Abstract
The molecular mechanisms mediating CYLD tumor suppressor function appear to be manifold. Here, we demonstrated that, in contrast to the increased levels of pJNK, CYLD was decreased in a majority of melanoma cell lines and tissues examined. Exogenous expression of CYLD but not its catalytically deficient mutant markedly inhibited melanoma cell proliferation and migration in vitro and subcutaneous tumor growth in vivo. In addition, the melanoma cells expressing exogenous CYLD were unable to form pulmonary tumor nodules following tail-vein injection. At the molecular level, CYLD decreased β1-integrin and inhibited pJNK induction by TNFα or cell-attachment to collagen IV. Moreover, CYLD induced an array of other molecular changes associated with modulation of the ‘malignant’ phenotype, including a decreased expression of cyclin D1, N-cadherin and nuclear Bcl3, and an increased expression of p53 and E-cadherin. Most interestingly, co-expression of the constitutively active MKK7 or c-Jun mutants with CYLD prevented the above molecular changes, and fully restored melanoma growth and metastatic potential in vivo. Our findings demonstrate that JNK/AP-1 signaling pathway underlies the melanoma growth and metastasis that is associated with CYLD loss-of-function. Thus, restoration of CYLD and inhibition of JNK and β1-integrin function represent potential therapeutic strategies for treatment of malignant melanoma.
Keywords: CYLD, JNK, AP-1, β1-integrin and human melanoma
INTRODUCTION
Melanoma represents the most deadly type of skin cancer whose incidence has been on the rise worldwide at a rate faster than most other cancers. In 2010, over 68,130 new cases of invasive melanoma were diagnosed in the US alone, where approximately 8,700 people die of this disease annually (Alexandrescu et al., 2010; Linos et al., 2009). Despite recent advances in this field, a cure for melanoma is still beyond reach for many patients (Gray-Schopfer et al., 2007). To understand better the molecular mechanisms underlying melanoma pathogenesis and to shed light on novel therapeutic strategies, we focused on exploring how the tumor suppressor CYLD controls melanoma cell growth and metastasis through the JNK/AP-1 and β1-integrin signaling pathways.
CYLD was initially discovered as a tumor suppressor due to its dominant genetic linkage to cylindromas (Bignell et al., 2000). Recent genetic and biomarker studies have linked CYLD loss-of-function to a broad range of non-epidermal cancers, including melanoma, myeloma, cervix, colon, breast, liver, kidney and prostate cancers (Hellerbrand et al., 2007; Hirai et al., 2004; Hutti et al., 2009; Jenner et al., 2007; Kikuno et al., 2008; Massoumi, 2011; Massoumi et al., 2009; Strobel et al., 2002; Wang et al., 2005). Nevertheless, little is understood about the functional importance and the nature of the molecular intricacies between CYLD and its downstream targets in these cancers. In normal cells, CYLD functions as a deubiquitinase that preferentially removes the activating K63-linked polyubiquitin chains from an array of target proteins involved in signal transduction and gene regulation. Examples of CYLD-targets include TRAF2/6, Bcl3, plk1, Tak1, lck, HDAC6 and Dvl (Bignell et al., 2000; Brummelkamp et al., 2003; Kovalenko et al., 2003; Regamey et al., 2003; Reiley et al., 2004; Reiley et al., 2007; Reiley et al., 2006; Stegmeier et al., 2007; Trompouki et al., 2003; Wilkinson, 1997). Downstream of TRAF2/6 are the classical IKK/NF-κB and JNK/AP-1 signaling pathways, which are subject to additional controls by CYLD at IKKγ and AP-1 levels, respectively (Brummelkamp et al., 2003; Kovalenko et al., 2003; Miliani de Marval et al., 2011; Regamey et al., 2003; Reiley et al., 2004; Trompouki et al., 2003). Of particular relevance to melanoma, the NF-κB pathway plays an essential role in cell survival and tissue invasion (Ueda and Richmond, 2006). Likewise, Bcl3, a non-canonical NF-κB subunit, is activated in response to CYLD-downregulation by Snail1 and contributes to the subsequent upregulation of N-cadherin and cyclin D1 in melanoma cells (Massoumi et al., 2009). In contrast, the role of the JNK/AP-1 signaling pathway in melanoma is not well-defined. Expression of the dominant negative c-Jun or c-Fos mutant enhances melanoma soft-agar colony formation, suggesting that AP-1 suppresses melanoma growth (Huang et al., 2003; Yang et al., 2004). On the other hand, elevated JNK/c-Jun signaling is characterized as a central process in melanoma cells harboring an hyperactive MEK-ERK signaling cascade (Lopez-Bergami et al., 2007). In addition, JNK1 is involved in promoting cell cycle progression or preventing apoptosis in various melanoma cell lines (Alexaki et al., 2008). These later findings suggest that the JNK/AP-1 pathway is important for melanoma tumorigenesis.
In this study, we investigated the functional importance of JNK/AP-1 in melanoma malignancy in response to CYLD gain- and loss-of-function. We demonstrated that CYLD was significantly downregulated, whereas JNK activation was increased in a majority of human melanoma tissues and cell lines examined. Exogenous expression of CYLD inhibited melanoma cell growth and migration in vitro. Consistently, CYLD but not its catalytically deficient mutant inhibited subcutaneous melanoma growth in immunodeficient mice, which is accompanied by the downregulation of cyclin D1 and N-cadherin and the upregulation of E-cadherin and p53. Moreover, CYLD downregulated expression of the β1-integrin and suppressed JNK activation during cell adhesion to collagen IV. Both JNK/AP-1 and β1-integrin signaling pathways were required for cell spreading and migration and c-Jun was also required for upregulating β1-integrin expression. Most surprisingly, restoring JNK/AP-1 function by expression of the constitutively active mutants of either MKK7 or c-Jun prevented CYLD-effects on cyclin D1, p53 and nuclear Bcl3. Consistently, cells transduced to coexpress CYLD along with active MKK7 or c-Jun fully demonstrated the ability to form tumor nodules in the lung when introduced through tail vein injection. Taken together, our findings indicate that JNK/AP-1 underlies melanoma malignancy in lieu of CYLD-deficiency by acting upstream of β1-integrin and Bcl3.
MATERIAL AND METHODS
Reagents, cell culture and gene transduction
Cell culture media and secondary antibodies were purchased from (Invitrogen, Carlsbad, CA) and primary antibodies were from (Santa Cruz Biotechnology, Santa Cruz, CA) unless otherwise noted. Human melanoma cell lines, including A2058, SKmel28 and A375, were obtained from ATCC. DM733, DM598, DM738 and DM833 were kindly provided Dr. Hilliard Seigler (Duke University Medical Center), and were derived from primary biopsies of metastatic melanoma obtained under a Duke University Institutional Review Board approved protocol. All cell lines were cultured with 10% FBS/DMEM and were not authenticated in this study other than the observation of melanin expression. Primary human melanocytes were isolated from neonatal foreskin and cultured in M-254 media. Retroviruses encoding LacZ, CYLD, CYLD.754, MKK7 or c-Jun were packaged in 293T phoenix cells as described in previous studies (Jin et al., 2011; Ke et al., 2010; Miliani de Marval et al., 2011; Zhang et al., 2007). For gene transduction, melanoma cell cultures were pretreated with 8 ug/ml polybrene for 15min and then fed with retroviral media supplemented with 8 ug/ml polybrene. Cell culture plates were spun at 500g for 1h at a pre-warmed (32°C) Beckman centrifuge and then incubated for another 3h at a tissue culture incubator prior to media replacement. Multiplex gene transduction was performed at 16h intervals.
Cell growth, adhesion, scratch-wounding and soft-agar formation analysis
Cells (5×104) transduced as above were plated in triplicates onto 35-mm dishes and trypsinized for counting at d1, d2 and d3. For cell attachment and spreading assays, cells are plated along with DMSO solvent, the JNK inhibitors (10uM BI78D3 or SP600125, Sigma-Aldrich, St. Louis, MO) or 20 ug/ml mAb13 or 12G10 monoclonal antibodies against β1-integrin (Fogerty et al., 1990; Mould et al., 1995). Images were taken 1h after plating. Soft-agar formation and scratch-wounding analysis were performed as described previously (Ke et al., 2010; Miliani de Marval et al., 2011).
Animal studies
Animal studies were conducted in accordance with protocols approved by the Duke Animal Care and Use Committee. Immunodeficient athymic NCr-nu/nu mice (n≥ 4 per group) were purchased from Duke animal facility. For subcutaneous tumor growth, A2058 cells were harvested at d2 after gene transduction and suspended (2×106 cells /200µl PBS) for each injection. Tumors were measured weekly for 4 weeks and then collected from sacrificed animals. For tail-vein injection, cells were suspended at (1×106/100µl PBS) for each mouse. Animals were sacrificed for pulmonary tumor growth analysis eight weeks later.
Real-time RT-PCR
Total RNA was isolated from melanoma cells transduced for expression of LacZ or CYLD using Qiagen RNA-isolation kit. Standard real-time RTPCR for β1-integrin was performed with the forward 5′-TTTCGATGCCATCATGCAA-3′ and reverse 5′-ACCAGCAGCCGTGTAACATTC-3′ primers. RT-PCR for 18S RNA was performed as a control with the forward 5′-TCTCCGGAATCGAACCCTGATT-3′ and reverse 5′-CCCATTCGAACGTCTGCCCTATC-3′ primers.
Immunoblotting
Human foreskin and primary melanoma tissues were obtained from Duke Hospital in accordance with an approved IRB protocol. Tissue lysates were prepared with RIPA buffer using a bullet blender (Next Advance Inc. NY). Protein lysates (20µg each) of tissues or cultured cells were analyzed by immunoblotting with antibodies against CYLD(H-419), p-JNK(G7), JNK1(F-3), JNK2(N-18) and actin (Santa Cruz Biotechnology, Santa CruZ, CA) followed by Alexa IRDye-conjugated secondary antibodies (Invitrogen, Carlsbad, CA). For β1-integrin blots, protein lysates were prepared and analyzed in the absence of DTT and blotted with the 12G10 monoclonal antibody. Subcellular fractionation was performed as described (Ke et al., 2010), and the cytoplasmic and nuclear fractions were analyzed by immunoblotting with antibodies against Bcl3, c-Jun, actin and lamin A/C (GenScript USA Inc, Piscataway, NJ).
Histological analysis
H&E staining was performed with paraffin sections by the Duke Pathology lab. Immunostaining was performed with frozen tissue sections as previously described (Ke et al., 2010; Zhang et al., 2007). Fluorescent pictures were taken and processed using Olympus BX41 microscopic imaging system.
RESULTS
CYLD is reduced whereas pJNK is increased in melanoma
We first examined the clinical relevance of CYLD to melanoma by immunoblotting. We found that CYLD was readily detectable in normal human melanocytes and skin tissues but was significantly reduced in the melanoma cell lines and tissues examined (Fig. 1a-b). Since the JNK signaling pathway is among the downstream targets of CYLD (Miliani de Marval et al., 2011; Reiley et al., 2004), we predicted that JNK activation might be increased in these melanoma samples. As expected, pJNK was expressed at higher levels in all melanoma cell lines and tissues than that of the normal melanocytes and skin tissues, respectively (Fig. 1a-b). Interestingly, pJNK was predominantly detected at the p46 position while the total JNK1 and JNK2 levels vary at the p46 and p54 position between different melanoma cell lines and tissue samples. Further JNK kinase analysis revealed that JNK1 but not JNK2 activity was increased in a majority of melanoma cell lines (Supplementary Fig. S1a). These findings indicate that JNK1 is the predominant isoform activated in melanoma cells, which is in agreement with the data showing that JNK1 is essential for melanoma growth and survival (Alexaki et al., 2008). Consistent with the increased pJNK levels, p-c-Jun was readily detected in melanoma tissues (Supplementary Fig. S1b). To determine whether CYLD is downregulated at the transcriptional level, we examined a previously generated gene array data set of melanoma cell lines (GSE10916). This analysis revealed that CYLD mRNA was nearly absent in over 90% of 50 melanoma cell lines examined. These data indicate that CYLD is reduced at both protein and mRNA levels, whereas JNK activation is increased in melanoma.
Fig. 1. CYLD is reduced while pJNK is increased in melanoma.
(a-b) Immunoblotting for CYLD, pJNK, JNK1 and JNK2 and Actin with protein lysates from (a) primary human melanocytes of passage 4 and 6 (Me1 and Me2) and melanoma cell lines, including DM733, Skmel28, A375, A2058, DM598 and DM738, and (b) normal human skin (S1 and S2) and melanoma tissues (M1-M11). The relative densitometry is shown below each of p46 band of pJNK with the values of Me1 and S1 set as 1.0.
CYLD inhibits melanoma cell proliferation and migration
To examine how CYLD affects melanoma tumorigenesis, we expressed LacZ control or CYLD in A2058 and SKmel28 human melanoma cell lines through retroviral gene transduction. Expression of CYLD inhibited cell proliferation in monolayer cell culture and reduced the capacity of anchorage-independent growth as assessed by soft agar colony formation (Fig. 2a-b). In addition, CYLD substantially slowed down scratchwounding induced cell migration (Fig. 2c). These findings indicate that CYLD inhibits growth and migration of melanoma cells.
Fig. 2. CYLD inhibits melanoma cell growth, colony formation and migration.
(a) Monolayer cell growth, (b) soft agar colony formation and (c) scratch-wounding assay. Malignant A2058 and SKmel28 human melanoma cell lines were transduced with retrovirus encoding LacZ or CYLD and then used for growth and wounding assays. Efficiency of gene transduction was confirmed by immunoblotting for CYLD with actin for control. Representative data of triplicate experiments were shown in the graphs as average numbers of (a) cell number or (b) soft agar colonies +/− SD. For scratch-wounding assay, cells were serum starved for 16h and then scratch-wounded. Images were taken at 0 and 24h post-wounding. Scale bar=200 um.
CYLD inhibits melanoma growth and progression in vivo
To determine the in vivo growth effects of CYLD expression in melanoma, we performed subcutaneous tumor growth analysis with A2058 cells that have undergone gene transduction for expression of LacZ or CYLD. Cells expressing CYLD displayed a reduced tumor growth kinetic as compared to the control cells (Fig. 3a). Consistently, CYLD-expressing tumors had features of reduced cell proliferation and enhanced cell apoptosis as indicated by the decreased number of Ki-67-positive cells and the increased number of caspase-3-positive cells, respectively (Fig. 3b-c, Supplementary Fig. S2). In contrast, tumors expressing CYLDm (CYLD.754), a catalytically deficient C-terminal deletion mutant of CYLD, had an increased number of Ki-67-positive cells and a reduced number of caspase-3-positive cells (Fig. 3a-c, Supplementary Fig. S2). In addition, E-cadherin, an epithelial cell marker was increased, while N-cadherin, a mesenchymal cell marker, was reduced in tumors expressing CYLD but not CYLDm (Fig. 3c). These data indicate that CYLD but not CYLDm inhibits melanoma growth and progression.
Fig. 3. CYLD inhibits melanoma growth in vivo.
(a) Subcutaneous tumor growth kinetics. A2058 cells transduced to express LacZ, CYLD or CYLDm were injected subcutaneously into immunodeficient nude mice (2×106 cells in 200 µl PBS/mouse). Graph represents the average tumor volume of 4–8 mice +/− SD/group. Expression of CYLD and CYLDm was confirmed by immunoblotting for CYLD and Actin. (b-c) Histological analysis of the 4-week old subcutaneous tumors by H&E staining and immunostaining for Ki-67, E-cadherin or N-cadherin [orange], nuclei [blue]. Scale bar=100 um.
β1-integrin and pJNK are downregulated by CYLD and display crosstalk
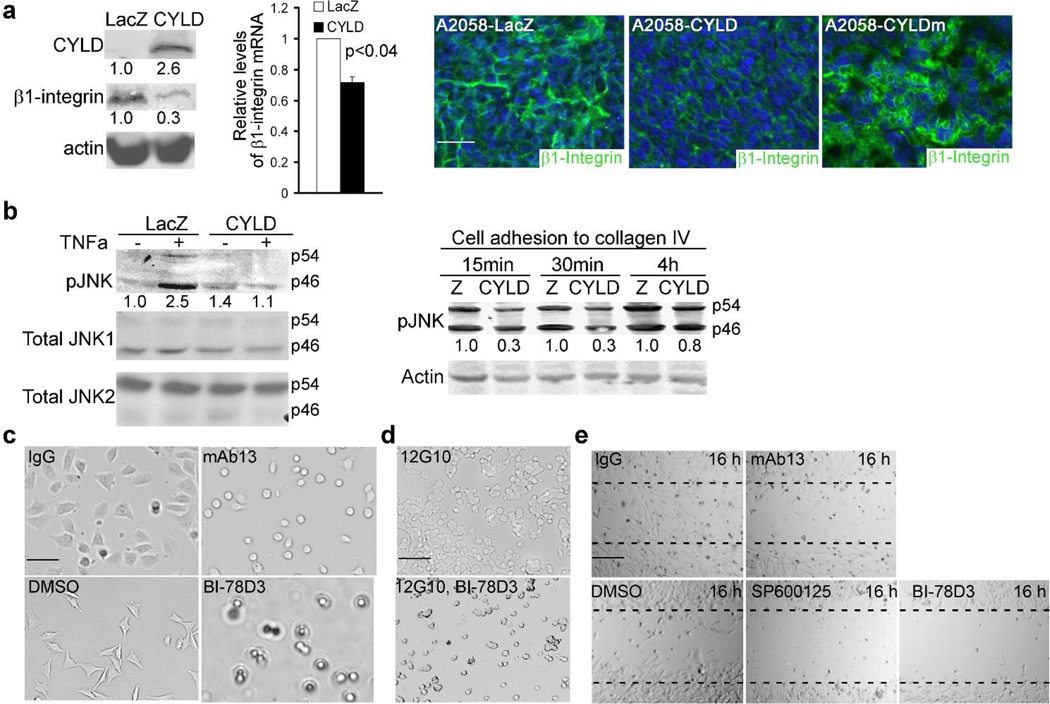
In order to explore how CYLD inhibits invasive tumor growth, we examined the expression status of β1-integrin which is a member of the heterodimeric transmembrane receptors involved in melanoma migration and chemotaxis (Hegerfeldt et al., 2002; Hodgson and Dong, 2001). Immunoblotting revealed that β1-integrin was highly expressed in melanoma cell lines and tissues (Supplementary Fig. S3). Exogenous expression of CYLD downregulated β1-integrin at both protein and mRNA levels as shown by RT-PCR and immunoblotting, respectively (Fig. 4a). Consistently, subcutaneous tumors expressing CYLD, but not those expressing CYLDm, expressed a significantly reduced level of β1-integrin as shown by immunostaining (Fig. 4a). Further in line with these data, CYLD inhibited pJNK induction not only by TNFα but also by cell adhesion to collagen IV, a natural substrate and activator of β1-integrin (Fig. 4b). On the other hand, siRNA-mediated gene silencing of c-Jun markedly decreased β1- integrin (Supplementary Fig. S4a), indicating that the JNK downstream target AP1 is required for the expression of β1-integrin. Similarly, pharmacological JNK-specific inhibition with BI-78D3 prevented β1-integrin-induction by TPA (Supplementary Fig. S4b). These data indicate that the JNK/AP-1 and β1-integrin signaling pathways can crosstalk and are negatively regulated by CYLD. Interestingly, JNK inhibition induced a minimal effect on the expression levels of CYLD (Supplementary Fig. S4c), implicating that JNK function is not directly involved in regulating CYLD expression.
Fig. 4. CYLD downregulates β1-integrin and p-JNK, both of which are required for cell adhesion and migration.
(a) β1-integrin expression by immunoblotting, RT-PCR and immunostaining. Protein and RNA samples were collected from A2058 cells at 48h post-transduction and were analyzed by immunoblotting for β1-integrin, CYLD and actin and by RT-PCR for β1-integrin and 18S RNA for control. The relative densitometry was shown below each β1-integrin band. The relative mRNA fold changes +/− SD shown in the graph represent data from duplicate experiments. A p-value <0.04 is shown in the graph. Immunostaining was performed with subcutaneous tumors generated with transduced A2058 cells. β1-integrin [green], nuclei [blue]. Scale bar=100 um. (b) Immunoblotting for pJNK, JNK1, JNK2 and actin. A2058 cells were treated with or without 50 ng/ml TNFα or plated onto the collagen IV-coated dishes and then collected at 15m, 30m and 1h time-points for protein analysis. (c-d) Cell attachment. A2058 cells were plated onto (c) collagen IV-coated dishes in the presence of DMSO, 20 ug/ml mab13 or 10 uM BI-78D3 and imaged 1h later, or (d) plain culture dishes in the presence of 20 ug/ml 12G10 along with or without 10 uM BI-78D3 and imaged 3h later. (e) Cell migration. Near confluent A2058 cell cultures were serum starved for 16h and then scratch-wounded in the presence of indicated reagents. Images were taken imaged at 16h post-wounding. (c-d), scale bar=50 um; (e) scale bar=200 um.
β1-integrin and pJNK are essential for melanoma cell attachment and migration
The functional link between β1-integrin and JNK in melanoma cell lines was next tested via cell attachment and migration assays. As expected from the previous studies (Fogerty et al., 1990), β1-integrin inhibition with the neutralizing monoclonal antibody mAb13 inhibited cell attachment, as was observed for pharmacological JNK-inhibition with BI-78D3 (Fig. 4c). Conversely, activation of β1-integrin with the monoclonal antibody 12G10 enhanced cell attachment, which was abolished by the presence of BI- 78D3 (Fig. 4d). Scratch-wounding assay revealed that mAb13 and the JNK-inhibitors BI-78D3 and SP600125 inhibited cell migration. These findings indicate that JNK function is essential for β1-integrin-mediated cell-attachment and migration (Fig. 4e). Taken together, these data underscore an important regulatory loop between JNK and β1-integrin in melanoma.
JNK/AP-1 activation restores normal expression of cyclin D1 and p53
To determine whether JNK-suppression is important for CYLD-inhibition of melanoma tumorigenesis, we expressed CYLD with or without the constitutively active mutants of c-Jun or MKK7, an immediate upstream activator of JNK. Expression of CYLD alone resulted in a downregulation of cyclin D1 both at the basal state and upon cell cycle reentry following starvation. Surprisingly, coexpression of either MKK7 or c- Jun with CYLD restored normal expression of cyclin D1 (Fig. 5). Additionally, CYLD upregulated p53 by about 2-fold, which was diminished by c-Jun and MKK7 (Fig. 5). These findings indicate that JNK/AP-1 is sufficient to overcome CYLD-effects on cyclin D1 and p53.
Fig. 5. JNK/AP-1 activation prevents CYLD-effect on cyclin D1 and p53.
(a-b) Immunoblotting for CYLD, cyclin D1, p53 and Actin. (a) A2058 cells transduced to express LacZ or CYLD were incubated with serum free media for 16h and then incubated with 10% FBS for 4h prior to protein collection. (b) Protein lysates were collected from A2058 cells transduced to express LacZ or CYLD along with or without the active mutants of c-Jun or MKK7. Relative densitometry was shown below each cyclin D1 and p53 band.
Activation of JNK/AP-1 restores melanoma growth and metastatic potential
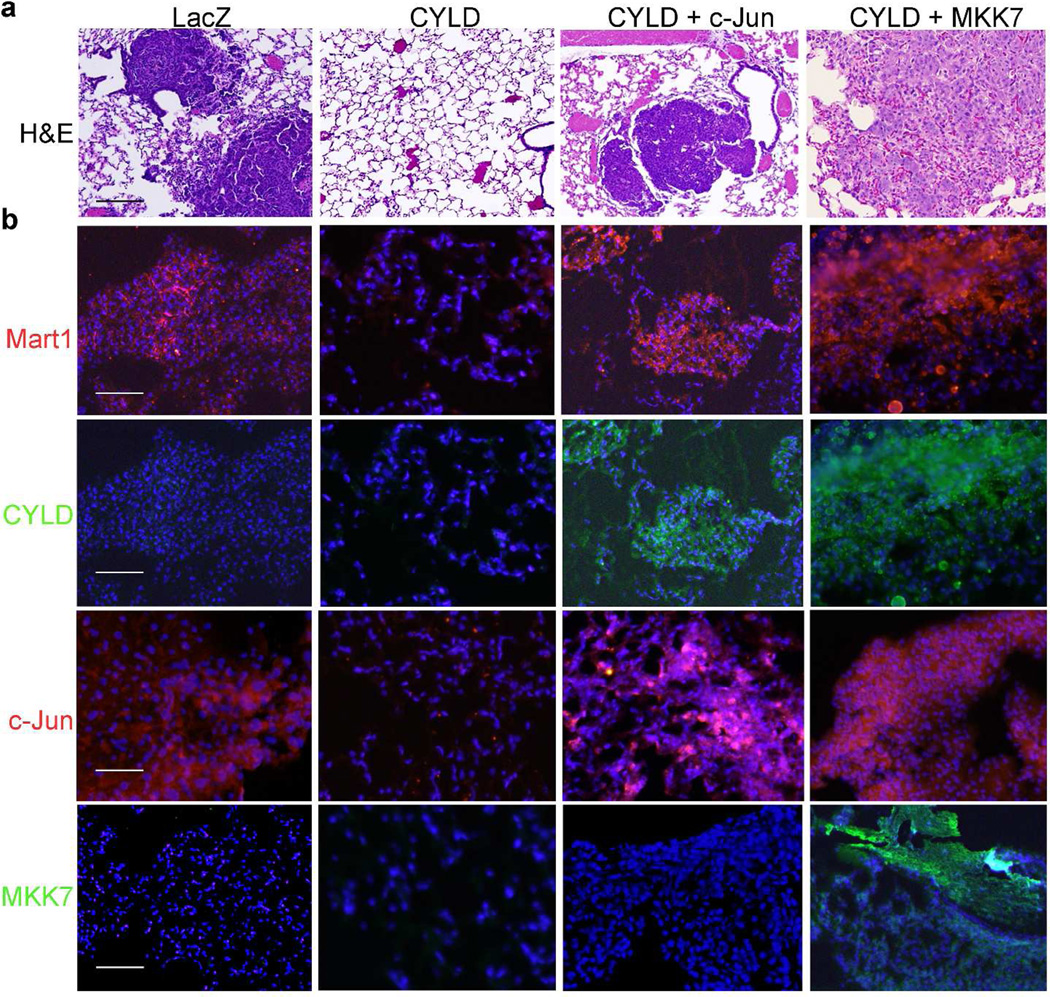
To further validate the functional importance of JNK/AP-1, we introduced A2058 cells that had undergone various gene transductions into immunodeficient nude mice through tail-vein injection. At 8 weeks post-injection, animals were sacrificed for pulmonary tumor growth analysis. We found that none of the 7 (0/7) animals injected with A2058- CYLD cells developed visible melanoma nodules in the lung tissue. In contrast, apparent pulmonary nodules were observed in 6/11, 4/5, 5/7 and 6/7 animals injected with cells expressing LacZ, c-Jun or CYLD together with MKK7 or c-Jun, respectively (Table 1). These tumor nodules were confirmed to be of melanoma origin as indicted by the dark and compact histological appearance of the tumor islands and the positive detection of Mart1, a melanocyte specific marker (Fig. 6a-b). Expression of CYLD, c- Jun and MKK7 were validated by immunostaining of the respective tumor tissues (Fig. 6b). Thus, JNK/AP-1 induction is sufficient to overcome CYLD-inhibition of melanoma progression. In agreement with the gain-of-function effects, pharmacological JNK-inhibition with BI-78D3 markedly reduced the capacity of anchorage-independent cell proliferation of A2058 cells, as demonstrated by soft agar colony formation analysis (Supplementary Fig. S5). Taken together, our findings indicate that the JNK/AP1 plays a dominant role in tumorigenesis and represents a potential therapeutic target for melanoma.
Table 1. JNK/AP-1 activation retores melanoma growth potential.
A2058 cells were transduced for expression of LacZ, CYLD, c-Jun or CYLD together with c-Jun or MKK7 and then injected into immunodeficient nude mice through tail vein. Animals were sacrificed at 8 weeks following cell-injection. The two-tailed T.Test p-values were ≤0.03 as obtained with Microsoft Excel.
| Transduced genes |
Total number of mice for tail vein injection |
Number of mice with lung tumor |
|---|---|---|
| LacZ | 11 | 6 |
| CYLD | 7 | 0 |
| CYLD + c-Jun | 7 | 6 |
| CYLD + MKK7 | 7 | 5 |
| c-Jun | 5 | 4 |
Fig. 6. JNK/AP-1 activation restores melanoma malignancy.
(a) H&E staining of the pulmonary tissues collected from nude mice at 8 weeks post-intravenous injection of A2058 cells. (b) Immunostaining of the pulmonary tumor nodules. Mart1, CYLD, c-Jun, MKK7 [orange], nuclei [blue]. Note, melanoma clusters were present in the lungs of animals injected with cells expressing LacZ or CYLD together with MKK7 or c-Jun but not CYLD alone. Scale bar=100 um.
Bcl3, an NF-κB family transcription factor whose nuclear translocalization is subject to negative regulation by CYLD, has been previously characterized as a key oncogenic effector and inducer of cyclin D1 in response to CYLD loss-of-function (Massoumi et al., 2006; Massoumi et al., 2009). As expected, Bcl3 was predominantly detected in the nuclear of the subcutaneous tumors generated with A2058-LacZ control cells; expression of CYLD resulted in the cytoplasmic presence of Bcl3 (Supplementary Fig. S6). Interestingly, coexpression of MKK7 and CYLD restored the nuclear presence of Bcl3 in melanoma tissues (Supplementary Fig. S6). These results implicate that JNK/AP-1 function acts upstream of Bcl3 to overcome CYLD-inhibition of tumorigenesis.
DISCUSSION
We have shown that CYLD loss-of-function and JNK activation are clinically relevant in all melanoma tissues and cell lines examined. Restoring CYLD expression not only retards melanoma growth in vitro and in vivo but also markedly diminishes melanoma metastatic potential in vivo. Most importantly, we have established a mechanistic link between CYLD-deficiency and JNK/AP-1 activation by characterizing JNK/AP-1 as critical effectors acting downstream of CYLD and upstream of β1-integrin. Additionally, we have demonstrated that JNK/AP-1 can crosstalk with the β1-integrin signaling pathway in response to altered CYLD function.
JNK/AP-1 proteins are involved in regulating an array of cellular processes, including cell proliferation, migration and survival, and are indispensable for epidermal cancers (Jin et al., 2011; Ke et al., 2010; Miliani de Marval et al., 2011; Zhang et al., 2007). In this study, we have shown that JNK/AP-1 is subject to CYLD control and its deregulation promotes melanoma tumorigenesis. In response to CYLD loss-of-function, JNK/AP-1 remains constitutively active, leading to the increased expression of the Ncadherin and cyclin D1 and the reduced expression of p53 tumor suppressor. We have also demonstrated that JNK/AP-1 is able to overcome CYLD-blockade of Bcl3 nuclear entry. However, our findings do not exclude the possibility of a synergistic effect between JNK/AP-1 and Bcl3 in inducing other target genes. On this regard, it has been previously shown that Bcl3 interacts with c-Jun and c-Fos in fibroblast and Hela cells, thereby potentiating AP-1-induction of cell proliferation (Na et al., 1999). Presumably, the physical interaction between AP1 and Bcl3 facilitates Bcl3-nuclei entry upon AP1 activation. It will be of great interest to characterize the nature of potential crosstalk between Bcl3 and JNK/AP-1 signaling pathways in melanoma.
Integrin family receptors have been implicated as feasible therapeutic targets due to their cell surface localization and their role in controlling melanocyte adhesion, migration and proliferation (Juliano et al., 2011; Pinon and Wehrle-Haller, 2010). We showed that CYLD downregulated β1-integrin at a transcriptional level via suppression of AP-1 function. Blocking β1-integrin through an antibody-mediated approach in turn dampened JNK signaling in response to cell-substrate attachment. Moreover, inhibition of either JNK or β1-integrin function impaired cell adhesion and migration. These findings indicate that JNK and β1-integrin signaling pathways function in a regulatory loop to mediate cell migration. It is intriguing to point out that the role of β1-integrin in melanoma is rather complex due to the complexity of heterodimerization with the α- integrin subunits (Nakahara et al., 1996; Ziober et al., 1999). Future efforts may be directed to further characterize the CYLD-effects on the dynamics of β1-integrin heterodimerization and the function of other integrin subunits in melanoma. In summary, our findings establish that CYLD-deficiency has a wide clinical relevance to melanoma and that the JNK/AP-1 signaling pathway underlies melanoma malignancy. These findings underscore the potential of novel therapeutic strategies, including approaches to restore CYLD expression and to inhibit the JNK/AP-1 and β1-integrin signaling pathways in melanoma.
Supplementary Material
ACKNOWLEDGEMENT
This work was supported in part by NIH/NIAMS (R01AR057746) and grants from to Duke School of Medicine and Duke Dermatology to Jennifer Y. Zhang and the Intramural Research Program of the National Institute of Environmental Health Sciences. We thank M. Angelica Selim (Duke University) for her guidance in histological analysis, Chuan-Yuan Li, Kelly Nelson and Shirley Zhang (Duke University) for suggestions and critical reading of the manuscript. We also thank Rene Bernard (Netherland Cancer Institute), Dirk Bohmann (University of Rochester Medical Center) and Michael Kracht (Medical School of Hanover, Hanover, NH) for sharing the CYLD, c- Jun and MKK7 expression constructs, respectively. In addition, we are indebted to Jonathan L Cook and Stephen Ray (Duke University) for providing surgically discarded melanoma tissues.
Footnotes
CONFLICT OF INTEREST
The authors state no conflict of interest.
REFERENCES
- Alexaki VI, Javelaud D, Mauviel A. JNK supports survival in melanoma cells by controlling cell cycle arrest and apoptosis. Pigment Cell Melanoma Res. 2008;21:429–438. doi: 10.1111/j.1755-148X.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- Alexandrescu DT, Ichim TE, Riordan NH, Marincola FM, Di Nardo A, Kabigting FD, et al. Immunotherapy for melanoma: current status and perspectives. J Immunother. 2010;33:570–590. doi: 10.1097/CJI.0b013e3181e032e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignell GR, Warren W, Seal S, Takahashi M, Rapley E, Barfoot R, et al. Identification of the familial cylindromatosis tumour-suppressor gene. Nat Genet. 2000;25:160–165. doi: 10.1038/76006. [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Nijman SM, Dirac AM, Bernards R. Loss of the cylindromatosis tumour suppressor inhibits apoptosis by activating NF-kappaB. Nature. 2003;424:797–801. doi: 10.1038/nature01811. [DOI] [PubMed] [Google Scholar]
- Fogerty FJ, Akiyama SK, Yamada KM, Mosher DF. Inhibition of binding of fibronectin to matrix assembly sites by anti-integrin (alpha 5 beta 1) antibodies. J Cell Biol. 1990;111:699–708. doi: 10.1083/jcb.111.2.699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray-Schopfer V, Wellbrock C, Marais R. Melanoma biology and new targeted therapy. Nature. 2007;445:851–857. doi: 10.1038/nature05661. [DOI] [PubMed] [Google Scholar]
- Hegerfeldt Y, Tusch M, Brocker EB, Friedl P. Collective cell movement in primary melanoma explants: plasticity of cell-cell interaction, beta1-integrin function, and migration strategies. Cancer Res. 2002;62:2125–2130. [PubMed] [Google Scholar]
- Hellerbrand C, Bumes E, Bataille F, Dietmaier W, Massoumi R, Bosserhoff AK. Reduced expression of CYLD in human colon and hepatocellular carcinomas. Carcinogenesis. 2007;28:21–27. doi: 10.1093/carcin/bgl081. [DOI] [PubMed] [Google Scholar]
- Hirai Y, Kawamata Y, Takeshima N, Furuta R, Kitagawa T, Kawaguchi T, et al. Conventional and array-based comparative genomic hybridization analyses of novel cell lines harboring HPV18 from glassy cell carcinoma of the uterine cervix. Int J Oncol. 2004;24:977–986. [PubMed] [Google Scholar]
- Hodgson L, Dong C. [Ca2+]i as a potential downregulator of alpha2beta1-integrin-mediated A2058 tumor cell migration to type IV collagen. Am J Physiol Cell Physiol. 2001;281:C106–C113. doi: 10.1152/ajpcell.2001.281.1.C106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y, Boskovic G, Niles RM. Retinoic acid-induced AP-1 transcriptional activity regulates B16 mouse melanoma growth inhibition and differentiation. J Cell Physiol. 2003;194:162–170. doi: 10.1002/jcp.10199. [DOI] [PubMed] [Google Scholar]
- Hutti JE, Shen RR, Abbott DW, Zhou AY, Sprott KM, Asara JM, et al. Phosphorylation of the tumor suppressor CYLD by the breast cancer oncogene IKKepsilon promotes cell transformation. Mol Cell. 2009;34:461–472. doi: 10.1016/j.molcel.2009.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenner MW, Leone PE, Walker BA, Ross FM, Johnson DC, Gonzalez D, et al. Gene mapping and expression analysis of 16q loss of heterozygosity identifies WWOX and CYLD as being important in determining clinical outcome in multiple myeloma. Blood. 2007;110:3291–3300. doi: 10.1182/blood-2007-02-075069. [DOI] [PubMed] [Google Scholar]
- Jin JY, Ke H, Hall RP, Zhang JY. c-Jun Promotes whereas JunB Inhibits Epidermal Neoplasia. J Invest Dermatol. 2011 doi: 10.1038/jid.2011.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL, Ming X, Nakagawa O, Xu R, Yoo H. Integrin targeted delivery of gene therapeutics. Theranostics. 2011;1:211–219. doi: 10.7150/thno/v01p0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke H, Harris R, Coloff JL, Jin JY, Leshin B, Miliani de Marval P, et al. The c-Jun NH2-terminal kinase 2 plays a dominant role in human epidermal neoplasia. Cancer Res. 2010;70:3080–3088. doi: 10.1158/0008-5472.CAN-09-2923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuno N, Shiina H, Urakami S, Kawamoto K, Hirata H, Tanaka Y, et al. Genistein mediated histone acetylation and demethylation activates tumor suppressor genes in prostate cancer cells. Int J Cancer. 2008;123:552–560. doi: 10.1002/ijc.23590. [DOI] [PubMed] [Google Scholar]
- Kovalenko A, Chable-Bessia C, Cantarella G, Israel A, Wallach D, Courtois G. The tumour suppressor CYLD negatively regulates NF-kappaB signalling by deubiquitination. Nature. 2003;424:801–805. doi: 10.1038/nature01802. [DOI] [PubMed] [Google Scholar]
- Linos E, Swetter SM, Cockburn MG, Colditz GA, Clarke CA. Increasing burden of melanoma in the United States. J Invest Dermatol. 2009;129:1666–1674. doi: 10.1038/jid.2008.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Bergami P, Huang C, Goydos JS, Yip D, Bar-Eli M, Herlyn M, et al. Rewired ERK-JNK signaling pathways in melanoma. Cancer Cell. 2007;11:447–460. doi: 10.1016/j.ccr.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massoumi R. CYLD: a deubiquitination enzyme with multiple roles in cancer. Future Oncol. 2011;7:285–297. doi: 10.2217/fon.10.187. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Chmielarska K, Hennecke K, Pfeifer A, Fassler R. Cyld inhibits tumor cell proliferation by blocking Bcl-3-dependent NF-kappaB signaling. Cell. 2006;125:665–677. doi: 10.1016/j.cell.2006.03.041. [DOI] [PubMed] [Google Scholar]
- Massoumi R, Kuphal S, Hellerbrand C, Haas B, Wild P, Spruss T, et al. Down-regulation of CYLD expression by Snail promotes tumor progression in malignant melanoma. J Exp Med. 2009;206:221–232. doi: 10.1084/jem.20082044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miliani de Marval P, Lutfeali S, Jin JY, Leshin B, Selim MA, Zhang JY. CYLD Inhibits Tumorigenesis and Metastasis by Blocking JNK/AP1 Signaling at Multiple Levels. Cancer Prev Res (Phila) 2011 doi: 10.1158/1940-6207.CAPR-10-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mould AP, Garratt AN, Askari JA, Akiyama SK, Humphries MJ. Identification of a novel anti-integrin monoclonal antibody that recognises a ligand-induced binding site epitope on the beta 1 subunit. FEBS Lett. 1995;363:118–122. doi: 10.1016/0014-5793(95)00301-o. [DOI] [PubMed] [Google Scholar]
- Na SY, Choi JE, Kim HJ, Jhun BH, Lee YC, Lee JW. Bcl3, an IkappaB protein, stimulates activating protein- 1 transactivation and cellular proliferation. J Biol Chem. 1999;274:28491–28496. doi: 10.1074/jbc.274.40.28491. [DOI] [PubMed] [Google Scholar]
- Nakahara H, Nomizu M, Akiyama SK, Yamada Y, Yeh Y, Chen WT. A mechanism for regulation of melanoma invasion. Ligation of alpha6beta1 integrin by laminin G peptides. J Biol Chem. 1996;271:27221–27224. doi: 10.1074/jbc.271.44.27221. [DOI] [PubMed] [Google Scholar]
- Pinon P, Wehrle-Haller B. Integrins: versatile receptors controlling melanocyte adhesion, migration and proliferation. Pigment Cell Melanoma Res. 2010 doi: 10.1111/j.1755-148X.2010.00806.x. [DOI] [PubMed] [Google Scholar]
- Regamey A, Hohl D, Liu JW, Roger T, Kogerman P, Toftgard R, et al. The tumor suppressor CYLD interacts with TRIP and regulates negatively nuclear factor kappaB activation by tumor necrosis factor. J Exp Med. 2003;198:1959–1964. doi: 10.1084/jem.20031187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley W, Zhang M, Sun SC. Negative regulation of JNK signaling by the tumor suppressor CYLD. J Biol Chem. 2004;279:55161–55167. doi: 10.1074/jbc.M411049200. [DOI] [PubMed] [Google Scholar]
- Reiley WW, Jin W, Lee AJ, Wright A, Wu X, Tewalt EF, et al. Deubiquitinating enzyme CYLD negatively regulates the ubiquitin-dependent kinase Tak1 and prevents abnormal T cell responses. J Exp Med. 2007;204:1475–1485. doi: 10.1084/jem.20062694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiley WW, Zhang M, Jin W, Losiewicz M, Donohue KB, Norbury CC, et al. Regulation of T cell development by the deubiquitinating enzyme CYLD. Nat Immunol. 2006;7:411–417. doi: 10.1038/ni1315. [DOI] [PubMed] [Google Scholar]
- Stegmeier F, Sowa ME, Nalepa G, Gygi SP, Harper JW, Elledge SJ. The tumor suppressor CYLD regulates entry into mitosis. Proc Natl Acad Sci U S A. 2007;104:8869–8874. doi: 10.1073/pnas.0703268104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel P, Zettl A, Ren Z, Starostik P, Riedmiller H, Storkel S, et al. Spiradenocylindroma of the kidney: clinical and genetic findings suggesting a role of somatic mutation of the CYLD1 gene in the oncogenesis of an unusual renal neoplasm. Am J Surg Pathol. 2002;26:119–124. doi: 10.1097/00000478-200201000-00016. [DOI] [PubMed] [Google Scholar]
- Trompouki E, Hatzivassiliou E, Tsichritzis T, Farmer H, Ashworth A, Mosialos G. CYLD is a deubiquitinating enzyme that negatively regulates NF-kappaB activation by TNFR family members. Nature. 2003;424:793–796. doi: 10.1038/nature01803. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Richmond A. NF-kappaB activation in melanoma. Pigment Cell Res. 2006;19:112–124. doi: 10.1111/j.1600-0749.2006.00304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Baiocchi RA, Pal S, Mosialos G, Caligiuri M, Sif S. The BRG1- and hBRM-associated factor BAF57 induces apoptosis by stimulating expression of the cylindromatosis tumor suppressor gene. Mol Cell Biol. 2005;25:7953–7965. doi: 10.1128/MCB.25.18.7953-7965.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson KD. Regulation of ubiquitin-dependent processes by deubiquitinating enzymes. Faseb J. 1997;11:1245–1256. doi: 10.1096/fasebj.11.14.9409543. [DOI] [PubMed] [Google Scholar]
- Yang S, McNulty S, Meyskens FL., Jr During human melanoma progression AP-1 binding pairs are altered with loss of c-Jun in vitro. Pigment Cell Res. 2004;17:74–83. doi: 10.1046/j.1600-0749.2003.00114.x. [DOI] [PubMed] [Google Scholar]
- Zhang JY, Adams AE, Ridky TW, Tao S, Khavari PA. Tumor necrosis factor receptor 1/c-Jun-NH2-kinase signaling promotes human neoplasia. Cancer Res. 2007;67:3827–3834. doi: 10.1158/0008-5472.CAN-06-4017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziober BL, Chen YQ, Ramos DM, Waleh N, Kramer RH. Expression of the alpha7beta1 laminin receptor suppresses melanoma growth and metastatic potential. Cell Growth Differ. 1999;10:479–490. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.