Abstract
OBJECTIVE
It is generally believed that muscle weakness in patients with polymyositis and dermatomyositis is due to autoimmune and inflammatory processes. However, it has been observed that there is a poor correlation between the suppression of inflammation and a recovery of muscle function in patients. We have therefore hypothesized that non-immune mechanisms also contribute to muscle weakness. In particular, it has been suggested that an acquired deficiency of AMP deaminase (AMPD1) may be responsible for muscle weakness in myositis.
METHODS
We have used comprehensive functional, behavioral, histological, molecular, enzymatic and metabolic assessments before and after the onset of inflammation in MHC class I mouse model of autoimmune inflammatory myositis.
RESULTS
We found that muscle weakness and metabolic disturbances were detectable in the mice prior to the appearance of infiltrating mononuclear cells. Force contraction analysis of muscle function revealed that weakness was correlated with AMDP1 expression and was myositis-specific. We also demonstrated that decreasing AMPD1 expression results in decreased muscle strength in healthy mice. Fiber typing suggested that fast-twitch muscles are converted to slow-twitch muscles as myositis progresses, and microarray results indicated that AMPD1 and other purine nucleotide pathway genes are suppressed, along with genes essential to glycolysis.
CONCLUSION
These data suggest that an AMPD1 deficiency is acquired prior to overt muscle inflammation and is responsible, at least in part, for the muscle weakness that occurs in the mouse model of myositis. AMPD1 is therefore a potential therapeutic target in myositis.
Introduction
Idiopathic inflammatory myopathies are typically characterized by a spontaneous onset of symptoms, autoimmune reactivity, symmetrical proximal muscle weakness, and progressive muscle degeneration. These symptoms can become life-threatening if the progressive muscle weakness causes defects in swallowing and respiratory function. However, in these myopathies (collectively called myositis) there is a dissociation between inflammation and muscle weakness (1). For example, studies have shown that a) there is a lack of correlation between the degree of inflammation and the degree of muscle weakness (2, 3) b) a subgroup of myositis patients do not respond to large doses of steroids (4, 5); and c) in some patients steroid treatment effectively eliminates the inflammatory cells in the myositis muscle tissue, with little improvement in clinical disease (6). Patients with chronic myositis show clinical disease without any identifiable inflammation (as assessed by histological analysis or magnetic resonance imaging) (7). These observations suggest that the autoimmune response arises in parallel with a myopathy that is not completely reversed by immunosuppression. Thus, the molecular mechanisms that account for muscle weakness in the absence of inflammation are still unknown.
One clue to this puzzle can be found in individuals with an inherited deficiency of AMPD1, a rate-limiting enzyme in the catabolism of adenosine monophosphate (AMP) to inosine 5′-phosphate (IMP) and NH3. These individuals can exhibit impaired energy production and a rapid onset of fatigue during moderate exercise (8, 9). These similarities led to the hypothesis that an acquired deficiency of AMPD1 (myoadenylate deaminase; muscle isoform) may be responsible for muscle weakness in myositis patients (9, 10).
AMPD1 is preferentially expressed at high levels in type II skeletal muscle, where it influences the levels of Pi, AMP, ADP, and phosphocreatine. All other tissues express low levels of either AMPD2 or AMPD3 (11–14). Patients with an inherited defect in AMPD1 expression often show significantly diminished muscle performance, suggesting that the purine nucleotide catabolic pathway plays a role in short-term energy production (15, 16). Myositis patients have been found to have low AMPD enzyme activity along with reduced levels of AMPD1 protein and mRNA (9, 10, 17). Taken together, these observations suggest that the refractory symptoms of muscle weakness in myositis patients might be explained by an acquired deficiency of the AMPD1 enzyme.
The pathogenic process leading to an acquired AMPD1 deficiency, however, is still poorly understood. Therefore, we have utilized a mouse model of inflammatory myositis to assess the potential relationship between AMPD1 expression and muscle weakness in myositis. The symptoms seen in the mouse model of myositis closely mimic those of the human disease, including muscle weakness, overexpression of MHC class I in the muscle, infiltration of mononuclear cells, autoantibodies (in some mice), and a higher prevalence of disease in females (18–21).
We now demonstrate that an acquired AMPD1 deficiency indeed exists in the MHC class I transgenic mouse model of myositis, but not mouse models of other myopathies. In addition, our results indicate that a drop in AMPD1 activity and muscle strength can be detected prior to the onset of inflammation in the muscle. Furthermore, a reduction (knockdown via morpholinos) in the expression of the AMPD1 enzyme resulted in the loss of muscle strength in otherwise healthy animals. These observations suggest that a loss of AMPD1 activity occurs prior to the infiltration of mononuclear cells, but not as a consequence of muscle inflammation, and this loss of activity may be a potential cause of weakness in myositis.
MATERIALS AND METHODS
Animals
The generation and genotyping of the HT myositis mouse model was described previously (18). All mice were handled according the local IACUC guidelines. Single-transgenic animals (designated “H mice”) do not develop myositis. Double-transgenic animals (“HT mice”) spontaneously develop myositis after doxycycline withdrawal. For controls, age-matched single-transgenic littermates were used. Only female mice were utilized for our experiments and data analysis. At 5 weeks of age, doxycycline was withdrawn from the water supply. Mice 15 weeks of age or older were designated infiltrated mice, and those 8 to 13 weeks old, pre-infiltrated. Muscle tissue was dissected and flash-frozen in isopentane (Sigma-Aldrich) pre-chilled with liquid nitrogen or fixed in 10% neutral buffered formalin (Fisher Scientific) for further analysis.
Functional and behavioral activities
Rotarod test
The latency-to-fall from a Rotarod apparatus (Ugo Basile) was assessed as described previously (22).
Grip strength test
Grip strength for both fore- and hindlimbs was assessed by using a grip strength meter, consisting of a horizontal forelimb mesh and an angled hind limb mesh (Columbus Instruments, Columbus, OH), according to a previously published protocol (23).
Behavioral activity
Voluntary activity in an open field was measured using an open field Digi-Scan apparatus (Omnitech Electronics, Columbus, OH) as described previously (23).
Force contractions on isolated skeletal muscle
Force contraction experiments were conducted on the EDL and soleus muscles of the right hindlimb of mice as described previously (24). The muscle-specific force, a measure of the intrinsic force generation of the muscle, was calculated according the following equation: specific force = maximal isometric force/ (muscle mass * (density of muscle tissue * fiber length)−1). The muscle tissue density was 1.056 kg/L.
Measurement of skeletal muscle AMPD enzymatic activity
Enzyme purification and determination of activity was performed as described previously (25). While AMPD1 is highly abundant in the skeletal muscle, a small amount of AMPD3 is expected to be found in whole muscle preparations. Consequently, AMPD enzymatic assays measure the combined activity from both enzymes. Soluble protein (20-μg samples) was assayed in 25mM imidazole, pH 6.5, containing 1mg/ml bovine serum albumin (BSA), 145mM KCl, and 20mM AMP (1000-μl total volume). The assay was initiated by the addition of substrate (AMP). After 10 min, substrate (AMP) and product (IMP) were separated on a Whatman Partisil 10-SAX anion exchange column and developed with a linear gradient. Column eluate was recorded at 254 nm, and peaks were quantified based on the correspondence of retention times to external standards of known concentrations.
Quantification of metabolites
The concentrations of fumarate and hypoxanthine were determined with a Fumarate Assay Kit (BioVision) and an Amplex Red Xanthine / Xanthine Oxidase Kit (Invitrogen), respectively, using 50 μl of muscle tissue lysate according to the manufacturer’s protocol. The levels of fumarate and hypoxanthine were then normalized to the protein concentration of each sample and plotted relative to metabolite levels in control mice.
Real-time PCR analysis
For RT-PCR, RNA was isolated from frozen muscle tissue by dicing tissue with a sterile razor, then homogenizing tissue in Trizol (Invitrogen) using a Kontes pestle. A total of 750 ng of RNA was used to produce cDNA using a Promega Reverse Transcription System kit. The relative expression of AMPD1 mRNA was quantified with Taqman primers for AMPD1 (Mm01308676_m1) and GAPDH (Mm99999915_g1) in an ABI 7900HT Fast Real-Time PCR machine. Relative AMPD1 expression was calculated using the ΔΔCt method, with GAPDH as the internal reference gene.
Fiber typing by immunohistochemical staining
Fiber typing was performed using primary antibodies recognizing slow-twitch fibers (VP-M667) and fast-twitch fibers (VP-M665) (Vector Laboratories). Antiboides were diluted 1/50 and 1/25, respectively, and incubated overnight at 4°C, then labeled with Alexafluor 488 (A-21121) for one hour before washing and mounting in Vectashield with DAPI (H-1200). Images were captured on a Zeiss LSM510 confocal microscope using ZEN 2009 software.
Gene expression profiling
Total RNA was extracted from gastrocnemius (n=4) and soleus (n=4) muscle of both HT and control mice using Trizol (Invitrogen) reagent, and 10μg of each total RNA sample was processed for microarray analysis as previously described (26). Data were normalized by quantile normalization and the GC-RMA procedure implemented in Partek Genomics Suite (Partek Incorporated) and GeneSpring GX (Agilent Technologies).
Inhibition of AMPD1 translation
Knockdown of AMPD1 protein levels was accomplished by systemic (IV) injection of custom Vivo-morpholino oligos (GeneTools Inc., Philomath, OR). The AMPD1 Vivo-morpholino (abbreviated moAMPD1) 5′-GAC CTG TTA GTT TGA ATA GAG GCAT -3′ and standard Vivo-morpholino control 5′-CCT CTT ACC TCA GTT ACA ATT TATA -3′ were designed by Gene Tools Inc. Vivo-morpholino uptake was enhanced by exercise on a horizontal treadmill (30 min at 12 m/min) for 2 days prior to injection. Vivo-Morpholinos were diluted into 100 μl of 0.9% saline and injected at 10 mg/kg into the lateral tail vein of the mice. Injections were performed twice weekly for 2 weeks, for a total of four injections. Mice were 14 weeks old when injections began, and 16 weeks old at the time of sacrifice.
Western blotting
Western blots were performed as described previously (27). Primary antibodies specific for AMPD1 (1:2000 dilution, Imgenex IMG-6068A) and vinculin (1:5000 dilution, Abcam ab18058) were utilized. Densitometry analysis was carried out on a BioRad GS-800 Calibrated Densitometer running the Quantity One software package.
Statistical analysis
Where appropriate, statistical significance was calculated using Student’s t-test for independent samples with Prism v4 software (GraphPad Software). Within each figure, p values are indicated as follows: p<0.05 is indicated by (*), p<0.01 by (**), and p<0.001 by (***). Graphed data are presented as means ± standard error.
RESULTS
Overt damage to skeletal muscle does not occur until the infiltrated phase
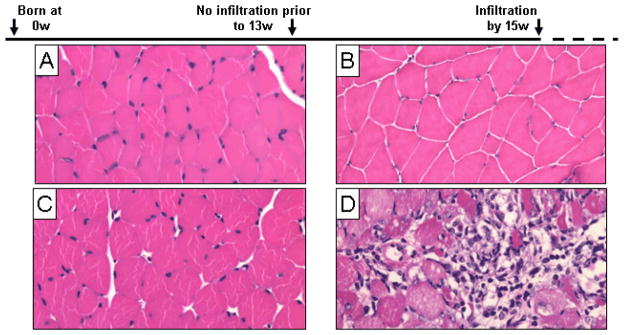
We and others have previously shown that conditional overexpression of syngeneic MHC class I (H-2Kb) in mouse skeletal muscle closely mimics the disease phenotypes of myositis patients (18, 20, 21, 28). Mice overexpressed MHC class I after doxycycline was withdrawn at 5 weeks of age, while visible and histological disease symptoms were not apparent until about 15 weeks of age. Histological hallmarks of myositis include infiltration of mononuclear cells and muscle fiber degeneration. No sign of infiltration or degeneration was present in healthy control mice (Fig. 1A, C) or in pre-infiltrated HT mice (3–8 weeks without doxycycline) (Fig. 1B). In contrast, 16 week old HT mice showed all of the hallmarks of myositis (Fig. 1D) including mononuclear infiltration and extensive muscle fiber degeneration. Infiltrated mice were also found to have below-average body weights, while pre-infiltrated mice did not differ from controls (Fig. S1B). These results show that lymphocyte infiltration and overt histological damage to skeletal muscle do not occur until the infiltrated phase of the disease.
Figure 1. Histology of pre-infiltrated and infiltrated phases of HT mice.
In this mouse model of myositis, single-transgenic mice are designated “H mice” and remain healthy (A, B), while double-transgenic animals are designated “HT mice” and develop myositis over time (C, D). Gastrocnemius muscles were fixed in formalin and stained with H&E during the pre-infiltrated and infiltrated stages. Characteristic inflammation and degeneration was seen in infiltrated HT mice at 16 weeks of age (D). No infiltrating mononuclear cells are seen during the pre-infiltration phase in HT mice at 8 weeks of age (C).
The soleus and EDL muscles in HT mice display weakness upon force contraction analysis
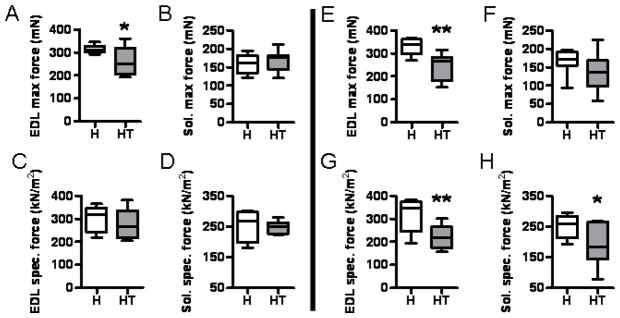
Assessing muscle strength is best measured by in vitro force contraction analysis on isolated muscles. Additionally, force contraction analysis can be performed separately on predominantly fast-twitch (i.e., EDL) and slow-twitch (i.e., soleus) muscles. Infiltrated HT mice showed significantly diminished EDL strength in terms of both maximal force and specific force when compared to controls (Fig. 2E, G). When we assessed the pre-infiltrated mice, we found significant weakness in the EDL (Fig. 2A) despite the otherwise healthy appearance of the muscle histology at this age (Fig. 1C).
Figure 2. The EDL and soleus muscles of HT mice are severely weakened as the disease progresses.
The EDL and soleus muscles were dissected from anesthetized mice and subjected to force contraction analysis. The contractile force of both the EDL and the soleus was quantified for both pre-infiltrated (A–D, on the left of the central dividing line) and infiltrated time points (E–H, on the right of the central dividing line). A significant drop in EDL max force was observed in the infiltrated phase (A), which worsened as the disease progressed (E).
Conversely, the soleus muscles of pre-infiltrated mice were not weak (Fig. 2B, D), but infiltrated mice showed a significantly reduced level of soleus specific force (Fig. 2H). There was a similar drop in the average soleus maximal force in infiltrated mice versus control mice (Fig. 2F), but this decrease was not statistically significant.
HT mice did not show any significant changes in behavior until the infiltrated phase of the disease. Specifically, infiltrated HT mice showed a significant loss of fore- and hindlimb grip strength, fell from the Rotarod more frequently, and stood vertically less often (Fig. S1C–F). Taken together, these results suggest that a measurable loss of strength in the fast-twitch muscles precedes the infiltration of mononuclear cells or obvious impairments in normal movement and the strength further deteriorates during the infiltration phase of the disease.
AMPD enzyme activity and expression are decreased in pre-infiltrated HT mice
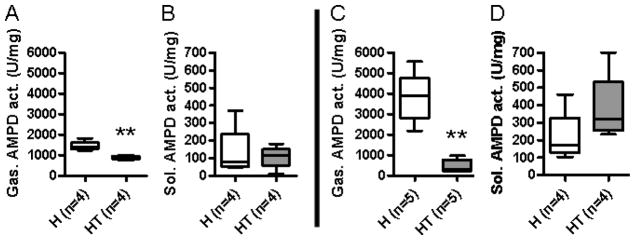
AMPD1 expression is highest in the fast-twitch fibers (e.g., EDL, gastrocnemius) of skeletal muscle. When we measured AMPD enzyme activity of the gastrocnemius, infiltrated HT mice showed a severe decrease (−88.2%) in AMPD activity compared to control mice (Fig. 3C), but the enzymatic activity was not significantly altered in the slow-twitch soleus muscle (Fig. 3D). Pre-infiltrated mice also showed a significant decrease (−39.7%) in AMPD activity in the gastrocnemius muscle fibers when compared to the control mice (Fig. 3A). This pattern of AMPD enzyme deficiency coincided and mimicked the pattern of muscle weakness seen in Figure 2.
Figure 3. A loss of AMPD enzyme activity is detectable prior to the onset of myositis symptoms.
AMPD enzyme activity in the gastrocnemius (A, C) and soleus (B, D) muscles was quantified in both pre-infiltrated (A, B) and infiltrated HT mice (C, D). Both infiltrated and pre-infiltrated HT mice showed significantly reduced AMPD enzyme activity in the gastrocnemius.
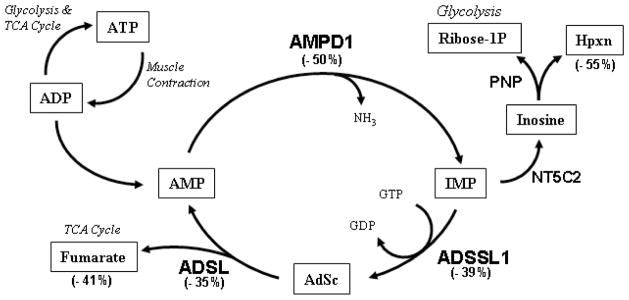
We next examined the effects of AMDP1 deficiency on downstream metabolites by quantifying both fumarate and hypoxanthine levels in skeletal muscle lysates. The relationship between AMPD1 and these metabolites is illustrated in Figure 8. Fumarate is a molecule that can fuel the TCA cycle, and healthy mice were found to have 42.26 ± 2.57 mmol of fumarate per 50 μg of soluble protein, whereas infiltrated HT mice (n=10) were observed to have significantly less fumarate; only 32.11 ± 3.14 mmol (Fig. S4A). Similarly, healthy mice had 27.11 ± 3.51 mmol of hypoxanthine per 50 μg of soluble protein, while infiltrated HT mice had only 16.01 ± 1.52 mmol; a significant decrease (Fig. S4B). It is pertinent to note that hypoxanthine and ribose-1P (a metabolite for PPP metabolism and glycolysis) are generated simultaneously during AMP catabolism (Fig. 8). The loss of these metabolites indicates that the loss of AMPD1 activity has a significant impact on the production of metabolites that can fuel glycolysis and the TCA cycle during muscle contractions.
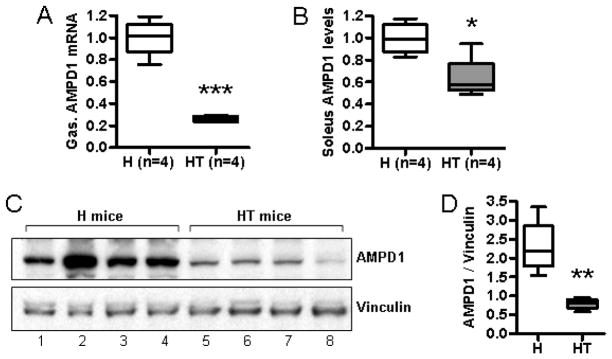
We then analyzed AMPD1 expression by quantitative PCR and Western blotting. An examination of mRNA levels for AMPD1 in pre-infiltrated mice did not show significant changes in mRNA levels for AMPD1 (data not shown), but infiltrated mice showed significant reduction in AMPD1 expression in fast-twitch muscle (Fig. 4A) and a moderate reduction in slow-twitch muscle (Fig 4B). A similar loss of AMPD1 protein in the fast-twitch muscle of infiltrated mice was observed by Western blotting (Fig. 4C).
Figure 4. Infiltrated HT mice have lowered levels of AMPD1 protein and mRNA.
RNA was isolated from the muscle tissue of infiltrated HT mice and healthy H controls. The relative quantity of AMPD1 mRNA in determined for both the gastrocnemius (A) and soleus (B) muscles. AMPD1 levels were normalized to expression levels in healthy littermate controls. Protein levels for AMPD1 in the gastrocnemius of healthy mice and infiltrated HT mice were determined by Western blotting (C) and quantified by densitometry (D), using vinculin as a loading control. Down-regulation of the AMPD1 gene coincides with a drop in protein levels.
AMPD enzyme activity is unaltered in other genetic myopathic models
In order to verify that the loss of AMPD enzyme activity was specific to myositis, and not a general feature of other mouse myopathies, we assessed AMPD activity in the mouse model of muscular dystrophy (the mdx mouse) and the mouse model of limb-girdle muscular dystrophy 2B (the SJL mouse) (Fig. S2). The results indicated that there was no significant difference in AMPD activity between the control C57/BL10, mdx, or SJL mice in either the fast-twitch (gastrocnemius) or slow-twitch (soleus) muscle fibers. These results support the conclusion that the loss of AMPD activity is specific to myositis.
Fast-twitch fibers in HT mice undergo fiber-type conversion
Quantification of slow-twitch muscle fibers was performed by staining with antibodies that recognize either the fast or slow myosin heavy chain protein. The results for pre-infiltrated mice showed no significant difference from control values in terms of the percentage of MHC-positive fibers within either the fast-twitch (Fig. S3A) or the slow-twitch muscle (Fig. S3B). However, infiltrated mice showed a significant increase in the number of slow MHC-positive fibers in both the gastrocnemius (Fig. S3C) and soleus (Fig. S3D).
The twitch parameters of the EDL muscle (fast-twitch) support fiber-type conversion in HT mice
In order to verify the functional consequences of fiber type conversion, we performed twitch parameter analysis in the range of 50–200 Hz on EDL and soleus muscles from infiltrated mice. The EDL muscles showed a significant reduction in the kinetics of peak force (39% weaker) and dtpt values (44% lower) when compared to the control mouse EDL muscles (Table S1). There was also a significant drop in peak force (29% weaker) for the soleus muscle when compared to that in control mice. Neither the EDL nor the soleus showed changes in the recovery half-rate time. Overall, the data are consistent with a loss of type II fibers in the EDL, as shown in Figure S3.
Gene expression profiling indicates an absence of inflammatory markers and presence of metabolic changes in pre-infiltrated mice
Given our observation that AMPD1 enzymatic activity decreased significantly before the onset of overt symptoms, we chose to examine the mRNA profile of pre-infiltrated mice using a gene expression array. Unsupervised hierarchical clustering analysis showed significant changes in the gene expression in the pre-infiltrated phase of the disease (Fig. S5). Expression array analysis showed no detectable levels of mRNA for CD3zeta, CD3epsilon, CD4, CD8, CD20, elastase, CD83, or CD94, suggesting that the muscle tissue from pre-infiltrated mice contained no significant populations of T cells, B cells, neutrophils, or NK cells. Macrophage-restricted genes (e.g. EMR1, LGALS3) were detected in both healthy and pre-infiltrated HT mice. The absence of lymphocyte-specific transcripts is consistent with our histological analyses (Fig. 1) and supports the hypothesis that significant muscle weakness seen in pre-infiltrated mice is not a result of the action of lymphocytes.
We reasoned that since an acquired loss of AMPD1 can be seen at the mRNA level, other genes related to purine nucleotide catabolism (see Fig. 5) or ATP production could similarly be decreased. The expression array indicated that the AMPD1 mRNA levels were halved (−fold change = −2.01; p<0.05) compared to normal transcript levels, consistent with Q-PCR data presented in Figure 4. Other genes in the purine catabolic pathway, such as adenylosuccinate synthetase (ADSSL1; −fold change = −1.62; p<0.05) and adenylosuccinate lyase (ADSL; −fold change = −1.52; p<0.05) were decreased in HT mice
Figure 5. Diagram of the role of AMPD1 in purine catabolism.
A number of enzymes involved in AMP catabolism are shown in relation to each other (A). The number next to each enzyme or molecule name indicates the percent decrease in gene expression or metabolite abundance in HT mice compared healthy controls. Large quantities of AMP are produced during muscle contractions and converted into IMP by AMPD1. The downstream products of IMP can either fuel oxidative phosphorylation (via fumarate) or glycolysis (via ribose-1P). Abbreviations: IMP: inosine monophosphate, AdSc: adenylosuccinate, L-Asp: L-aspartate, Hpxn: hypoxanthine.
An examination of the genes required for both glycolysis and oxidative phosphorylation revealed that there was a moderate but significant downregulation of these two energy production pathways in pre-infiltrated mice. Specifically, we found a significant (−fold change <−1.5; p < 0.05) decrease in the expression of transcripts for phoshpofructokinase (PFKM), aldolase (ALDOA), phosphoglycerate mutase (PGAM2), enolase (ENO3), pyruvate kinase (PKM2), and lactate dehydrogenase (LDHA). Known mitochondrial enzymes were also significantly downregulated, but very few transcripts for mitochondrial enzymes exceeded the (−fold change <−1.5) threshold. Cumulatively, these changes in mRNA regulation are consistent with other observations that a metabolic deficiency exists in pre-infiltrated muscles.
Knockdown of AMPD1 protein levels in healthy mice results in decreased muscle strength
Next, we tested the hypothesis that knocking down AMPD1 levels would induce muscle weakness in healthy mice. Vivo-morpholinos were used to knock down AMPD1 expression in vivo. The Vivo-morpholino was targeted to occlude a 24-bp region centered on the start codon of the AMPD1 mRNA in order to block translation. This inhibition did not affect mRNA levels but did lower protein levels of the targeted gene.
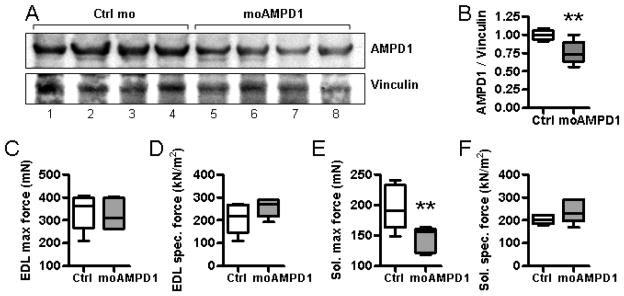
Healthy C57BL/6 mice were injected intravenously with either control (Ctrl mo) or AMPD1-targeting morpholinos (moAMPD1). Western blot analysis revealed that moAMPD1 was able to partially block protein translation in the quadriceps, while the control mice showed normal levels of AMPD1 (Fig 6A). Densitometry analysis of confirmed that the moAMPD1 knockdown resulted in a significant in drop in AMPD1 protein levels (Fig 6B). The amount of inhibition achieved was sufficient to induce weakness in the soleus muscle (Fig. 6E), where basal AMPD1 activity is low. Conversely, the EDL muscles (where AMPD1 is abundant) were not significantly weakened by this treatment (Fig 6A, B).
Figure 6. Morpholinos targeted against AMPD1 can significantly reduce AMPD1 protein levels and reduce force generation in the soleus muscle.
Healthy, 14-week-old C57BL/6 mice were given a total of four injections of control morpholinos (Ctrl mo) or anti-AMPD1 morpholinos (moAMPD1) at 10 mg/kg. Twenty-four hours after the final injection, the mice were subjected for force contraction analysis and sacrificed. Western blot analysis for AMPD1 revealed that the morpholinos were able to enter skeletal muscle and block the translation of AMPD1 (A). Densitometry analysis for AMPD1 and the loading control, vinculin, confirmed that the AMPD1 levels were significantly reduced (B). The level of knockdown achieved was not sufficient to cause weakness in the EDL (C, D) but did induce weakness in the soleus (E, F).
Discussion
This study examined the contribution of AMPD1 to the muscle function in the mouse model of myositis. Our findings support the hypothesis that an acquired AMPD1 deficiency could contribute to the muscle weakness seen in myositis. This metabolic deficiency can explain how muscle weakness may persist independent of autoimmune inflammation. The data obtained from our enzymatic assays and gene expression array also indicated that muscle weakness begins early in the pathologic process and loss of AMPD enzyme activity is one of the earliest markers of disease.
This work provides a basis for understanding the AMPD1-related muscle weakness in inflammatory myopathies. Force contraction analysis revealed weakness in both type I and type II fibers, although type II muscles (e.g., EDL) suffer worse weakness and degeneration than do type I fibers (e.g., soleus) in mice. Importantly, the signs of weakness can be detected during the pre-infiltrated stage of the disease. Healthy mice have a very tight distribution of values for both EDL maximal force and specific force, and the onset of disease causes a significant loss of muscle strength in HT mice. However, the progression of myositis is not uniform even between littermates, leading to a wider distribution of values for muscle strength in HT mice. Nevertheless, comparisons between pre-infiltrated and infiltrated mice make it clear that muscle weakness in the HT mice is detectable even before the appearance of infiltrating lymphocytes and other histological signs of disease.
Previous studies have demonstrated that the type II (fast) fibers are more prone to inflammation-induced degeneration or atrophy than are type I (slow) muscle fibers (29, 30). We also found that the proportion of slow-twitch fibers was increased in the EDL of infiltrated mice. Our twitch parameter analysis corroborated the finding that the EDL in HT mice does not behave like a typical fast-twitch muscle. The most telling change was observed in the dpdt value, where the ascending slope of the twitch was flattened (more like a slow-twitch muscle) when compared to the healthy EDL muscles. The values for TPT are a less reliable measure of fiber type composition, since TPT values can be influenced by a lower peak force (Pt) and, indeed, the Pt values for the EDL and soleus were reduced in infiltrated mice. Overall, these results support the conclusion that the skeletal muscle tissue becomes weaker and takes on some of the characteristics of slow twitch fibers as the disease progresses.
Unfortunately, the mechanistic link between AMPD1, myositis, and muscle weakness in humans has been confounded by a common mutant allele of AMPD1 in Caucasians known as C34T. This allele introduces a premature stop codon in the second exon of AMPD1, but many investigators ignore the fact that AMPD1 has two major splice variants, the second of which drops exon 2 without affecting enzyme activity (31). Several human studies have assumed that a homozygous C34T individual possesses 0% enzyme activity, resulting in potentially erroneous conclusions. Importantly, two different mutations (R388W and R425H) of AMPD1 have been identified in Japanese patients with very low AMPD1 enzyme activity and symptoms of myalgia, weakness, and exercise intolerance(32).
One of the novel findings in this paper was that AMPD1 enzyme activity is significantly decreased in pre-infiltrated mice. While it has been reported that AMPD1 levels are decreased in human myositis patients, there are no data concerning weakness occurring prior to lymphocyte infiltration in human muscle, leaving some researchers to question whether the AMPD1 deficiency is congenital or acquired during disease onset. Unlike humans, our mouse model has no known mutation in the AMPD1 gene, and we were able to examine the mice both before and after the onset of inflammation. Our data show that AMPD1 levels normally increase with age, but the onset of myositis causes a loss of AMPD1 activity, especially in type II fibers. Furthermore, enzymatic data show that a significant decrease in AMPD activity can be detected in the pre-infiltrated phase of the disease. The decline in AMPD enzyme activity prior to lymphocyte infiltration strongly suggests that the overexpression of MHC class I in skeletal muscle contributes to a non-immune-mediated pathology within the muscle fibers.
We used systemic administration of Vivo-morpholinos to specifically block the translation of AMPD1. AMPD activity is more than 10 times higher in fast-twitch muscles (EDL, quadriceps) than in slow-twitch muscles (soleus). Consequently, the degree of knockdown achieved was sufficient to produce observable weakness in slow-twitch muscles because the enzyme is expressed at lower levels in these muscles. Based on our data, we anticipate that a 50% knockdown of AMPD1 could have produced weakness in the EDL. Unfortunately, higher doses of Vivo-morpholinos are known to be cytotoxic in vivo and could not be tested. Our data demonstrate that a transient knockdown of AMPD1 in wildtype mice can cause muscle weakness and support the hypothesis that an acquired deficiency of AMPD1 can result in muscle weakness.
Currently, the initiating factors that drive the early expression of MHC class I molecules and inflammation in humans are still unknown. Earlier work by our group has revealed that in the HT mice, the surface expression of MHC class I molecules also coincides with increased expression of markers for ER stress, lysosome formation, and autophagy (20, 21, 28, 33). These previous results suggest that ER stress and the unfolded protein response play a part in the non-immune-mediated degeneration muscle fibers in myositis. We set out to gain insight into this self-sustaining pathology by examining pre-infiltrated muscle tissue from HT mice with a gene expression array. The results of our analysis indicated that AMPD1 was significantly down-regulated prior to the infiltration of lymphocytes, as were its downstream metabolites. Also, the expression array analysis revealed a global deregulation of energy production in the muscle fibers. These findings are consistent with prior evidence of the disruption of glycolysis in human myositis patients (34).
In summary, the results of our research support the hypothesis that decreased AMPD1 activity contributes to muscle weakness in inflammatory myopathies. These results further support the hypothesis that the loss of muscle strength can be due to an acquired loss of AMPD1, rather than simply due to autoimmune muscle damage. We also presented evidence that the energy production capacity of the skeletal muscle cells may be diminished in inflammatory myopathies. Overall, these data support the contention that a non-immune-mediated pathology could be responsible for the muscle weakness in these patients and that targeting these pathways may have therapeutic value.
Supplementary Material
Acknowledgments
This research was supported by NIH grant RO1-AR050478; K26RR032082; and DOD W81XWH-11-1-0809.
We would like to thank Arpana Sali for her work in submitting and maintaining our animal protocols, as well as Dr. Deborah McClellan for reviewing this manuscript. William Coley is a predoctoral student in the Microbiology and Immunology Program of the Institute for Biomedical Sciences at the George Washington University. This work is integral to a dissertation to be presented in partial fulfillment of the requirements for his Ph.D. degree. Dr. Nagaraju’s work was supported by NIH grant RO1-AR050478; K26RR032082; and DOD W81XWH-11-1-0809)
References
- 1.Dorph C, Englund P, Nennesmo I, Lundberg IE. Signs of inflammation in both symptomatic and asymptomatic muscles from patients with polymyositis and dermatomyositis. Ann Rheum Dis. 2006;65(12):1565–71. doi: 10.1136/ard.2005.051086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Englund P, Lindroos E, Nennesmo I, Klareskog L, Lundberg IE. Skeletal muscle fibers express major histocompatibility complex class II antigens independently of inflammatory infiltrates in inflammatory myopathies. Am J Pathol. 2001;159(4):1263–73. doi: 10.1016/S0002-9440(10)62513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li CK, Varsani H, Holton JL, Gao B, Woo P, Wedderburn LR. MHC Class I overexpression on muscles in early juvenile dermatomyositis. J Rheumatol. 2004;31(3):605–9. [PubMed] [Google Scholar]
- 4.Mastaglia FL, Phillips BA, Zilko P. Treatment of inflammatory myopathies. Muscle Nerve. 1997;20(6):651–64. doi: 10.1002/(sici)1097-4598(199706)20:6<651::aid-mus1>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 5.Zilko PJ, Mastaglia FL, Phillips BA. Idiopathic inflammatory myopathies: optimum immunosuppressive treatment. BioDrugs. 1997;7(4):262–72. doi: 10.2165/00063030-199707040-00003. [DOI] [PubMed] [Google Scholar]
- 6.Lundberg I, Kratz AK, Alexanderson H, Patarroyo M. Decreased expression of interleukin-1alpha, interleukin-1beta, and cell adhesion molecules in muscle tissue following corticosteroid treatment in patients with polymyositis and dermatomyositis. Arthritis Rheum. 2000;43(2):336–48. doi: 10.1002/1529-0131(200002)43:2<336::AID-ANR13>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 7.Nyberg P, Wikman AL, Nennesmo I, Lundberg I. Increased expression of interleukin 1alpha and MHC class I in muscle tissue of patients with chronic, inactive polymyositis and dermatomyositis. J Rheumatol. 2000;27(4):940–8. [PubMed] [Google Scholar]
- 8.Sabina RL, Swain JL, Olanow CW, Bradley WG, Fishbein WN, DiMauro S, et al. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984;73(3):720–30. doi: 10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fishbein WN. Myoadenylate deaminase deficiency: inherited and acquired forms. Biochem Med. 1985;33(2):158–69. doi: 10.1016/0006-2944(85)90024-9. [DOI] [PubMed] [Google Scholar]
- 10.Sabina RL, Fishbein WN, Pezeshkpour G, Clarke PR, Holmes EW. Molecular analysis of the myoadenylate deaminase deficiencies. Neurology. 1992;42(1):170–9. doi: 10.1212/wnl.42.1.170. [DOI] [PubMed] [Google Scholar]
- 11.Bausch-Jurken MT, Mahnke-Zizelman DK, Morisaki T, Sabina RL. Molecular cloning of AMP deaminase isoform L. Sequence and bacterial expression of human AMPD2 cDNA. J Biol Chem. 1992;267(31):22407–13. [PubMed] [Google Scholar]
- 12.Mahnke-Zizelman DK, Eddy R, Shows TB, Sabina RL. Characterization of the human AMPD3 gene reveals that 5′ exon useage is subject to transcriptional control by three tandem promoters and alternative splicing. Biochim Biophys Acta. 1996;1306(1):75–92. doi: 10.1016/0167-4781(95)00231-6. [DOI] [PubMed] [Google Scholar]
- 13.Mahnke-Zizelman DK, Sabina RL. Cloning of human AMP deaminase isoform E cDNAs. Evidence for a third AMPD gene exhibiting alternatively spliced 5′-exons. J Biol Chem. 1992;267(29):20866–77. [PubMed] [Google Scholar]
- 14.van Kuppevelt TH, Veerkamp JH, Fishbein WN, Ogasawara N, Sabina RL. Immunolocalization of AMP-deaminase isozymes in human skeletal muscle and cultured muscle cells: concentration of isoform M at the neuromuscular junction. J Histochem Cytochem. 1994;42(7):861–8. doi: 10.1177/42.7.8014469. [DOI] [PubMed] [Google Scholar]
- 15.Fischer H, Esbjornsson M, Sabina RL, Stromberg A, Peyrard-Janvid M, Norman B. AMP deaminase deficiency is associated with lower sprint cycling performance in healthy subjects. J Appl Physiol. 2007;103(1):315–22. doi: 10.1152/japplphysiol.00185.2007. [DOI] [PubMed] [Google Scholar]
- 16.Norman B, Nygren AT, Nowak J, Sabina RL. The effect of AMPD1 genotype on blood flow response to sprint exercise. Eur J Appl Physiol. 2008;103(2):173–80. doi: 10.1007/s00421-008-0683-0. [DOI] [PubMed] [Google Scholar]
- 17.Sabina RL, Sulaiman AR, Wortmann RL. Molecular analysis of acquired myoadenylate deaminase deficiency in polymyositis (idiopathic inflammatory myopathy) Adv Exp Med Biol. 1991;309B:203–5. doi: 10.1007/978-1-4615-7703-4_46. [DOI] [PubMed] [Google Scholar]
- 18.Nagaraju K, Raben N, Loeffler L, Parker T, Rochon PJ, Lee E, et al. Conditional up-regulation of MHC class I in skeletal muscle leads to self-sustaining autoimmune myositis and myositis-specific autoantibodies. Proc Natl Acad Sci U S A. 2000;97(16):9209–14. doi: 10.1073/pnas.97.16.9209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li CK, Knopp P, Moncrieffe H, Singh B, Shah S, Nagaraju K, et al. Overexpression of MHC Class I Heavy Chain Protein in Young Skeletal Muscle Leads to Severe Myositis. Implications for Juvenile Myositis. Am J Pathol. 2009 doi: 10.2353/ajpath.2009.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Salomonsson S, Grundtman C, Zhang SJ, Lanner JT, Li C, Katz A, et al. Upregulation of MHC class I in transgenic mice results in reduced force-generating capacity in slow-twitch muscle. Muscle Nerve. 2009;39(5):674–82. doi: 10.1002/mus.21129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagaraju K, Casciola-Rosen L, Lundberg I, Rawat R, Cutting S, Thapliyal R, et al. Activation of the endoplasmic reticulum stress response in autoimmune myositis: potential role in muscle fiber damage and dysfunction. Arthritis Rheum. 2005;52(6):1824–35. doi: 10.1002/art.21103. [DOI] [PubMed] [Google Scholar]
- 22.Raben N, Nagaraju K, Lee E, Plotz P. Modulation of disease severity in mice with targeted disruption of the acid alpha-glucosidase gene. Neuromuscul Disord. 2000;10(4–5):283–91. doi: 10.1016/s0960-8966(99)00117-0. [DOI] [PubMed] [Google Scholar]
- 23.Spurney CF, Gordish-Dressman H, Guerron AD, Sali A, Pandey GS, Rawat R, et al. Preclinical drug trials in the mdx mouse: assessment of reliable and sensitive outcome measures. Muscle Nerve. 2009;39(5):591–602. doi: 10.1002/mus.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rayavarapu S, Van der Meulen JH, Gordish-Dressman H, Hoffman EP, Nagaraju K, Knoblach SM. Characterization of dysferlin deficient SJL/J mice to assess preclinical drug efficacy: fasudil exacerbates muscle disease phenotype. PLoS One. 2010;5(9):e12981. doi: 10.1371/journal.pone.0012981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sims B, Mahnke-Zizelman DK, Profit AA, Prestwich GD, Sabina RL, Theibert AB. Regulation of AMP deaminase by phosphoinositides. J Biol Chem. 1999;274(36):25701–7. doi: 10.1074/jbc.274.36.25701. [DOI] [PubMed] [Google Scholar]
- 26.Di Giovanni S, Knoblach SM, Brandoli C, Aden SA, Hoffman EP, Faden AI. Gene profiling in spinal cord injury shows role of cell cycle in neuronal death. Ann Neurol. 2003;53(4):454–68. doi: 10.1002/ana.10472. [DOI] [PubMed] [Google Scholar]
- 27.Casciola-Rosen L, Nagaraju K. Immunoblotting of single cell types isolated from frozen sections by laser microdissection. Methods Enzymol. 2002;356:70–9. doi: 10.1016/s0076-6879(02)56924-x. [DOI] [PubMed] [Google Scholar]
- 28.Li CK, Knopp P, Moncrieffe H, Singh B, Shah S, Nagaraju K, et al. Overexpression of MHC class I heavy chain protein in young skeletal muscle leads to severe myositis: implications for juvenile myositis. Am J Pathol. 2009;175(3):1030–40. doi: 10.2353/ajpath.2009.090196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lunemann JD, Schmidt J, Schmid D, Barthel K, Wrede A, Dalakas MC, et al. Beta-amyloid is a substrate of autophagy in sporadic inclusion body myositis. Ann Neurol. 2007;61(5):476–83. doi: 10.1002/ana.21115. [DOI] [PubMed] [Google Scholar]
- 30.Dastmalchi M, Alexanderson H, Loell I, Stahlberg M, Borg K, Lundberg IE, et al. Effect of physical training on the proportion of slow-twitch type I muscle fibers, a novel nonimmune-mediated mechanism for muscle impairment in polymyositis or dermatomyositis. Arthritis Rheum. 2007;57(7):1303–10. doi: 10.1002/art.22996. [DOI] [PubMed] [Google Scholar]
- 31.Morisaki H, Morisaki T, Newby LK, Holmes EW. Alternative splicing: a mechanism for phenotypic rescue of a common inherited defect. J Clin Invest. 1993;91(5):2275–80. doi: 10.1172/JCI116455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morisaki H, Higuchi I, Abe M, Osame M, Morisaki T. First missense mutations (R388W and R425H) of AMPD1 accompanied with myopathy found in a Japanese patient. Hum Mutat. 2000;16(6):467–72. doi: 10.1002/1098-1004(200012)16:6<467::AID-HUMU3>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 33.Alger HM, Raben N, Pistilli E, Francia DL, Rawat R, Getnet D, et al. The role of TRAIL in mediating autophagy in myositis skeletal muscle: a potential nonimmune mechanism of muscle damage. Arthritis Rheum. 2011;63(11):3448–57. doi: 10.1002/art.30530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parker KC, Kong SW, Walsh RJ, Salajegheh M, Moghadaszadeh B, Amato AA, et al. Fast-twitch sarcomeric and glycolytic enzyme protein loss in inclusion body myositis. Muscle Nerve. 2009;39(6):739–53. doi: 10.1002/mus.21230. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.