnetic stimulation of the femoral nerve in man. Muscle Nerve. 1996;19:549–55. doi: 10.1002/(SICI)1097-4598(199605)19:5<549::AID-MUS1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Frank LR, Haseler LJ. Dynamic knee-extensor and cycle exercise: functional MRI of muscular activity. Int J Sports Med. 1998;19:182–7. doi: 10.1055/s-2007-971901. [DOI] [PubMed] [Google Scholar]
- Richardson RS, Noyszewski EA, Kendrick KF, Leigh JS, Wagner PD. Myoglobin O2 desaturation during exercise. Evidence of limited O2 transport. J Clin Invest. 1995;96:1916–26. doi: 10.1172/JCI118237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson RS, Wagner H, Mudaliar SR, Saucedo E, Henry R, Wagner PD. Exercise adaptation attenuates VEGF gene expression in human skeletal muscle. Am J Physiol Heart Circ Physiol. 2000;279:H772–8. doi: 10.1152/ajpheart.2000.279.2.H772. [DOI] [PubMed] [Google Scholar]
- Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol. 1988;64:2306–13. doi: 10.1152/jappl.1988.64.6.2306. [DOI] [PubMed] [Google Scholar]
- Saey D, Michaud A, Couillard A, Cote CH, Mador MJ, LeBlanc P, Jobin J, Maltais F. Contractile fatigue, muscle morphometry, and blood lactate in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2005;171:1109–15. doi: 10.1164/rccm.200408-1005OC. [DOI] [PubMed] [Google Scholar]
- Sandiford SD, Green HJ, Duhamel TA, Schertzer JD, Perco JD, Ouyang J. Muscle Na-K-pump and fatigue responses to progressive exercise in normoxia and hypoxia. Am J Physiol Regul Integr Comp Physiol. 2005;289:R441–R449. doi: 10.1152/ajpregu.00652.2004. [DOI] [PubMed] [Google Scholar]
- Vagg R, Mogyoros I, Kiernan MC, Burke D. Activity-dependent hyperpolarization of human motor axons produced by natural activity. J Physiol. 1998;507(Pt 3):919–25. doi: 10.1111/j.1469-7793.1998.919bs.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanhatalo A, Fulford J, DiMenna FJ, Jones AM. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: a 31P magnetic resonance spectroscopy study. Exp Physiol. 2010;95:528–40. doi: 10.1113/expphysiol.2009.050500. [DOI] [PubMed] [Google Scholar]
- Verges S, Maffiuletti NA, Kerherve H, Decorte N, Wuyam B, Millet GY. Comparison of electrical and magnetic stimulations to assess quadriceps muscle function. J Appl Physiol. 2009;106:701–10. doi: 10.1152/japplphysiol.01051.2007. [DOI] [PubMed] [Google Scholar]
- Westerblad H, Allen DG. Cellular mechanisms of skeletal muscle fatigue. Adv Exp Med Biol. 2003;538:563–70. doi: 10.1007/978-1-4419-9029-7_50. discussion 571. [DOI] [PubMed] [Google Scholar]