SUMMARY
Alphaherpesvirus virions infect neurons and are transported in axons for long distance spread within the host nervous system. The assembly state of newly made herpesvirus particles during anterograde transport in axons is an essential question in alphaherpesvirus biology. The structure of the particle has remained both elusive and controversial for the past two decades, with conflicting evidence from EM, immunofluorescence, and live cell imaging studies. Two opposing models have been proposed—the Married and Separate Models. Under the Married Model, infectious virions are assembled in the neuronal cell body before sorting into axons and then traffic inside a transport vesicle. Conversely, the Separate Model postulates that vesicles containing viral membrane proteins are sorted into axons independent of capsids, with final assembly of mature virions occurring at a distant egress site. Recently, a complementary series of studies employing high-resolution EM and live cell fluorescence microscopy have provided evidence consistent with the Married Model, whereas other studies offer evidence supporting the Separate Model. In this review, we compare and discuss the published data and attempt to reconcile divergent findings and interpretations as they relate to these models.
TWO MODELS FOR SORTING AND TRANSPORT OF ALPHAHERPES VIRUS PARTICLES IN AXONS
Members of the Alphaherpesvirinae subfamily, including PRV and HSV, infect a wide range of vertebrate species. Infection initiates by virion infection of epithelial cells, followed by invasion of nerve terminals and retrograde transport of the capsid using host axonal trafficking machinery to PNS neuronal cell bodies [1]. A complex interaction between infected neurons and natural host defenses leads to the establishment of a latent infection in PNS neurons with only occasional reactivation [2–4].
Active viral replication produces infectious progeny virions as well as a variety of intermediates and virus-like structures from the assembly pathway [5–8]. The term “particle” is a loosely defined reference to this cohort of viral structures (Table 1). A “virion” is a mature virus particle containing a genome within a proteinaceous capsid, wrapped in a layer of viral proteins termed tegument, and enclosed within a host derived membrane. Spread of infection requires assembly of the virion structure, subsequent egress of that mature virion from the infected cell, entry of the capsid into a new susceptible cell, and delivery of the viral genome to the nucleus to initiate a new round of replication. Alphaherpes viruses spread between synaptically connected neurons in two distinct directions: from the pre-synaptic neuron to the post-synaptic neuron (anterograde spread) or from the post-synaptic neuron to the pre-synaptic neuron (retrograde spread). Consequently, we can define two distinct sites where egress occurs to achieve directional transneuronal spread: the cell body and dendrites (retrograde spread) or the axon terminus and varicosities along axon shafts (anterograde spread).
Table 1.
Key terms and definitions, as used in this review
| Term | Definition |
|---|---|
| Anterograde/retrograde spread | Transneuronal spread of infectious virions across synaptically connected neurons in a circuit |
| Anterograde transport | Trafficking of the structural components of a virion, including capsid, tegument, and glycoproteins, down an axon along microtubules in a plus-end-directed manner toward the axon terminus |
| Retrograde transport | Transport of an infecting capsid in a minus-end-directed manner within axons toward the soma |
| Virion | Infectious unit of an alphaherpes virus, consisting of a capsid surrounded by tegument, surrounded by a host-derived lipid bilayer containing viral glycoproteins |
| Transport vesicle | A host-derived vesicle capable of associating with microtubule motors and transporting cargo in its lumen |
| Particle | Any of the structures that exist during the alphaherpes virus life cycle (entry through assembly and egress) including: |
| 1. Naked capsids | |
| 2. Virions resident in the lumen of a transport vesicle | |
| 3. L-particles resident in the lumen of a transport vesicle | |
| 4. Empty transport vesicles containing viral glycoproteins |
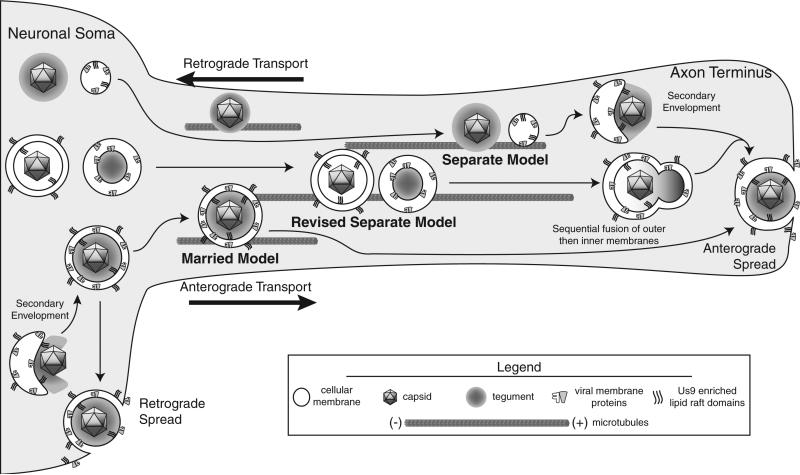
The sorting mechanism that partitions virus particles to either retrograde or anterograde egress sites is not well understood. Retrograde spread events from cell bodies are far more frequent than anterograde spread events from axons [9]. The viral particles involved in retrograde spread are presumed to be mature virions contained in transport vesicles, which fuse at the post-synaptic cell membrane of the dendrite or soma analogous to transport/egress from epithelial cells. In contrast, there is much disagreement over the structure of the particle that is involved in anterograde spread. The disagreement stems from differing observations and interpretations of viral particles visualized in the axons of infected neurons (compiled in Table 2). Two models have been proposed based on the structure of the viral particle: the “Married” and “Separate” models (Figure 1). Under the Married Model, mature virions are assembled in the cell body and sorted into axons in transport vesicles for egress and spread at distal sites. Conversely, the Separate Model holds that naked capsids, and viral glycoprotein vesicles are transported down the axon separately, with final virion assembly occurring at sites of egress.
Table 2.
Summary of notable studies and their evidence for or against the Married and Separate Models
| Reference | Viral strain and infection timepoint | Neuronal culture | Assay type and axon location | Findings/model supported |
|---|---|---|---|---|
| Taylor et al., 2012 [20] | PRV 8–10hpi | Rat SCG dissociated cultures | Live cell imaging; proximal and distal axon regions | 96% colocalization of capsid/membrane protein signals during anterograde transport events→Married Model |
| Ibiricu et al., 2011 [60] | HSV-1 F, KOS 16hpi | Rat hippocampal dissociated cultures | Cryo-electron tomography; mid-axon(?) | Predominantly naked capsids (67 of 73 total axonal structures); secondary envelopment events at axon termini, no quantitation→Separate Model |
| Wisner et al., 2011 [25] | HSV-1 YK304; 19–23hpi | Rat DRG in microfluidic chambers, SK-N-SH (induced neuron-like human cells) dissociated cultures | Live cell imaging; undescribed region of axon | Variable colocalization (~50% max) of capsid/glycoprotein signals during anterograde transport events→Married and Separate Models |
| Huang et al., 2011 [22] | PRV, HSV-1 NS, HSV-2 2.12 24, 48 hpi | Rat SCG Campenot Chambered Cultures | TEM; distal axon contained within Campenot chamber | Enveloped capsids in axons across all three viral strains; no quantitation→Married Model |
| Antinone et al., 2010 [24] | HSV-1 18–24hpi | Chick and Rat DRG, mouse Cath.A-differentiated (induced neuron-like cells) dissociated cultures | Live cell imaging; proximal and distal axon | 65%–70% colocalization of capsid/glycoprotein signals during clear anterograde transport events→Married Model |
| Negatsch et al., 2010 [34] | HSV-1 HFEM, SC16, 17+16–24hpi | Rat SCG dissociated cultures | TEM; proximal and distal axon | 75% enveloped virions in axons→Married Model |
| Maresch et al., 2010 [23] | PRV 18hpi | Rat SCG dissociated cultures | TEM; proximal and distal axon | <10% naked capsids in axons→Married Model |
| Miranda-Saksena et al., 2009 [64] | HSV-1 CW1 28hpi | Human DRG explant | TIEM; undefined region of axon | Colocalization of Vp16 and Vp22 on large dense-core vesicles, no colocalization with gB→Separate Model |
| Snyder et al., 2008 [39] | HSV-1 F 18hpi | SK-N-SH dissociated cultures | IF; proximal and distal axon | No detection of glycoproteins (gD/gB) or capsids in axons in Us9-null background; colocalization of Us9, but not gE/gI with capsids in axons→Separate Model |
| Lyman et al., 2007 [15] | PRV 24hpi | Rat SCG explant cultures; dual chamber | Live cell imaging; proximal and distal axon | No viral structural components (tegument, capsid, or glycoprotein) detected in axon in Us9-null background→Married Model |
| Feierbach et al., 2007 [21] | PRV 20hpi | Rat SCG, dual chamber culture | TEM; mid-axon | Mostly enveloped virions in axons, no quantitation→Married Model |
| Miranda-Saksena et al., 2006 [62] | HSV-1 CW1 24 and 48 hpi | Human DRG explant cultures | TEM; varicosities and growth cones | Enveloped and unenveloped capsids at sites of egress; 6 partially enveloped; 1974 “axon processes and regions” examined total→Separate Model |
| Snyder et al., 2006 [38] | HSV-1 F 15–18hpi | SK-N-SH dissociated cultures | IF; proximal and distal axon | Only 3%–6% colocalization of capsid (anti-Vp5) and glycoprotein (anti-gD, gB, gE/gI) signals→Separate Model |
| Antinone et al., 2006 [19] | PRV 10-13hpi | Chick DRG dissociated cultures | Live cell imaging; proximal and distal axon | 85% colocalization of capsid/glycoprotein signals→Married Model |
| Ch'ng and Enquist, 2005 [14] | PRV 14hpi | Rat SCG Campenot Chamber Cultures | TEM; mid-axon | Mostly enveloped virions in axons, no quantitation→Married Model |
| Del Rio et al., 2005 [52] | PRV 12hpi | Rat SCG dissociated cultures | TEM; proximal and distal axon | Mostly enveloped virions in axons, no quantitation→Married Model |
| Tomishima and Enquist, 2001 [13] | PRV 12, 16hpi | Rat SCG dissociated cultures | IF, proximal and distal axon | Capsids and tegument (Vp22, UL25) can be detected in axons despite nonfunctional Us9→Separate Model |
| Miranda-Saksena et al., 2000 [51] | HSV-1 CS1 17 and 24 hpi | Human and Rat DRG dissociated cultures; Brefeldin pretreatment | TEM, TIEM; proximal axon | No glycoproteins (gC) or tegument (Vp16) detected in axons following Brefeldin-A treatment; unenveloped capsids present in axon; no quantitation→Separate Model |
| Ohara et al., 2000 [33] | HSV-1 F 3dpi | Murine trigeminal ganglia axons, in vivo | TIEM; undefined axonal region | Mature virions in vesicles in axons visualized by day 3 of infection→Married Model |
| Holland et al., 1999 [59] | HSV-1 CW1 24hpi | Human DRG, dual chamber cultures | TEM, TIEM; undefined axonal region | Only unenveloped capsids detected, no quantitation; 95% colocalization of Vp5 with Vp16 signal; dense-core vesicles resembling naked capsids detected in uninfected axons→Separate Model |
| LaVail et al., 1997 [32] | HSV-1 H129 3dpi | Murine trigeminal ganglia axons, in vivo | TEM; undefined axonal region | Enveloped virions detected in axonal profiles; no quantitation→Married Model |
| Penfold et al., 1994 [35] | HSV-1 WM1 and WM2 16, 24, 48 hpi | Human DRG explant cultures; dual chamber | TEM; undefined axonal region | Only unenveloped capsids detected in axons (>200), enveloped capsids in soma (20)→Separate Model |
| Lycke et al., 1988 [31] | HSV-1 McIntyre 20hpi | Human DRG dissociated cultures | TEM; undefined axonal region | Enveloped virions detected in axonal profiles; exocytosis of virion transport vesicles; no quantitation→Married Model |
| Kristensson et al., 1974 [29] | HSV-2 G 3dpi | Rabbit retinal ganglia and right superior colliculus | TEM; undefined axonal region | Predominantly enveloped virions detected in axonal processes; no quantitation—> Married Model |
| Cook and Stevens, 1973 [28] | HSV-1 McIntrye 4dpi | Murine DRG, in vivo | TEM; undefined axonal region | Enveloped virions detected in axonal profiles; no quantitation→Married Model |
| Yamamoto et al., 1973 [30] | HSV-1 HF 1–4dpi | Murine coeliac ganglia, in vivo | TEM; undefined axonal region | Enveloped virions detected in groups in axonal profiles; no quantitation→Married Model |
| Hill et al., 1972 [26] | HSV-1, –2 6–7dpi | Murine sciatic nerve ganglia | TEM; undefined axonal region | Enveloped virions detected in axonal endoplasmic reticulum; no quantitation→Married Model |
Key points relating to viral strain, cell type, and location are noted. The key findings have been summarized as well as whether the interpretation supports the Married or Separate models. The studies have been organized by date published, most recent first.
SCG, superior cervical ganglia; DRG, dorsal root ganglia; TEM, transmission electron microscopy; TIEM, transmission immuno-electron microscopy;IF, immuno-fluorescence.
Figure 1.
Schematic of HSV anterograde transport models. A schematic visualization of the Married, Separate, and Revised Separate Models of anterograde transport. Particles are diagramed at three stages: cell body assembly/sorting, axonal transport, and egress. Distribution of tegument is not accounted for by the Revised Separate Model and therefore not presented
It is important to resolve the discrepancies between the two models of alphaherpesvirus transport if we are to understand viral spread and pathogenesis. In this review, we examine the evidence describing anterograde transport of PRV and HSV viral particles within axons. We also discuss experimental limitations that may have given rise to conflicting interpretations and suggest experiments that may reconcile the differences between the Married and Separate models of viral anterograde transport.
MEMBRANE ACQUISITION PRECEDES ANTEROGRADE TRANSPORT OF PRV: THE MARRIED MODEL
PRV is an alphaherpesvirus of pigs utilized in experimental models because of its robust replication and spread within neurons. It is often compared with HSV to illuminate and assay the shared properties of alphaherpes viruses [10]. Understanding the assembly state of PRV virions undergoing antero-grade transport in axons is an important step toward establishing a generalized model for alphaherpes virus trafficking. To date, a consensus model has been reached for PRV: viral particles trafficking in the anterograde direction are mature virions in transport vesicles, consistent with the Married Model. However, early experimental results were interpreted to support the Separate Model. In the following analysis, we will discuss the basis for this prior assertion and the experimental evidence that now supports the Married Model for PRV anterograde transport.
Molecular mechanisms of PRVaxonal sorting and anterograde transport
Directional spread of alphaherpes viruses is modulated by the action of several viral proteins. The PRV proteins gE, gI, and Us9 are necessary for anterograde spread of infection in vivo [11,12]. In cultured neurons, these proteins facilitate sorting of viral particles into axons [13–15]. The membrane protein Us9 plays a dominant role in axonal sorting and transport [15]. Initial observations during a Us9-null PRV infection of PNS cultures detected capsids, but not membrane proteins, in axons by IF microscopy [13]. This finding was interpreted to show that newly made capsids and envelope proteins were transported by separate mechanisms in axons. However, the dispersed cultures of neurons used in these studies have an inherent confounding variable: the extensive secondary retrograde spread of infection between infected cells that occurs during infection. The axon network in these cultures forms functional connections between adjacent axons and cells such that a single axon transports not only viral particles from the initial inoculum but also newly made virions egressing from other infected cells [16,17]. This connectivity makes it impossible to deduce the directionality of viral particles in fixed images of axons. Studies using modified Campenot chambers or microfluidic devices where cell bodies are physically separated from axons minimized the confounding effect of retrograde spread and confirmed that Us9 plays a central role in sorting all virion components into axons [15,18].
Live cell fluorescence microscopy
PRV recombinants expressing fluorescent proteins fused to structural proteins have been invaluable for labeling PRV particles as well as simultaneously imaging their movement in living cells. Antinone et al. [19] characterized the co-localization and dynamics of two fluorescent fusion proteins in PRV infected neurons: GFP fused to the glycoprotein gD and mRFP fused to the capsid-associated protein Vp26. The two fluorescent signals co-localized in more than 86% of particles trafficking in axons in the anterograde direction. However, none of the mRFP-labeled capsids moving in the retrograde direction were labeled with gD-GFP. An important concern is the limit of detection: how many gDGFP molecules need to be present in a particle to be visible by fluorescence microscopy. This study attempted to answer this question by imaging extracellular virions. The expectation was that every capsid will have an envelope and therefore should show detectable red and green signals. The authors found that 86% of extracellular virions identified by an mRFP signal (capsids) were also GFP positive (capsids with membranes). However, 14% of the particles with an mRFP signal had no apparent GFP signal. This limit of detectability indicates that the 14% population of mRFP puncta undergoing anterograde transport without associated GFP fluorescence might not be naked capsids but rather virions with insufficient gD-GFP signal to be detected. A similar dual fluorescent protein imaging study with a PRV recombinant expressing a GFP-US9 membrane fusion protein reported that anterograde directed capsids always co-localized with the GFP-Us9 signal [20]. Both studies are consistent with the Married Model of axonal sorting and transport where viral membrane proteins and capsid proteins traffic together in the anterograde direction.
Importantly, these studies also noted important characteristics related to capsid dynamics during infection. Both studies reported abundant mRFPVP26 capsid puncta moving the retrograde direction with no membrane protein signal. One explanation is that these particles originate from secondary or delayed primary infection. A second shared observation was a large population of immobile capsids present in the axon at all times during infection. The origin of these stationary capsids is unclear, but they might arise from dissociation of the microtubule-motor complex from transporting particles or fusion of the transport vesicle with the surrounding axonal membrane. All these particles would cofound interpretation of fixed cells visualized by EM or optical IF imaging. The evidence that first supported the Separate Model for PRV was obtained in fixed cells [13]. Only after studies were performed with live-cell microscopy did it become clear that most of the naked capsids in axons were retrograde moving or stalled particles.
Electron microscopy
Ultrastructural studies of PRV-infected neurons have also been performed to explore the assembly state of particles in axons. Two complementary studies employing neuronal cultures whose cell bodies and axons are physically separated in a modified Campenot chamber provided strong evidence for the Married Model [21,22]. Enveloped PRV virions were identified throughout the length of the axon after infection, with less than 10% unenveloped capsids observed at any point [21,22]. Furthermore, work by Maresch and colleagues [23] using a gB-null PRV strain incapable of initiating a secondary round of infection also visualized predominantly enveloped virions throughout the axon using high resolution EM. This study limited the confounding effect of secondary infection and also efficiently discriminated between cellular dense-core neurovesicles and capsids, which can be quite similar in appearance—a factor that is considered for HSV infection in the succeeding text. Taken together, these EM studies provided the compelling evidence for the Married Model of anterograde transport for PRV.
EVIDENCE FOR THE MARRIED MODEL OF HSV ANTEROGRADE TRANSPORT
Research on axonal transport of HSV has utilized immunofluorescence, EM, and live cell fluorescence microscopy techniques as with PRV. Table 2 provides a compilation of these reports and the different experimental approaches. Much of the most recent work supports the Married Model of anterograde transport where mature HSV virions undergo ante-rograde transport in a transport vesicle.
Live cell imaging of dual-fluorescent constructs
Recent live cell imaging of neurons or neuronal cell lines infected with dual-fluorescent HSV-1 constructs is consistent with the Married Model of anterograde axonal transport. Using an HSV strain expressing GFP-gB and mRFP-Vp26 fusion proteins, Antinone et al. [24] reported 65%–70% colocalization of the membrane protein and capsid signals for particles moving in the anterograde direction throughout the length of chick and rat dorsal root ganglia axons, as well as axons from mouse neuronal cell line. They observed co-localized fluorescent puncta moving in the anterograde direction and some red puncta with no GFP signal moving in the retrograde direction. Furthermore, the authors also imaged extracellular virions to establish baseline detection limits for fluorescent proteins in these enveloped particles. They found that 58% of the red capsid puncta co-labeled with green puncta of variable intensity (GFP-gB), whereas the remainder lacked GFP signal. They concluded that the incorporation of GFP-gB in virion envelopes is heterogeneous and more than 42% have insufficient GFP-gB to be visible in their system. On the basis of these data, they interpreted the anterograde motion of some red puncta in axons without associated green fluorescence to reflect virions with insufficient GFP-gB for detection. Given these findings, the authors concluded that the Married Model holds for these HSV-1 mutants. A complementary study from Wisner et al. [25] using an HSV recombinant expressing both mRFP-gB and Vp26-Venus fusion proteins reported more variable colocalization of the membrane (red) and capsid (Venus) markers in rat superior cervical ganglia (SCG) neurons. The authors concluded that both Married and Separate particles could traffic in rat SCG axons, and the majority were separate. This study did not include imaging of extracellular virions of the dual recombinant virus to establish the detection limits. It is therefore difficult to establish if Venus puncta with no red signal are particles without envelopes or if the red signal is simply below the detection level in these particles. Some of their data (Figure 6 of [25]) display consistent anterograde-directed kinetic profiles consistent with the Married Model. One of the representative separate particles (Figure 6 of [25]) engages in net retrograde transport over the time course presented. Given the fluorescence profiles of the anterograde transport events available from both studies, it is possible that most HSV-1 capsids of these mutant viruses are co-transported with glycoproteins.
Electron microscopy of HSV-1 in axons
Early EM studies visualizing HSV-1 particles in axons consistently reported that the particles were not naked capsids but rather enveloped virions [26–33]. Recent EM studies also reported the presence of enveloped HSV virions resident in the lumen of a transport vesicle in axons [22,34]. Using the tri-chamber Campenot neuronal culture system with rat or mouse SCG neurons, Huang et al. [22] described enveloped capsids in axons during infection with PRV, HSV-1, and HSV-2, using identical multiplicity of infection and culture conditions. Similarly, Negatsch et al. [34] found ~70% enveloped capsids in axons of SCG neurons during infection with the HSV-1 HFEM, SC16, and 17+ strains. Their high-resolution EM work enabled efficient discrimination between cellular dense-core neurovesicles and true capsids. This combination of a high resolution, large sample size study, and tri-chamber isolation of infection offer complementary evidence that the majority of HSV capsids found in axons, inferred to be undergoing anterograde transport, are indeed enveloped.
EVIDENCE FOR THE SEPARATE MODEL OF HSV ANTEROGRADE TRANSPORT
The Separate Model was first proposed by Penfold et al. [35] through an EM visualization of naked capsids in HSV-1 infected axons. Since then, a number of studies have provided evidence for and against this model (reviewed in [36]; Table 2). It has been difficult to find experiments that disprove one model or the other because imaging techniques have differing limitations and therefore can reach different conclusions.
Immunofluorescence assays and epitope masking
Investigators have used a number of antibodies to viral glycoproteins in conjunction with an anti-Vp5 capsid antibody to assay the assembly state of particles in axons by IF. Several studies, notably work by Snyder and colleagues [37–39], have shown minimal colocalization between capsid and glyco-protein signals for particles in HSV-infected axons. Separate punctate signals in axons were interpreted as segregated transport complexes consistent with the Separate Model. An important issue regarding the interpretation of negative IF data concerns the accessibility of viral antigens in assembled particles. Does lack of an IF signal indicate that the structure is absent or does it mean that the antibody cannot interact with the epitope in question? The Separate Model holds that naked capsids, perhaps covered only by the inner tegument layer, are transported in the anterograde direction. The Married Model posits that mature virions are contained inside transport vesicles moving in the anterograde direction. The accessibility of capsid antigens to antibody is quite different in each model. For the Married Model, two membranes and the inner/outer tegument layer constitute physical barriers that may limit accessibility of the capsid to antibodies, even after permeabilization. IF protocols are designed to facilitate penetration of antibodies through the plasma membrane, but not necessarily through a second or third set of lipid bilayers associated with tegument proteins. Indeed, experiments presented by Antinone et al. [24] demonstrate that extracellular virions from an HSV strain expressing an mRFPVp26 capsid fusion protein show less than 50% colocalization of mRFP/antibody signal when probed with the anti-VP5 capsid antibody used in IF analyses of axonal transport [37–39]. A recent review suggests that in any study requiring fixation of cells, care must be taken to avoid treatment that alters the particle in question [40]. Schnell and colleagues [40] detail how variability in fixation and permeabilization efficacy, particularly with aldehydes, can alter protein conformations and organelle structure. They argue for use of complementary live-cell imaging assays where function can be correlated with structure.
The presence or absence of proteins as determined by antibodies or by fluorescent fusion proteins is also limited by the minimal amount of protein that can be detected. Data describing the distribution of viral proteins across the membrane of the transport vesicle as compared with the virion envelope are largely unavailable. Until the detection limits for each assay are verified, the absence of a signal does not necessarily indicate that the protein is absent, thus preventing durable conclusions from being made.
The role of Us9 in axonal targeting of HSV capsids
The PRV Us9 protein is crucial for sorting and transport of virions in axons. For HSV, Us9 is also implicated in axonal targeting of capsids, tegument, and glycoproteins [39,41]. However, conflicting results have been reported regarding both its biochemistry and contribution to anterograde spread of HSV infection [42]. HSV Us9 exhibits the same type II membrane topology as PRV Us9 [43] and is not a component of the tegument as had been previously suggested [44]. It is unclear how a membrane protein could govern anterograde transport of naked capsids, if the Separate Model is correct. Snyder et al. [39] initially proposed “the Loading Model” where trans-Golgi-network-derived vesicles containing Us9 would facilitate the transfer of naked capsids and vesicles containing viral glycoproteins onto microtubule tracks near the axon initial segment. However, such a model does not account for the observed colocalization of HSV Us9 with capsids in axons [39]. The authors therefore suggest a Revised Separate Model in which naked capsids are not entirely naked but rather enclosed in a transport vesicle containing Us9 but not other viral glycoproteins (Figure 1). Such a mechanism is not consistent with other data. First, all EM data supporting the Separate Model shows capsids completely devoid of a membrane. Second, such a structure is incompatible with any simple model of final virion assembly (Figure 1). Finally, assembly of HSV virions is believed to be mediated by direct interactions between gM and tegument proteins [45–48], and the Revised Separate Model precludes a direct role for gM, or any other viral glycoprotein, in the formation of the special capsid-Us9 vesicle. To account for these discrepancies, a novel mechanism of capsid envelopment, for which there is currently no evidence, must take place separate from the well-described processes of the secondary envelopment. As such, the Revised Separate Model has inconsistencies that remain to be resolved.
Brefeldin-A pretreatment inhibits axonal localization of capsids, tegument, and glycoproteins
Brefeldin-A (BFA) is a small molecule inhibitor that blocks assembly of virions by interfering with the secretory system and disrupting the Golgi apparatus [49,50]. It has been used to test the Separate Model because it should block cytoplasmic assembly of virions by disrupting viral membrane protein traf-ficking without affecting production of cytoplasmic naked capsids and their subsequent sorting into axons. Indeed, Dasgupta and Wilson found an abrogation of secondary envelopment following short-course BFA treatment along with an accumulation of nuclear and cytoplasmic naked capsids [50]. Notably then, Miranda-Saksena et al. [51] reported the presence of naked HSV-1 capsids in axons after a long-course BFA treatment. They concluded that capsids and glycoprotein vesicles engage separate sorting and transport complexes. They also reported an accumulation of enveloped capsids in the cell bodies, an unexpected observation given the published action of BFA on both PRV and HSV virion assembly [49,50]. Subsequent work by Snyder et al. [38] using various concentrations of BFA, however, found that BFA treatment induced a complete abrogation of capsid sorting into axons even when BFA was added at 3hpi. For PRV, del Rio et al. [52] also showed that BFA treatment completely blocked entry of both capsids and envelope proteins into axons. Taken together, these two reports are incompatible with the Separate Model in that the naked cytoplasmic capsids formed during BFA treatment are unable to undergo anterograde transport. It is possible that the capsids seen in axons after BFA treatment by Miranda-Saksena and colleagues [51] were newly infecting capsids undergoing retrograde transport toward the cell body.
Given what is known about how BFA affects HSV-1 virion assembly [50], it is not clear how BFA would block axonal entry of the Vp16, Vp22, and Vp13/14 tegument proteins to varying degrees [51,53]. Us11, another component of the mature virion tegument, has been visualized in axons in the presence of BFA [54]. As previously noted for IF analyses, it cannot be determined if the structures labeled by Us11 antibody in EM micrographs are moving toward or away from the cell body. Us11 and the conventional kinesin heavy chain are known to interact [54], suggesting that at least one tegument component could facilitate anterograde transport of unenveloped capsids. However, there is no evidence that Us11 is required for anterograde transport of HSV. Us11 is a multi-functional protein, involved in regulating viral mRNA translation and ribosome binding, as well as inhibition of apoptosis [55–58]. Kinesin may be required to move Us11 to various sites in the cell to achieve these functions. Importantly, any mechanism involving Us11 is unique to HSV-1 because neither PRV nor Varicella Zoster Virus, another related human alphaherpes virus, encodes a Us11 gene.
Electron microscopy of proximal and distal axons
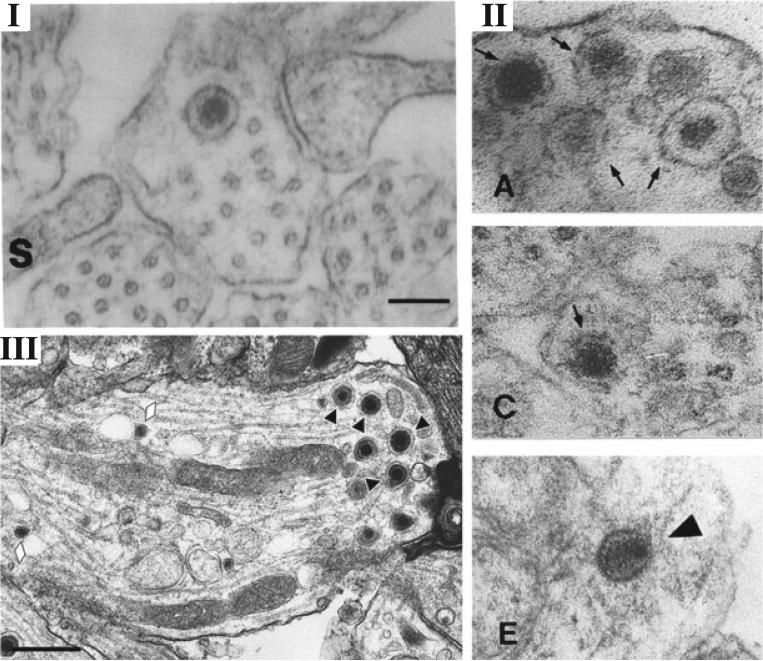
Penfold et al. [35] through EM analysis, first proposed the Separate Model for axonal spread of HSV infection. Using a two-chamber neuronal culture system with explants seeded in a center compartment projecting axons into the outer compartment, the Cunningham group reported significant numbers (>200) of unenveloped capsids in the proximal axon [35]. Furthermore, they noted the presence of a small number of naked capsids in distal regions of the axon 16 h after the addition of inoculum. The resolution of the EM images presented, notably Figure 3 of [35] (reproduced here in Figure 2), precludes a conclusive assessment of the assembly state of these capsids. For example, the representative image of a naked capsid in this figure could be interpreted to be enclosed by a membrane-like boundary and may not have the icosahedral shape typical of capsids. These particles may be dense-core vesicles, common structures in axons [59] (Figure 2). If many of these particles are indeed dense-core vesicles, then the quantitation of capsids and enveloped particles (20 enveloped in the soma vs >200 naked in the proximal axon) may be more consistent with the Married Model and other studies showing that most of the particles in axons are newly entering capsids and not egressing progeny particles [9,24,25]. The Campenot chambers employed do not prevent secondary re-infection events following egress of newly synthesized progeny outside the chamber wall or between explanted cell bodies. Control images of mock-infected cells are necessary to resolve this issue.
Figure 2.
Comparison of EM micrographs of putative naked capsids, dense-core vesicles, and enveloped virions in axons. (I) Figure 3, from Penfold et al. [35]—“unenveloped nucleocapsid in close proximity to microtubules” Copyright (1994) National Academy of Sciences, USA, reproduced with permission. (II) Figure 4, Panels A,C,E, from Holland et al. [59]—“TEM of axons in the . . . noninfected (A to C) model at 24h postinfection; (E) Viral nucleocapsids in the infected model” Copyright (1999) American Society for Microbiology, reproduced with permission. (III) Figure 1, Panel D, from Negatsch et al. [34]—“Black triangles indicate enveloped virions; . . . Neurovesicles are marked by lozenges.” Copyright (2010) American Society for Microbiology, reproduced with permission. Note: quotations indicate excerpt from authors’ original figure legend. The ability to discern between enveloped and naked capsids in axons through EM has been confounded by the presence of dense-core vesicles. Note the similarity between the naked capsid described in Penfold et al. [35] and the dense-core vesicles in Holland et al. [59]. Note the absence of naked capsids and the clear distinction between enveloped virions and dense core vesicles made possible by high-resolution EM in Negatsch et al. 2010 [34]
Recent work by Ibiricu et al. [60] employing live cell imaging and cryo-electron tomography visualization of HSV-infected hippocampal axons reported the presence of predominantly naked capsids in axons as well as putative secondary envelopment sites at axon terminals. Their compelling data support the Separate Model, though there is at least one confounding issue: it is unclear if the structures observed are in axons or in dendrites. Hippocampal neurons in culture develop numerous long dendrites and usually only one axon [61], and the authors presented no identification of the sections of axons where imaging was performed. Transport of viral particles in dendrites has not been previously characterized and may not exhibit the same polarity as axons. It is therefore possible that aberrant structures, such as naked capsids, may enter dendrites though they are not involved in transneuronal spread. However, the alternative possibility worth consideration may be that central and peripheral nervous system neurons support different modes of viral transport, reflecting the inherent differences between these classes of neurons.
CONCLUSIONS AND FUTURE DIRECTIONS
How do we resolve the current state of affairs? All available evidence from studies with PRV and some from HSV studies are consistent with the Married Model. Support for the Separate Model tends to be grounded in negative evidence: the inability to detect enveloped virions or components of mature virions in axons. Some of the negative data may reflect the limits of detection of these visual assays. The absence of an antibody or fluorescent signal may only indicate that the protein content is too low to be detected. Furthermore, the need to measure both the type of particle and its direction of movement is essential. Video microscopy coupled with quantitative data on detection limits is essential.
An important implication of the Separate Model is the final assembly of virions at axon termini and varicosities. However, the evidence for secondary envelopment of naked capsids at distal axon sites relies on interpretation of fixed images. The relative rarity of egress and entry events and the rapidity with which they occur present significant problems for quantitation and imaging. In the reports published to date, only five such events have been observed in axon termini and varicosities out of a sampling of 1206 total “axon processes and regions of axons” from an unspecified number of replicate samples [62]. It is possible that these structures may be assembly intermediates, or they may be intermediates of endocytosis or entry of incoming particles. EM studies of PRV and HSV secondary envelopment have visualized it as a sequestration process, with a distinct horseshoe-shaped vesicle intermediate as the naked capsid invaginates the membrane (notably Figure 3, [6]). Without video microscopy to correlate with fixed images, the interpretation of static virion structures in axons, in terminals, or in varicosities remains an open question. Stalled particles, particles that have egressed along the axon shaft, particles that have entered via axons and are on the way to the cell body, and particles that are on the surface of axons (e.g. failed entry), all can confound imaging techniques using fixed cells. Furthermore, live cell imaging studies can be hampered by spatially limited regions, frequent movement of particles outside the focal plane, low membrane protein detectability, and the propensity of particles toward stalling and reversals [25].
Resolution of the conflicting interpretations of experimental results requires the consistent application of conditions and a convergence of methodologies. Unambiguous live-cell imaging of anterograde transport and egress of a naked particle would be critical steps toward validating the Separate Model as a physiologically relevant pathway for antero-grade transport. Importantly, studies must apply rigorous reproducible conditions for classifying anterograde transport and decrease any ambiguity by removing particles that frequently stall or reverse direction. Further support of the Separate model could be found using live cell imaging of fluorescent virus strains to visualize the coupled secondary envelopment and egress events predicted by the Model. Beyond live-cell imaging, the use of systems that provide physical isolation of cell bodies from distal axons (e.g. modified Campenot chambers or microfluidic devices) is critical for reducing the confounding effects of retrograde transport and input inoculum. Recently, novel gB/gD-null mutant strains have been characterized as deficient in secondary but not primary envelopment and accumulate naked cytoplasmic capsids [63]. Chambered infections with such mutants and subsequent assays of anterograde transport could directly probe the Separate Model and provide definitive evidence along the line of previous experimentation with BFA treatment.
The biology that supports the Separate Model would be quite interesting and important. For example, would separate adaptor proteins be required to sort capsids and membrane protein vesicles? How would these events be coordinated? Would different kinesin motor family members be used to move these different structures in axons? Perhaps more interesting is the question of final assembly in distal axons. Is it the same process as secondary envelopment in the cell body? The Married Model is simpler with respect to the processes of axonal sorting, anterograde motion, and egress because the relevant biology for assembly and sorting occurs in the cell body. However, viral processes are selected by evolution, not by human logic and are rarely simple or straightforward.
ACKNOWLEDGEMENTS
We thank Greg Smith, Northwestern University, for insightful comments in the preparation of this manuscript. L.W.E. and R.K. are supported by the US National Institutes of Health grants R37 NS033506-16 and R01 NS060699-03. M.P.T. is supported by an American Cancer Society Postdoctoral Research Fellowship (PF-10-057-01-MPC).
Abbreviations used
- GFP
green fluorescent protein
- mRFP
monomeric red fluorescent protein
- HSV
herpes simplex virus
- PRV
pseudorabies virus
- IF
immunofluorescence
- EM
electron microscopy
- BFA
Brefeldin-a
- PNS
peripheral nervous system
Footnotes
CONFLICT OF INTEREST The authors have no competing interest.
REFERENCES
- 1.Smith GA. Herpesvirus transport to the nervous system and back again. Annual Review of Microbiology. 2012;66 doi: 10.1146/annurev-micro-092611-150051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knipe DM, Cliffe A. Chromatin control of herpes simplex virus lytic and latent infection. Nature Reviews Microbiology. 2008;6:211–221. doi: 10.1038/nrmicro1794. DOI: 10.1038/nrmicro1794. [DOI] [PubMed] [Google Scholar]
- 3.Daheshia M, Feldman LT, Rouse BT. Herpes simplex virus latency and the immune response. Current Opinion in Microbiology. 1998;1:430–435. doi: 10.1016/s1369-5274(98)80061-1. [DOI] [PubMed] [Google Scholar]
- 4.Khanna KM, Lepisto AJ, Decman V, et al. Immune control of herpes simplex virus during latency. Current Opinion in Immunology. 2004;16:463–469. doi: 10.1016/j.coi.2004.05.003. DOI: 10.1016/j.coi.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 5.Johnson DC, Baines JD. Herpesviruses remodel host membranes for virus egress. Nature Reviews Microbiology. 2011;9:382–394. doi: 10.1038/nrmicro2559. DOI: 10.1038/nrmicro2559. [DOI] [PubMed] [Google Scholar]
- 6.Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: an update. Virus Research. 2009;143:222–234. doi: 10.1016/j.virusres.2009.03.018. DOI: 10.1016/j.virusres.2009.03.018. [DOI] [PubMed] [Google Scholar]
- 7.Mettenleiter TC, Klupp BG, Granzow H. Herpesvirus assembly: a tale of two membranes. Current Opinion in Microbiology. 2006;9:423–429. doi: 10.1016/j.mib.2006.06.013. DOI: 10.1016/j.mib.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 8.Mettenleiter TC. Herpesvirus assembly and egress. Journal of Virology. 2002;76:1537–1547. doi: 10.1128/JVI.76.4.1537-1547.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Curanovic D, Enquist LW. Virion-incorporated glycoprotein B mediates transneuronal spread of pseudorabies virus. Journal of Virology. 2009;83:7796–7804. doi: 10.1128/JVI.00745-09. DOI: 10.1128/JVI.00745-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pomeranz LE, Reynolds AE, Hengartner CJ. Molecular biology of pseudorabies virus: impact on neurovirology and veterinary medicine. Microbiology and Molecular Biology Reviews. 2005;69:462–500. doi: 10.1128/MMBR.69.3.462-500.2005. DOI: 10.1128/MMBR.69.3.462-500.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Husak PJ, Kuo T, Enquist LW. Pseudorabies virus membrane proteins gI and gE facilitate anterograde spread of infection in projection-specific neurons in the rat. Journal of Virology. 2000;74:10975–10983. doi: 10.1128/jvi.74.23.10975-10983.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brideau AD, Card JP, Enquist LW. Role of pseudorabies virus Us9, a type II membrane protein, in infection of tissue culture cells and the rat nervous system. Journal of Virology. 2000;74:834–845. doi: 10.1128/jvi.74.2.834-845.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomishima MJ, Enquist LW. A conserved alpha-herpesvirus protein necessary for axonal localization of viral membrane proteins. The Journal of Cell Biology. 2001;154:741–752. doi: 10.1083/jcb.200011146. DOI: 10.1083/jcb.200011146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ch'ng TH, Enquist LW. Efficient axonal localization of alphaherpesvirus structural proteins in cultured sympathetic neurons requires viral glycoprotein E. Journal of Virology. 2005;79:8835–8846. doi: 10.1128/JVI.79.14.8835-8846.2005. DOI: 10.1128/JVI.79.14.8835-8846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lyman MG, Feierbach B, Curanovic D, et al. Pseudorabies virus Us9 directs axonal sorting of viral capsids. Journal of Virology. 2007;81:11363–11371. doi: 10.1128/JVI.01281-07. DOI: 10.1128/JVI.01281-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McCarthy KM, Tank DW, Enquist LW. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathogens. 2009;5:e1000640. doi: 10.1371/journal.ppat.1000640. DOI: 10.1371/journal.ppat.1000640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith GA, Pomeranz L, Gross SP, et al. Local modulation of plus-end transport targets herpesvirus entry and egress in sensory axons. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:16034–16039. doi: 10.1073/pnas.0404686101. DOI: 10. 1073/pnas.0404686101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu WW, Goodhouse J, Jeon NL, et al. A microfluidic chamber for analysis of neuron-to-cell spread and axonal transport of an alpha-herpesvirus. PLoS One. 2008;3:e2382. doi: 10.1371/journal.pone.0002382. DOI: 10.1371/journal.pone.0002382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Antinone SE, Smith GA. Two modes of herpesvirus trafficking in neurons: membrane acquisition directs motion. Journal of Virology. 2006;80:11235–11240. doi: 10.1128/JVI.01441-06. DOI: 10.1128/JVI.01441-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor MP, Kramer T, Lyman MG, et al. Visualization of an alphaherpesvirus membrane protein that is essential for ante-rograde axonal spread of infection in neurons. MBio. 2012;3:e00063–12–e00063–12. doi: 10.1128/mBio.00063-12. DOI: 10.1128/mBio.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Feierbach B, Bisher M, Goodhouse J, et al. In vitro analysis of transneuronal spread of an alphaherpesvirus infection in peripheral nervous system neurons. Journal of Virology. 2007;81:6846–6857. doi: 10.1128/JVI.00069-07. DOI: 10.1128/JVI.00069-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang J, Lazear HM, Friedman HM. Completely assembled virus particles detected by transmission electron microscopy in proximal and mid-axons of neurons infected with herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology. 2011;409:12–16. doi: 10.1016/j.virol.2010.10.009. DOI: 10.1016/j.virol.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maresch C, Granzow H, Negatsch A, et al. Ultrastructural analysis of virion formation and anterograde intraaxonal transport of the alphaherpesvirus pseudorabies virus in primary neurons. Journal of Virology. 2010;84:5528–5539. doi: 10.1128/JVI.00067-10. DOI: 10.1128/JVI.00067-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antinone SE, Zaichick SV, Smith GA. Resolving the assembly state of herpes simplex virus during axon transport by live-cell imaging. Journal of Virology. 2010;84:13019–13030. doi: 10.1128/JVI.01296-10. DOI: 10.1128/JVI.01296-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wisner TW, Sugimoto K, Howard PW, et al. Anterograde transport of herpes simplex virus capsids in neurons by both Separate and Married mechanisms. Journal of Virology. 2011:1–10. doi: 10.1128/JVI.00116-11. DOI: 10.1128/JVI.00116-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hill TJ, Field HJ, Roome APC. Intra-axonal location of herpes simplex virus particles. Journal of General Virology. 1972;15:253–255. doi: 10.1099/0022-1317-15-3-253. DOI: 10.1099/0022-1317-15-3-253. [DOI] [PubMed] [Google Scholar]
- 27.Hill TJ, Field HJ. The interaction of herpes simplex virus with cultures of peripheral nervous tissue: an electron microscopic study. Journal of General Virology. 1973;21:123–133. doi: 10.1099/0022-1317-21-1-123. [DOI] [PubMed] [Google Scholar]
- 28.Cook ML, Stevens JG. Pathogenesis of herpetic neuritis and ganglionitis in mice: evidence for intra-axonal transport of infection. Infection and Immunity. 1973;7:272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kristensson K, Ghetti B, Wiśniewski HM. Study on the propagation of herpes simplex virus (type 2) into the brain after intraocular injection. Brain Research. 1974;69:189–201. doi: 10.1016/0006-8993(74)90001-8. [DOI] [PubMed] [Google Scholar]
- 30.Yamamoto T, Otani S, Shiraki H. Ultrastructure of herpes simplex virus infection of the nervous system of mice. Acta Neuropathologica. 1973;26:285–299. doi: 10.1007/BF00688077. [DOI] [PubMed] [Google Scholar]
- 31.Lycke E, Hamark B, Johansson M, et al. Herpes simplex virus infection of the human sensory neuron. Archives of Virology. 1988;101:87–104. doi: 10.1007/BF01314654. DOI: 10.1007/BF01314654. [DOI] [PubMed] [Google Scholar]
- 32.LaVail JH, Topp KS, Giblin PA, et al. Factors that contribute to the transneuronal spread of herpes simplex virus. Journal of Neuroscience Research. 1997;49:485–496. [PubMed] [Google Scholar]
- 33.Ohara PT, Chin MS, LaVail JH. The spread of herpes simplex virus type 1 from trigeminal neurons to the murine cornea: an immunoelectron microscopy study. Journal of Virology. 2000;74:4776–4786. doi: 10.1128/jvi.74.10.4776-4786.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Negatsch A, Granzow H, Maresch C, et al. Ultrastructural analysis of virion formation and intraaxonal transport of herpes simplex virus type 1 in primary Rat neurons. Journal of Virology. 2010;84:13031–13035. doi: 10.1128/JVI.01784-10. DOI: 10. 1128/JVI.01784-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penfold M, Armati P, Cunningham A. Axonal transport of herpes simplex virions to epidermal cells: evidence for a specialized mode of virus transport and assembly. Proceedings of the National Academy of Sciences. 1994;91:6529. doi: 10.1073/pnas.91.14.6529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diefenbach RJ, Miranda-Saksena M, Douglas MW, et al. Transport and egress of herpes simplex virus in neurons. Reviews in Medical Virology. 2008;18:35–51. doi: 10.1002/rmv.560. DOI: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 37.Snyder A, Bruun B, Browne HM, et al. A herpes simplex virus gD-YFP fusion glyco-protein is transported separately from viral capsids in neuronal axons. Journal of Virology. 2007;81:8337–8340. doi: 10.1128/JVI.00520-07. DOI: 10.1128/JVI.00520-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Snyder A, Wisner TW, Johnson DC. Herpes simplex virus capsids are transported in neuronal axons without an envelope containing the viral glycoproteins. Journal of Virology. 2006;80:11165–11177. doi: 10.1128/JVI.01107-06. DOI: 10.1128/JVI.01107-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Snyder A, Polcicova K, Johnson DC. Herpes simplex virus gE/gI and US9 proteins promote transport of both capsids and virion glycoproteins in neuronal axons. Journal of Virology. 2008;82:10613–10624. doi: 10.1128/JVI.01241-08. DOI: 10.1128/JVI.01241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schnell U, Dijk F, Sjollema KA, et al. Immuno-labeling artifacts and the need for live-cell imaging. Nature Methods. 2012;9:152–158. doi: 10.1038/nmeth.1855. DOI: 10.1038/nmeth.1855. [DOI] [PubMed] [Google Scholar]
- 41.LaVail JH, Tauscher AN, Sucher A, et al. Viral regulation of the long distance axonal transport of herpes simplex virus nucleocapsid. Neuroscience. 2007;146:974–985. doi: 10.1016/j.neuroscience.2007.02.010. DOI: 10.1016/j.neuroscience.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.McGraw HM, Awasthi S, Wojcechowskyj JA, et al. Anterograde spread of herpes simplex virus type 1 requires glycoprotein E and glycoprotein I but not Us9. Journal of Virology. 2009;83:8315–8326. doi: 10.1128/JVI.00633-09. DOI: 10. 1128/JVI.00633-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lyman MG, Kemp CD, Taylor MP, et al. Comparison of the pseudorabies virus Us9 protein with homologs from other veterinary and human alphaherpesviruses. Journal of Virology. 2009;83:6978–6986. doi: 10.1128/JVI.00598-09. DOI: 10.1128/JVI.00598-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Frame MC, McGeoch DJ, Rixon FJ, et al. The 10K virion phosphoprotein encoded by gene US9 from herpes simplex virus type 1. Virology. 1986;150:321–332. doi: 10.1016/0042-6822(86)90297-7. [DOI] [PubMed] [Google Scholar]
- 45.Fuchs W, Klupp BG, Granzow H, et al. Physical interaction between envelope glycoproteins E and M of pseudorabies virus and the major tegument protein UL49. Journal of Virology. 2002;76:8208–8217. doi: 10.1128/JVI.76.16.8208-8217.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Leege T, Fuchs W, Granzow H, et al. Effects of simultaneous deletion of pUL11 and glycoprotein M on virion maturation of herpes simplex virus type 1. Journal of Virology. 2009;83:896–907. doi: 10.1128/JVI.01842-08. DOI: 10.1128/JVI.01842-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Browne H, Bell S, Minson T. Analysis of the requirement for glycoprotein m in herpes simplex virus type 1 morphogenesis. Journal of Virology. 2004;78:1039–1041. doi: 10.1128/JVI.78.2.1039-1041.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kopp M, Granzow H, Fuchs W, et al. Simultaneous deletion of pseudorabies virus tegument protein UL11 and glycoprotein M severely impairs secondary envelopment. Journal of Virology. 2004;78:3024–3034. doi: 10.1128/JVI.78.6.3024-3034.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Whealy ME, Card JP, Meade RP, et al. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. Journal of Virology. 1991;65:1066–1081. doi: 10.1128/jvi.65.3.1066-1081.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dasgupta A, Wilson DW. Evaluation of the primary effect of brefeldin A treatment upon herpes simplex virus assembly. Journal of General Virology. 2001;82:1561–1567. doi: 10.1099/0022-1317-82-7-1561. [DOI] [PubMed] [Google Scholar]
- 51.Miranda-Saksena M, Armati P, Boadle RA, et al. Anterograde transport of herpes simplex virus type 1 in cultured, dissociated human and rat dorsal root ganglion neurons. Journal of Virology. 2000;74:1827–1839. doi: 10.1128/jvi.74.4.1827-1839.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rio TD, Chng TH, Flood EA, et al. Heterogeneity of a fluorescent tegument component in single pseudorabies virus virions and enveloped axonal assemblies. Journal of Virology. 2005:1–17. doi: 10.1128/JVI.79.7.3903-3919.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miranda-Saksena M, Boadle RA, Armati P, et al. In rat dorsal root ganglion neurons, herpes simplex virus type 1 tegument forms in the cytoplasm of the cell body. Journal of Virology. 2002;76:9934–9951. doi: 10.1128/JVI.76.19.9934-9951.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Diefenbach R, Miranda-Saksena M, Diefenbach E, et al. Herpes simplex virus tegument protein US11 interacts with conventional kinesin heavy chain. Journal of Virology. 2002;76:3282. doi: 10.1128/JVI.76.7.3282-3291.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chou J, Roizman B. The gamma 1(34.5) gene of herpes simplex virus 1 precludes neuroblastoma cells from triggering total shutoff of protein synthesis characteristic of programed cell death in neuronal cells. Proceedings of the National Academy of Sciences of the United States of America. 1992;89:3266–3270. doi: 10.1073/pnas.89.8.3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Roller RJ, Monk LL, Stuart D, et al. Structure and function in the herpes simplex virus 1 RNA-binding protein U(s)11: mapping of the domain required for ribosomal and nucleolar association and RNA binding in vitro. Journal of Virology. 1996;70:2842–2851. doi: 10.1128/jvi.70.5.2842-2851.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roller RJ, Roizman B. The herpes simplex virus 1 RNA binding protein US11 is a virion component and associates with ribosomal 60S subunits. Journal of Virology. 1992;66:3624–3632. doi: 10.1128/jvi.66.6.3624-3632.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mulvey M, Camarena V, Mohr I. Full resistance of herpes simplex virus type 1-infected primary human cells to alpha interferon requires both the Us11 and gamma(1)34.5 gene products. Journal of Virology. 2004;78:10193–10196. doi: 10.1128/JVI.78.18.10193-10196.2004. DOI: 10. 1128/JVI.78.18.10193-10196.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Holland DJ, Miranda-Saksena M, Boadle RA, et al. Anterograde transport of herpes simplex virus proteins in axons of peripheral human fetal neurons: an immunoelectron microscopy study. Journal of Virology. 1999;73:8503–8511. doi: 10.1128/jvi.73.10.8503-8511.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ibiricu I, Huiskonen JT, Döhner K, et al. Cryo electron tomography of herpes simplex virus during axonal transport and secondary envelopment in primary neurons. PLoS Pathogens. 2011;7:e1002406. doi: 10.1371/journal.ppat.1002406. DOI: 10.1371/journal.ppat.1002406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H, Cong R, Na L, et al. Long-term primary culture of highly-pure rat embryonic hippocampal neurons of low-density. Neurochemical Research. 2010;35:1333–1342. doi: 10.1007/s11064-010-0189-0. DOI: 10.1007/s11064-010-0189-0. [DOI] [PubMed] [Google Scholar]
- 62.Miranda-Saksena M, Wakisaka H, Tijono B, et al. Herpes simplex virus type 1 accumulation, envelopment, and exit in growth cones and varicosities in mid-distal regions of axons. Journal of Virology. 2006;80:3592–3606. doi: 10.1128/JVI.80.7.3592-3606.2006. DOI: 10.1128/JVI.80.7.3592-3606.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Johnson DC, Wisner TW, Wright CC. Herpes simplex virus glycoproteins gB and gD function in a redundant fashion to promote secondary envelopment. Journal of Virology. 2011;85:4910–4926. doi: 10.1128/JVI.00011-11. DOI: 10.1128/JVI.00011-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Miranda-Saksena M, Boadle RA, Aggarwal A, et al. Herpes simplex virus utilizes the large secretory vesicle pathway for antero-grade transport of tegument and envelope proteins and for viral exocytosis from growth cones of human fetal axons. Journal of Virology. 2009;83:3187–3199. doi: 10.1128/JVI.01579-08. [DOI] [PMC free article] [PubMed] [Google Scholar]