Abstract
The hypothalamic-pituitary-adrenocortical (HPA) axis is a major neuro-endocrine pathway that modulates the stress response. The glucocorticoid, cortisol is the principal end product of the HPA axis in humans, and plays a fundamental role in maintaining homeostasis and in fetal maturation and development. Antenatal administration of synthetic glucocorticoids (GCs) accelerates fetal lung maturation and has significantly decreased neonatal mortality and morbidity in infants born before 34 weeks of gestation. Exposure to excess levels of endogenous GCs and exogenous GCs (betamethasone and dexamethasone) has been shown to alter the normal development trajectory. The development and regulation of the fetal HPA axis is discussed and the experimental animal evidence presented suggests long-term adverse consequences of altered HPA function. The clinical data in infants exposed to GCs also suggests altered HPA axis function over the short term. The longer-term consequences of antenatal GC exposure on HPA axis function and subtler neurodevelopmental outcomes including adaptation to stress, cognition, behavior, and the cardiovascular and immune responses are poorly understood. Emerging clinical strategies and interventions may help in the selection of mothers at risk for preterm delivery who would benefit from existing or future formulations of antenatal GCs with a reduction in the associated risk to the fetus and newborn. Detailed longitudinal long-term follow up of those infants exposed to synthetic GCs are needed.
Keywords: Cortisol, fetal programming, glucocorticoids, HPA axis, pregnancy
Introduction
Over the past 40 years the administration of synthetic antenatal corticosteroids as initially proposed by Liggins and Howie in 19721 has incontrovertibly demonstrated significant reductions in neonatal mortality and morbidity in preterm infants born before 34 weeks gestation. Multiple worldwide clinical trials have been well summarized in the meta-analyses by Crowley, Chalmers and Keirse2 and in Cochrane Database Reviews.3–6 These analyses, consensus statements,7, 8 and practice guidelines have influenced changes in clinical practice for single and repeated courses of synthetic glucocorticoids (GCs) for women in preterm labor.
Dating back to the work of Levine and colleagues beginning in the 1950’s it has been known that the early environment has the potential to exert lasting consequences on the functioning of the hypothalamic-pituitary-adrenocortical (HPA) axis.9 Exposure to endogenous and exogenous GCs during pregnancy is one environmental influence that programs the development of the fetal HPA axis with long-term consequences. For this reason, exposure to excess GCs during gestation may have an adverse impact on stress responsive systems. This review will consider what is known about the effects of prenatal treatment with synthetic GCs on the functioning of the HPA axis.
The Hypothalamic-Pituitary-Adrenocortical (HPA axis)
GCs (cortisol in humans), the end product of the HPA axis, are steroid hormones that are physiologically important for maintaining homeostasis. GCs exert a wide array of key metabolic, endocrine and immune effects on most cells, perhaps more than any other biological ligand.10, 11 Further, GCs pass through the blood brain barrier and with consequences for brain structure and function.12 GCs serve as a major mediator of the stress response and promote survival in the face of threat. The cascade of events that produce changes in cortisol release by the adrenals begins with the release of corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) by cells in the paraventricular nuclei of the hypothalamus. CRH and AVP are released through capillary vessels to the anterior pituitary where they stimulate the release of adenocorticotropic (ACTH) hormone into circulation. ACTH stimulates the biosynthesis and release of cortisol from the adrenal cortex. Because of the damaging effects of chronic exposure to elevated levels of cortisol, the HPA axis is protected by a negative feedback loop whereby cortisol binds to receptors in the pituitary and hypothalamus as well as the hippocampus and prefrontal cortex inhibiting or turning off the HPA axis response. In contrast to the inhibitory role of cortisol on hypothalamic CRH production, cortisol increases the production of CRH in brain regions including the central nucleus of the amygdala, activation of which plays a role not only on stimulating the HPA axis, but also in stimulating increases in central norepinephrine and peripheral activation of the sympathetic nervous system.13 Activation of the HPA axis, including the release of GCs, involves a complex interaction of multiple central and peripheral stress response systems. The interaction between the HPA axis and the sympathetic nervous system has been critically reviewed by Kvetnansky and colleagues.14 This review will focus on the primary consequences of GC treatment for the functioning of the HPA axis.
Changes in the Maternal HPA Axis During Pregnancy
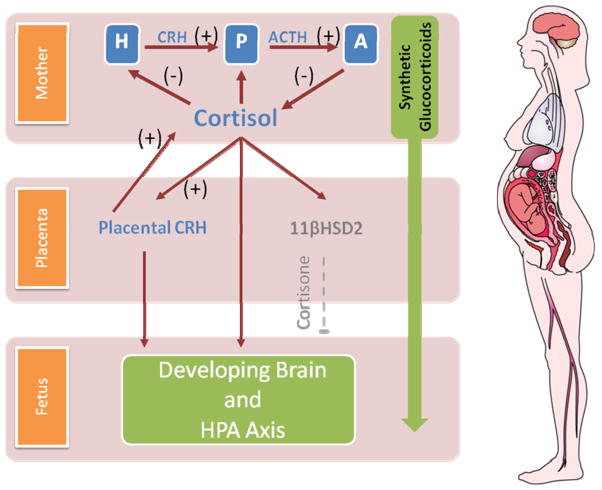
In the course of pregnancy dramatic changes in the functioning of the maternal HPA axis are observed because the placenta expresses the genes for CRH (hCRHmRNA) and the precursor for ACTH and beta-endorphin (proopiomelanocortin). Placental CRH production increases dramatically over gestation and placental CRH plays a central role in the regulation of fetal maturation and the timing of parturition.15–17 In contrast to the negative feedback regulation of hypothalamic CRH, cortisol stimulates the expression of hCRHmRNA in the placenta, establishing a positive feedback loop that allows for the simultaneous increase of placental CRH, ACTH, and cortisol over the course of gestation.18 For example maternal cortisol increases 2 to 4 fold during pregnancy.19 This physiologic increase exerts broad influences on the developing fetus including maturation of the fetal lungs, the central nervous system (CNS) and other organ systems. GCs influence fetal brain development by changing neuronal migration, synaptic plasticity, and neurotransmitter activity.20 Because of the positive feedback between GCs and placental CRH, the effects of excess endogenous or synthetic GCs may be amplified with potentially negative consequences for the developing fetus. The consequences of prenatal treatment with synthetic GCs such as betamethasone and dexamethasone may be more profound as they cross the placenta more easily because they are not readily metabolized by the placental enzyme, 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) that protects the fetus from maternal cortisol.21, 22 Illustrated in figure 1.
Figure 1.
Neuroendocrine interactions among the maternal, placental and fetal compartments. The top panel depicts the activation (+) and negative feedback inhibition (−) pathways of the HPA system. During pregnancy CRH is released from the placenta into both the maternal and fetal compartments. In contrast to the negative feedback regulation of hypothalamic CRH, cortisol increases the production of CRH from the placenta. Because of this positive feedback between cortisol and placental CRH the effects of GCs on the fetus may be amplified. In addition to its effects on placental CRH, maternal cortisol passes through the placenta. However, the effects of maternal cortisol on the fetus are modulated by the presence of a placental enzyme 11βHSD2 which oxidizes it into an inactive form, cortisone. Synthetic GCs used for fetal lung maturation (betamethasone and dexamethasone) cross the placenta more easily because they are not readily metabolized by 11β-HSD2. Structures of the HPA axis begin their development early in gestation and become increasingly functional with the progression towards term. See text for description. GCs= glucocorticoids, CRH=corticotropin releasing hormone, ACTH=adrenocorticotropic hormone, 11βHSD2=11β-hydroxysteroid dehydrogenase type 2
Development of the Fetal HPA Axis
Liggin’s observed that the fetal HPA axis played a major role in the onset of labor and its ablation resulted in underdevelopment of the fetal lungs.23 Since then a number of studies have shown that the fetal HPA axis is highly sensitive to excess levels of GC that could alter regulation of HPA function.12, 18, 24, 25 The fetal adrenal gland makes unique contributions to both the regulation of fetal development and the timing of parturition. Morphologically, the fetal adrenal gland is comprised of three zones: the outer, definitive zone, a large, inner fetal zone, and an intermediate transitional zone. The fetal adrenals undergo rapid growth throughout gestation and at term gestation the fetal adrenals are significantly larger, relative to body weight, than the adult adrenals.26 The adrenal gland has steroidogenic enzymes as early as the seventh gestational week27, 28 and there is evidence for a functional stress response involving an increased production of cortisol in response to pain during the latter half of gestation.29
Animal and human data strongly suggest that GCs program the fetal HPA axis with consequences for postnatal functioning.18, 30 This programming may occur in part through the alterations of brain regions that are both integral to the regulation of stress responses and vulnerable to exposure to stress hormones. Vulnerable regions such as the hippocampus and amygdala undergo rapid development during fetal life. Both are identifiable between 6 and 8 gestational weeks and by term the basic neuroanatomical architecture of these regions is present.31 Cortisol has affinity to two receptors, mineralocorticoid receptors (MRs) and glucocorticoid receptors (GRs). Limited information exists regarding the time course of prenatal development of cortisol receptors in humans. However, data indicates that both receptors types are present in the human hippocampus by 24 gestational weeks.32
Animal Data
Antenatal GC treatment has broad range of effects on the fetus including regulation of growth, organ maturation, immune functioning, and the sympathetic nervous system. Alterations to the functioning of the HPA axis is a primary consequences of prenatal GC treatment that is observed in the offspring of a variety of species including rats, sheep, guinea pigs and non-human primates. 30, 33–39 Data indicate that these changes in functioning of the HPA axis are associated with alterations of GC receptor number in regions including the prefrontal cortex, hippocampus, amygdala and pituitary that are important for activation and negative feedback regulation of the HPA axis.40–42 The nature and the magnitude of these effects are determined by the type of steroid used (e.g., betamethasone or dexamethasone), the number of doses, and the timing of exposure.35, 43–45 Specifically, the binding affinity differs for betamethasone and dexamethasone (e.g., dexamethasone has a greater affinity for GR receptors) and there is evidence that these steroids have differential consequences for offspring.30, 35 In terms of dose, data indicate that exposure to multiple doses is associated with more profound consequences.46, 47 Finally, organ systems may be most vulnerable to the consequences of GC treatment during times of rapid development.48, 49 Thus, the timing of treatment will likely determine the nature and the magnitude of the effects. In addition the type of synthetic GC and dose also has differential effects on lung maturation and surfactant synthesis.50 Carefully conducted studies with sheep have further documented that the phenotypic changes to the HPA axis are additionally determined by the developmental stage at the time of assessment. For example, although increased HPA axis activity is observed in the young animal, suppression of HPA axis activity is observed as the animal matures.34, 51
Exciting and important new work raises the possibility that prenatal exposure to synthetic GCs may alter reproductive functioning among female offspring and exert intergenerational effects on HPA axis functioning. Dunn and colleagues35 have recently demonstrated that females exposed to betamethasone in utero require a greater number of ovulatory cycles for successful reproduction as compared to their unexposed counterparts. These data raise the possibility that intrauterine exposure to synthetic GCs may have consequences for fertility among female offspring. Intergenerational consequences of early life experiences for the functioning of the HPA axis have been observed.52–54 Potential mechanisms for these effects include altered functioning of endocrine and cardiovascular systems among prenatally exposed females as well as epigenetic changes.30
Human Data
Less is known about the consequences of antenatal GC exposure in humans.48, 49 Assessments of cord blood and amniotic fluid indicate acute suppression of endogenous fetal cortisol production 55–57 and an acute increase in GC bioactivity 56, 58 in response to synthetic GCs. The suppression of endogenous fetal/neonatal cortisol production appears to persist into the immediate postnatal period. Several studies have shown that among preterm infants exposed to antenatal GCs, unstimulated (baseline) cortisol levels are suppressed for several days after treatment during the first postnatal week and then return to normal levels.58–61 The assessment of cortisol levels at a single time point during the day provides limited information regarding the functioning of the HPA axis. To better understand the influence of prenatal GC treatment on HPA axis functioning it is necessary to use serial assessments to evaluate both responses to stressors and the circadian regulation.
Few studies have evaluated the consequences of intrauterine exposure to synthetic GCs on the ability of the HPA axis to respond to a challenge. As reviewed in Table 1, the limited existing data, including studies from our own lab suggests that prenatal GC treatment may have persisting effects on the HPA axis and regulation of stress responses. 62–65 These studies indicate that although suppression of unstimulated /baseline levels of cortisol is only present during the first several days after treatment, effects on HPA axis regulation persist.55, 59 We have observed that prenatal treatment with a single course of betamethasone is associated with a suppression of the cortisol response to the painful stress of a heel-stick blood draw among preterm infants and that this effect persists for at least 4 to 6 weeks after birth.62, 64 Similarly in a study including a combined sample of term and preterm neonates, Schaffer and colleagues documented a suppressed cortisol response to heel-stick.63 These studies suggest that prenatal GC treatment has implications for HPA axis regulation that persist for at least 8 weeks after treatment with some evidence for greater consequences associated with exposure to multiple courses.66
TABLE 1.
Antenatal Glucocorticoid effects on Neonatal Stress Response
| Type of GC ; number of Courses | GAweeks at GC exposure | Post GC Days to birth | Maturity / GestAge at Birth | NeonatalStress Challenge Cortisolresponse | Behavioral response | Comme | |
|---|---|---|---|---|---|---|---|
| Davis. Psychoneurendo. 2004 ref 62 | betamethasone × 1 course | n/a | mean = 8.3 days, range = 1 – 21 days | preterm only 33–34 | Blunted / no cortisol response among GC treated infants as compaired to no treatment comparison group | similar to comparison group | HPA axis function alter |
| Davis. J of Perinatology. 2006 ref 64 | betamethasone × 1 course | n/a | mean = 5.1 days, range = 1–9 days | preterm only 28 –30 | Blunted cortisol response among GC treated infants as compaired to no treatment comparison group | similar to comparison group | HPA axis function alter |
| Ashwood. AJOG. 2006 ref 66 | betametasone × 1 course; compared to multiple courses. No control group | 23 – 31 | unknown | preterm only < 32 and > 32 | Blunted cortisol response in multiple course group compared to single course group Greater blunting of cortisol response at earlier gestational exposure Baseline cortisol lower in multiple course group at day 7 compared to single course ; baseline cortisol levels were similar in the two groups by day 14. |
not studied | Acute differences in HP in infants exposed to m |
| Schaffer. Obstet & Gynecol. 2009 ref 63 | betamethasone × 1 course | 29.4 | mean = 60 days n/a | term and preterm >34 weeks | Blunted cortisol and cortisone response among GC treated infants as compared to no treatment comparison group | not studied | HPA axis function alter |
| Davis. J Child Psychol and Psychiatry. 2011 ref 69 | Maternal Psychosocial stress; Exposed to elevated endogenous maternal cortisol in 2 and 3 trimesters | No exposure to synthetic GC treatment prenatal endogenous maternal GCs evaluated | n/a | term only 37–41 | larger cortisol response and slower recovery associated with elevated maternal endogenous cortisol levels | slower recovery to baseline state | HPA axis function alter |
| Davis. Dvlp Psychobiol. 2011 ref 65 | betamethasone × 1 course | 29.5 | mean = 60.5 days range = 28–105 days | term only 37–41 | Larger cortisol response among the GC treated infants compared to no treatment comparison group; with greater response when GC exposure was at earlier GA | similar to comparison group | HPA axis function alter |
Table1 summarizes studiesevaluating the effectsof antenatalglucocorticoid exposure on the HPA axis response tostress interm and preterm neonates. See text for details
One of the limitations of existing research is the focus on preterm infants who experience a range of acute medical complications and are exposed to multiple sources of stress as part of their care in neonatal intensive care units (NICU). Factors such as preterm birth, acute respiratory illness and exposure to painful medical procedures have been shown to affect the development of the HPA axis67, 68 and thus, may interfere with the ability to accurately identify and isolate the consequences of GC therapy. To address this issue, we evaluated the cortisol response to the painful stress of a heel-stick blood draw among full term infants treated with a single course of the GC betamethasone and a matched comparison group of infants born at term gestation.65 We found that prenatal treatment with the synthetic GC, betamethasone, was associated with a more pronounced cortisol response to the painful stress of the heel-stick procedure among healthy infants born at term gestation, despite no difference in baseline levels. Interestingly, this heightened cortisol response to the heel-stick procedure is similar to the one observed among term infants exposed to elevated levels of endogenous maternal cortisol during the third trimester.69 These data suggest a lasting influence of prenatal exposure to excess GCs that has persisting consequences for the functioning of the newborn HPA axis.
There is limited evidence regarding the long-term consequences of prenatal GC treatment for the functioning of the HPA axis. The two studies that evaluate the consequences of GC exposure for HPA axis functioning later in infancy yield conflicting results. This may be due to the inclusion of heterogeneous samples of preterm infants who were exposed to different types and doses of steroids at varying times during pregnancy and postpartum.70, 71
To our knowledge only one study has evaluated the consequences of antenatal GC treatment for adult HPA axis functioning. At 30 years of age morning cortisol levels were 7% higher among individuals exposed to antenatal steroids as compared to the control group. This increase was not significant after consideration of factors including sex, birth weight, gestational age at birth, trial type, body-mass index, and use of oral contraceptives.72 The fact that a tendency for an effect on the HPA axis was observed in the presence of multiple confounding variables and with a limited evaluation of the HPA axis leaves open the possibility that prenatal GC treatment may exert long-term consequences on the functioning of the HPA axis in humans.48
Based on the experimental animal data and human observations it is evident that the HPA axis is particularly vulnerable to exposure to elevated levels of endogenous and exogenous GCs. Dysregulation of HPA axis functioning during infancy may put children at risk for physiological and behavioral problems throughout the lifespan.73–75 A parallel set of observations in a model of prenatal stress having programming effects and adverse outcomes on the fetal HPA axis and the Hypothalamus-Adrenal-Gonadal Axis is with the Fetal Alcohol Spectrum Disorder, which further confirms that the fetal HPA axis is susceptible to changes secondary to prenatal stress with long-term consequences.76 The focus of this review is on the programming consequences of antenatal GC treatment. It is, however, noteworthy that other common antenatal therapies such as administration of beta 2 adrenergic agonists additionally exert developmental influences and may interact with the effects of GC treatment.77, 78
Clinical Considerations
The initial choice and dose of synthetic GCs for accelerating fetal lung maturation was not subject to formal evaluation and FDA approval for prenatal use. However based on multiple clinical trials and wide clinical experience, the current standard of practice is to administer either a single course of betamethasone or dexamethasone (i.e. two doses of betamethasone 12 mg im at 24 hour intervals or four doses of 6 mg of dexamethasone im at 12 hour intervals) to women in preterm labor between 24 – 34 weeks gestation.7 There is also evidence that incomplete or partial treatment with these formulations does provide some benefit in terms of decreasing morbidity.79, 80 While the effects of antenatal corticosteroids on lung maturation appear to be dose dependent, the biological effects and the optimal dose of antenatal GCs is unknown and under investigation especially with respect to the long-term effects on other organ systems, particularly the CNS and neuro-developmental outcomes.
Detailed studies in sheep suggest that comparable acceleration of fetal lung maturation (biochemical and functional) could be achieved with a single dose of maternal betamethasone acetate as compared to the conventional clinical 2-dose regimen.50 As suggested by Jobe et al, the hypothesis that low fetal exposure to betamethasone following maternal Beta-Acetate treatment might decrease risks of adverse neurodevelopmental and growth effects remains to be tested.50
The practice of repeated courses of antenatal corticosteroids has been widely used for over twenty-five years with the intent of maximizing maturation of the lungs and decreasing the incidence of acute lung disease in premature infants. Follow up studies have shown a decrease in the incidence and severity of lung disease along with decreases in fetal growth and head circumference. More recently, the NIH in its second consensus conference in 20008 and the American College of Obstetricians and Gynecologists,81 concluded that the repeat course of antenatal corticosteroids, including rescue therapy should be reserved for women enrolled in clinical trials. The majority of the meta-analyses of clinical trials comparing the standard single course regimen of antenatal GCs to repeated doses up to 34 weeks gestation also suggest caution in recommending the repeated course regimens.82–84
The latest updated systematic review by Crowther6 comparing single dose to repeated doses of synthetic GC betamethasone from 10 randomized clinical trials between 2001 and 2010 in 4730 mothers and their 5700 infants found a significant reduction in the risk of respiratory distress syndrome and serious neonatal morbidity. Long term, two to three year follow up of infant outcomes among 4170 of these infants showed no difference in major neurosensory disabilities, as well as in secondary outcomes in cerebral palsy, blindness and deafness. The authors concluded that repeated dose regimen in these trials “showed no evidence of harm or benefit” as compared to the standard single course of antenatal GC, and recommended consideration of repeated doses of antenatal GC as was used in these clinical trial protocols for women at risk of preterm delivery. Of note however, is that in one of the studies included in this review, NICHHD MFM Network study group85 reported higher number of infants with cerebral palsy in a subgroup of infants exposed to four or more courses of corticosteroids compared to either the single course or the placebo group. Though the difference in their two groups was not statistically significant, appropriate concern and caution was raised regarding the routine repeated courses of GCs. These clinical observations (along with those in animal literature) of repeated exposure of the fetus to synthetic GCs raises the possibility that the long-term effects on brain development and outcomes are not yet fully appreciated. The randomized studies to date have reported primarily on major neuro-sensory disabilities and overall neurodevelopmental outcomes. There is a need for detailed evaluations of the more subtle CNS effects of GC exposure such as those mediated through effects on the HPA axis, the Pituitary Gonadal Axis and other specialized pathways in the brain. The need to evaluate these subtle changes is substantiated by a recent review by Sandman and colleagues documenting the lasting influence on prenatal exposure to naturally occurring stress hormones for HPA axis functioning, cognitive and emotional regulation and brain development.86
An alternate approach being studied to reduce and minimize the potential risks of multiple courses of antenatal corticosteroids is to limit repeated exposures, with the administration of a single retreatment (rescue) course following the standard initial course, when preterm delivery seemed imminent before 33 weeks gestation.87 This rescue dosing strategy appears to have the same benefits as multiple courses of steroids in terms of overall postnatal morbidity, decrease in RDS, and postnatal surfactant use.
Current thinking on the choices of synthetic GC, use of repeated or rescue dosing and the use of antenatal corticosteroids for decreasing respiratory morbidity in the late preterm infant has recently been critically evaluated and summarized.88 The broadest clinical experience for single and repeat courses of antenatal corticosteroids is based largely on the use of currently formulated combined dose of betamethasone phosphate and betamethasone acetate. Although many of the observational cohort studies seem to indicate that treatment with betamethasone has better outcomes and less adverse effects than dexamethasone,89–91 the data are not conclusive.88, 92
A few clinical strategies currently being investigated have emerged which, when used in conjunction, show promise for the improved diagnosis and management of preterm labor. These include the use of biological markers such as fetal fibronectin93, 94 and measurement of cervical length by transvaginal ultrasonography95 for identifying women who are at risk for preterm labor and preterm delivery. Also of note is the recent FDA approval in 2011 of progesterone supplementation for the prevention of preterm birth. The evidence for the effectiveness of this treatment has been extensively reviewed by Norwitz and Caughey.96 A recent report97 also suggests a promising clinical strategy of using transvaginal ultrasound for identifying mothers at risk for preterm labor and the use of progesterone therapy to prevent preterm delivery. These emerging clinical strategies, if confirmed, are likely to assist the clinician in being more selective in the choice of patients who are candidates for one or more courses of antenatal corticosteroids.
Unanswered Questions and Future Directions
Because the early clinical diagnosis of preterm labor is difficult and subjective and the laboratory indicators of early preterm labor imprecise, there are a number of mothers who receive antenatal GCs for preterm labor who deliver beyond 37 weeks gestation. The potential risks of antenatal GC exposure to these infants born at term needs to be evaluated in the context of clear and substantial benefit to preterm infants. The risk or benefit of GC exposure to this group of infants is unknown and longitudinal follow up studies for this sub-group of infants are clearly needed.
Other clinical studies that would help us better evaluate the potential risks and consequences of antenatal GC exposure are ones that would adequately evaluate sexually dimorphic responses to antenatal GC.40, 52 Further, new evidence indicates that the long term programming effect on the HPA axis in females must include evaluation of the stage of the reproductive cycle.35 Separately, animal data34, 51 and observational human studies62, 65 indicate that the phenotype associated with exposure to antenatal GC treatment varies based on the developmental stage at the time of assessment of the HPA axis. These findings bring up the need for prospective longitudinal studies to evaluate HPA axis function at multiple time points. Further, comprehensive assessment of HPA axis function including responses to stress and circadian regulation in the human are needed to fully characterize the consequences of prenatal GCs on this system.
Summary and Conclusions
Based on the available animal data and ongoing clinical studies, exposure to single and repeated courses of synthetic GCs causes dysregulation of the fetal and neonatal HPA axis. Animal studies indicate that these HPA axis changes persist into adulthood and represent a “programming effect” that is trans-generational and sex specific. This altered (dysregulated) state of the HPA activity appears to be a function of timing of the exposure (gestational age) and is dose dependent.
Though the short term outcomes and benefits of exposure to single and repeated doses of synthetic GCs in humans have been largely re-assuring there is reason for caution in extrapolating these findings for the long term in humans based on the reported adverse long term and permanent changes on the HPA axis in multiple animal models and in select short term clinical outcomes. The long-term clinical outcomes for the human with regard to subtler neurodevelopmental outcomes, the response to stress, learning, cognition behavior and risk for adult onset diseases are presently unknown. More precise diagnosis and management of preterm labor and the careful selection of candidates for repeat courses of antenatal corticosteroids would potentially decrease the risk of long-term adverse outcomes in this group of infants. Careful and systematic longitudinal follow up of those infants exposed to single and repeated doses of antenatal GCs in present and future studies are also of vital importance in determining the risks and benefits of this important clinical intervention.
Acknowledgments
Supported in part by NIH awards HD50662 and HD65823to EPD.
Footnotes
DISCLOSURE: The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Liggins GC, Howie RN. A controlled trial of antepartum glucocorticoid treatment for prevention of the respiratory distress syndrome in premature infants. Pediatrics. 1972;50:515–25. [PubMed] [Google Scholar]
- 2.Crowley P, Chalmers I, Keirse MJ. The effects of corticosteroid administration before preterm delivery: an overview of the evidence from controlled trials. Brit J Obstet Gynaec. 1990;97:11–25. doi: 10.1111/j.1471-0528.1990.tb01711.x. [DOI] [PubMed] [Google Scholar]
- 3.Roberts D, Dalziel S. Antenatal corticosteroids for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2006;3:CD004454. doi: 10.1002/14651858.CD004454.pub2. [DOI] [PubMed] [Google Scholar]
- 4.Brownfoot FC, Crowther CA, Middleton P. Different corticosteroids and regimens for accelerating fetal lung maturation for women at risk of preterm birth. Cochrane Database Syst Rev. 2008:CD006764. doi: 10.1002/14651858.CD006764.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crowther CA, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for preventing neonatal respiratory disease. Cochrane Database Syst Rev. 2007:CD003935. doi: 10.1002/14651858.CD003935.pub2. [DOI] [PubMed] [Google Scholar]
- 6.Crowther CA, Mckinlay CJ, Middleton P, Harding JE. Repeat doses of prenatal corticosteroids for women at risk of preterm birth for improving neonatal health outcomes. Cochrane Database Syst Rev. 2011:CD003935. doi: 10.1002/14651858.CD003935.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consensus Development Panel on the Effect of Corticosteroids for Fetal Maturation on Perinatal Outcomes. Jama-J Am Med Assoc. 1995;273:413–8. doi: 10.1001/jama.1995.03520290065031. [DOI] [PubMed] [Google Scholar]
- 8.Antenatal corticosteroids revisited: repeat courses - National Institutes of Health Consensus Development Conference Statement, August 17–18, 2000. Obstet Gynecol. 2001;98:144–50. doi: 10.1016/s0029-7844(01)01410-7. [DOI] [PubMed] [Google Scholar]
- 9.Levine S. Infantile experience and resistance to physiological stress. Science. 1957;126:405–06. doi: 10.1126/science.126.3270.405. [DOI] [PubMed] [Google Scholar]
- 10.Chrousos GP, Kino T. Glucocorticoid signaling in the cell. Expanding clinical implications to complex human behavioral and somatic disorders. Ann Ny Acad Sci. 2009;1179:153–66. doi: 10.1111/j.1749-6632.2009.04988.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chrousos GP, Gold PW. The concepts of stress and stress system disorders. Overview of physical and behavioral homeostasis. Jama-J Am Med Assoc. 1992;267:1244–52. [PubMed] [Google Scholar]
- 12.Harris A, Seckl J. Glucocorticoids, prenatal stress and the programming of disease. Horm Behav. 2011;59:279–89. doi: 10.1016/j.yhbeh.2010.06.007. [DOI] [PubMed] [Google Scholar]
- 13.Rosen JB, Schulkin J. From normal fear to pathological anxiety. Psychol Rev. 1998;105:325–50. doi: 10.1037/0033-295x.105.2.325. [DOI] [PubMed] [Google Scholar]
- 14.Kvetnansky R, Pacak K, Fukuhara K, et al. Sympathoadrenal system in stress. Interaction with the hypothalamic-pituitary-adrenocortical system. Ann Ny Acad Sci. 1995;771:131–58. doi: 10.1111/j.1749-6632.1995.tb44676.x. [DOI] [PubMed] [Google Scholar]
- 15.Smith R, Smith JI, Shen X, et al. Patterns of plasma corticotropin-releasing hormone, progesterone, estradiol, and estriol change and the onset of human labor. J Clin Endocr Metab. 2009;94:2066–74. doi: 10.1210/jc.2008-2257. [DOI] [PubMed] [Google Scholar]
- 16.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol. 1999;180:257–63. doi: 10.1016/s0002-9378(99)70712-x. [DOI] [PubMed] [Google Scholar]
- 17.Sandman CA, Glynn L, Schetter CD, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides. 2006;27:1457–63. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 18.Sandman CA, Davis EP. Gestational stress influences cognition and behavior. Future Neurol. 2010;5:675–90. [Google Scholar]
- 19.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–48. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–86. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Brown RW, Diaz R, Robson AC, et al. The ontogeny of 11β-hydroxysteroid dehydrogenase type 2 and mineralicorticoid receptor gene expression reveal intricate control of glucocorticoid action in development. Endocrinology. 1996;137:794–97. doi: 10.1210/endo.137.2.8593833. [DOI] [PubMed] [Google Scholar]
- 22.Murphy VE, Clifton VL. Alterations in human placental 11beta-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–44. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 23.Liggins GC. The Foetal Role in the Initiation of Parturition in the Ewe. In: Wolstenholme GEW, O'Conner M, editors. Ciba Foundation Symposium - Foetal Autonomy. Churchill, London: John Wiley & Sons, Ltd; 1969. [Google Scholar]
- 24.Matthews SG. Antenatal glucocorticoids and programming of the developing CNS. Pediatr Res. 2000;47:291–300. doi: 10.1203/00006450-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 25.Kapoor A, Dunn E, Kostaki A, Andrews MH, Matthews SG. Fetal programming of hypothalamo-pituitary-adrenal function: prenatal stress and glucocorticoids. Am J Physiol. 2006;572:31–44. doi: 10.1113/jphysiol.2006.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ishimoto H, Jaffe RB. Development and function of the human fetal adrenal cortex: a key component in the feto-placental unit. Endocr Rev. 2011;32:317–55. doi: 10.1210/er.2010-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jaffe RB, Mesiano S, Smith R, Coulter CL, Spencer SJ, Chakravorty A. The regulation and role of fetal adrenal development in human pregnancy. Endocr Res. 1998;24:919–26. doi: 10.3109/07435809809032707. [DOI] [PubMed] [Google Scholar]
- 28.Kempna P, Fluck CE. Adrenal gland development and defects. Best Pract Res Clin Endocrinol Metab. 2008;22:77–93. doi: 10.1016/j.beem.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 29.Gitau R, Fisk NM, Teixeira JM, Cameron A, Glover V. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–9. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 30.Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic–pituitary–adrenal (HPA) axis function and behavior by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–95. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 31.Ulfig N, Setzer M, Bohl J. Ontogeny of the human amygdala. Ann Ny Acad Sci. 2003;985:22–33. doi: 10.1111/j.1749-6632.2003.tb07068.x. [DOI] [PubMed] [Google Scholar]
- 32.Noorlander CW, De Graan PN, Middeldorp J, Van Beers JJ, Visser GH. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499:924–32. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- 33.Uno H, Eisele S, Sakai A, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–48. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 34.Sloboda DM, Moss TJ, Gurrin LC, Newnham JP, Challis JR. The effect of prenatal betamethasone administration on postnatal ovine hypothalamic-pituitary-adrenal function. J Endocrinol. 2002;172:71–81. doi: 10.1677/joe.0.1720071. [DOI] [PubMed] [Google Scholar]
- 35.Dunn E, Kapoor A, Leen J, Matthews SG. Prenatal synthetic glucocorticoid exposure alters hypothalamic-pituitary-adrenal regulation and pregnancy outcomes in mature female guinea pigs. J Physiol. 2010;588:887–99. doi: 10.1113/jphysiol.2009.182139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.De Vries A, Holmes MC, Heijnis A, et al. Prenatal dexamethasone exposure induces changes in nonhuman primate offspring cardiometabolic and hypothalamic-pituitary-adrenal axis function. J Clin Invest. 2007;117:1058–67. doi: 10.1172/JCI30982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–18. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- 38.Hauser J, Dettling-Artho A, Pilloud S, Maier C, Feldon J, Pryce CR. Effects of prenatal dexamethasone treatment on postnatal physical, endocrine, and social development in the common marmoset monkey. Endocrinology. 2007;148:1813–22. doi: 10.1210/en.2006-1306. [DOI] [PubMed] [Google Scholar]
- 39.Segar JL, Lumbers ER, Nuyt AM, Smith OJ, Robillard JE. Effect of antenatal glucocorticoids on sympathetic nerve activity at birth in preterm sheep. Am J Physiol. 1998;274:R160–7. doi: 10.1152/ajpregu.1998.274.1.R160. [DOI] [PubMed] [Google Scholar]
- 40.Liu L, Li A, Matthews SG. Maternal glucocorticoid treatment programs HPA regulation in adult offspring: sex-specific effects. Am J Physiol Endocrinol Metab. 2001;280:E729–39. doi: 10.1152/ajpendo.2001.280.5.E729. [DOI] [PubMed] [Google Scholar]
- 41.Banjanin S, Kapoor A, Matthews SG. Prenatal glucocorticoid exposure alters hypothalamic-pituitary-adrenal function and blood pressure in mature male guinea pigs. J Physiol. 2004;558:305–18. doi: 10.1113/jphysiol.2004.063669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Diaz Heijtz R, Fuchs E, Feldon J, Pryce CR, Forssberg H. Effects of antenatal dexamethasone treatment on glucocorticoid receptor and calcyon gene expression in the prefrontal cortex of neonatal and adult common marmoset monkeys. Behav Brain Funct. 2010;6:18. doi: 10.1186/1744-9081-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Coe CL, Lubach GR. Developmental consequences of antenatal dexamethasone treatment in nonhuman primates. Neurosci Biobehav R. 2005;29:227–35. doi: 10.1016/j.neubiorev.2004.10.003. [DOI] [PubMed] [Google Scholar]
- 44.Jobe AH, Soll RF. Choice and dose of corticosteroid for antenatal treatments. Am J Obstet Gynecol. 2004;190:878–81. doi: 10.1016/j.ajog.2004.01.044. [DOI] [PubMed] [Google Scholar]
- 45.Braun T, Li S, Sloboda DM, et al. Effects of maternal dexamethasone treatment in early pregnancy on pituitary-adrenal axis in fetal sheep. Endocrinology. 2009;150:5466–77. doi: 10.1210/en.2009-0086. [DOI] [PubMed] [Google Scholar]
- 46.Antonow-Schlorke I, Helgert A, Gey C, et al. Adverse effects of antenatal glucocorticoids on cerebral myelination in sheep. Obstet Gynecol. 2009;113:142–51. doi: 10.1097/AOG.0b013e3181924d3b. [DOI] [PubMed] [Google Scholar]
- 47.Huang WL, Beazley LD, Quinlivan JA, Evans SF, Newnham JP, Dunlop SA. Effect of corticosteroids on brain growth in fetal sheep. Obstet Gynecol. 1999;94:213–18. doi: 10.1016/s0029-7844(99)00265-3. [DOI] [PubMed] [Google Scholar]
- 48.Tegethoff M, Pryce C, Meinlschmidt G. Effects of intrauterine exposure to synthetic glucocorticoids on fetal, newborn, and infant hypothalamic-pituitary-adrenal axis function in humans: a systematic review. Endocr Rev. 2009;30:753–89. doi: 10.1210/er.2008-0014. [DOI] [PubMed] [Google Scholar]
- 49.Sandman CA, Davis EP. Health risk is associated with gestational exposure to stress hormones. Expert Review of Endocrinology and Metabolism. doi: 10.1586/eem.12.33. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jobe AH, Nitsos I, Pillow JJ, Polglase GR, Kallapur SG, Newnham JP. Betamethasone dose and formulation for induced lung maturation in fetal sheep. Am J Obstet Gynecol. 2009;201:611, e1–7. doi: 10.1016/j.ajog.2009.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sloboda DM, Moss TJ, Li S, et al. Prenatal betamethasone exposure results in pituitary-adrenal hyporesponsiveness in adult sheep. Am J Physiol Endocrinol Metab. 2007;292:E61–70. doi: 10.1152/ajpendo.00270.2006. [DOI] [PubMed] [Google Scholar]
- 52.Bale TL. Sex differences in prenatal epigenetic programming of stress pathways. Stress. 2011;14:348–56. doi: 10.3109/10253890.2011.586447. [DOI] [PubMed] [Google Scholar]
- 53.Bertram C, Khan O, Ohri S, Phillips DI, Matthews SG, Hanson MA. Transgenerational effects of prenatal nutrient restriction on cardiovascular and hypothalamic-pituitary-adrenal function. J Physiol. 2008;586:2217–29. doi: 10.1113/jphysiol.2007.147967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Drake AJ, Tang JI, Nyirenda MJ. Mechanisms underlying the role of glucocorticoids in the early life programming of adult disease. Clin Sci. 2007;113:219–32. doi: 10.1042/CS20070107. [DOI] [PubMed] [Google Scholar]
- 55.Ballard PL, Granberg P, Ballard RA. Glucocorticoid levels in maternal and cord serum after prenatal betamethasone therapy to prevent respiratory distress syndrome. J Clin Invest. 1975;56:1548–54. doi: 10.1172/JCI108236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kajantie E, Raivio T, Janne OA, Hovi P, Dunkel L, Andersson S. Circulating glucocorticoid bioactivity in the preterm newborn after antenatal betamethasone treatment. J Clin Endocr Metab. 2004;89:3999–4003. doi: 10.1210/jc.2004-0013. [DOI] [PubMed] [Google Scholar]
- 57.Marinoni E, Korebrits C, Di Lorio R, Cosmi EV, Challis JR. Effect of betamethasone in vivo on placental corticotropin-releasing hormone in human pregnancy. Am J Obstet Gynecol. 1998;178:770–78. doi: 10.1016/s0002-9378(98)70490-9. [DOI] [PubMed] [Google Scholar]
- 58.Nykanen P, Raivio T, Heinonen K, Janne OA, Voutilainen R. Circulating glucocorticoid bioactivity and serum cortisol concentration in premature infants: The influence of exogenous glucocorticoids and clinical factors. Eur J Endocrinol. 2007;156:577–83. doi: 10.1530/EJE-06-0672. [DOI] [PubMed] [Google Scholar]
- 59.Ballard PL, Gluckman PD, Liggens GC, Kaplan SL, Grumbach MM. Steroid and growth hormone levels in premature infants after prenatal betamethasone therapy to prevent respiratory distress syndrome. Pediatr Res. 1980;14:122–27. doi: 10.1203/00006450-198002000-00011. [DOI] [PubMed] [Google Scholar]
- 60.Dorr HG, Versmold HT, Sippell WG, Bidlingmaier F, Knorr D. Antenatal betamethasone therapy: Effects on maternal, fetal, and neonatal mineralocorticoids, glucocorticoids, and progestins. J Pediatr. 1986;108:990–93. doi: 10.1016/s0022-3476(86)80946-5. [DOI] [PubMed] [Google Scholar]
- 61.Parker CR, Atkinson MW, Owen J, Andrews WW. Dynamics of the fetal adrenal, cholesterol, and apolipoprotein B responses to antenatal betamethasone therapy. Am J Obstet Gynecol. 1996;174:562–65. doi: 10.1016/s0002-9378(96)70428-3. [DOI] [PubMed] [Google Scholar]
- 62.Davis EP, Townsend EL, Gunnar MR, et al. Effects of prenatal betamethasone exposure on regulation of stress physiology in healthy premature infants. Psychoneuroendocrino. 2004;29:1028–36. doi: 10.1016/j.psyneuen.2003.10.005. [DOI] [PubMed] [Google Scholar]
- 63.Schaffer L, Luzi F, Burkhardt T, Rauh M, Beinder E. Antenatal betamethasone administration alters stress physiology in healthy neonates. Obstet Gynecol. 2009;113:1082–8. doi: 10.1097/AOG.0b013e3181a1f0e6. [DOI] [PubMed] [Google Scholar]
- 64.Davis EP, Townsend EL, Gunnar MR, et al. Antenatal betamethasone treatment has a persisting influence on infant HPA axis regulation. J Perinatol. 2006;26:147–53. doi: 10.1038/sj.jp.7211447. [DOI] [PubMed] [Google Scholar]
- 65.Davis EP, Waffarn F, Sandman CA. Prenatal treatment with glucocorticoids sensitizes the hpa axis response to stress among full-term infants. Dev Psychobiol. 2011;53:175–83. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ashwood PJ, Crowther CA, Willson KJ, et al. Neonatal adrenal function after repeat dose prenatal corticosteroids: a randomized controlled trial. Am J Obstet Gynecol. 2006;194:861–7. doi: 10.1016/j.ajog.2005.08.063. [DOI] [PubMed] [Google Scholar]
- 67.Grunau RE, Holsti L, Haley DW, et al. Neonatal procedural pain exposure predicts lower cortisol and behavioral reactivity in preterm infants in the NICU. Pain. 2005;113:293–300. doi: 10.1016/j.pain.2004.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Grunau RE, Whitfield MF, Petrie-Thomas J, et al. Neonatal pain, parenting stress and interaction, in relation to cognitive and motor development at 8 and 18 months in preterm infants. Pain. 2009;143:138–46. doi: 10.1016/j.pain.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Davis EP, Glynn LM, Waffarn F, Sandman CA. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–29. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Glover V, Miles R, Matta S, Modi N, Stevenson J. Glucocorticoid exposure in preterm babies predicts saliva cortisol response to immunization at 4 months. Pediatr Res. 2005;58:1233–7. doi: 10.1203/01.pdr.0000185132.38209.73. [DOI] [PubMed] [Google Scholar]
- 71.Miller NM, Williamson C, Fisk NM, Glover V. Infant cortisol response after prolonged antenatal prednisolone treatment. Brit J Obstet Gynaec. 2004;111:1471–4. doi: 10.1111/j.1471-0528.2004.00288.x. [DOI] [PubMed] [Google Scholar]
- 72.Dalziel SR, Walker NK, Parag V, et al. Cardiovascular risk factors after antenatal exposure to betamethasone: 30-year follow-up of a randomised controlled trial. Lancet. 2005;365:1856–62. doi: 10.1016/S0140-6736(05)66617-2. [DOI] [PubMed] [Google Scholar]
- 73.Buss K, Davidson RJ, Kalin NH, Goldsmith HH. Context specific freezing. Dev Psychol. 2004;40:583–94. doi: 10.1037/0012-1649.40.4.583. [DOI] [PubMed] [Google Scholar]
- 74.Gunnar MR, Davis EP. The developmental psychobiology of stress and emotion in early childhood. In: Lerner RM, Easterbrooks MA, Mistry J, editors. Developmental Psychology. Vol. 6 New York: Wiley; 2003. [Google Scholar]
- 75.Phillips DI. Programming of the stress response: a fundamental mechanism underlying the long-term effects of the fetal environment? J Intern Med. 2007;261:453–60. doi: 10.1111/j.1365-2796.2007.01801.x. [DOI] [PubMed] [Google Scholar]
- 76.Weinberg J, Sliwowska JH, Lan N, Hellemans KG. Prenatal alcohol exposure: foetal programming, the hypothalamic-pituitary-adrenal axis and sex differences in outcome. J Neuroendocrinol. 2008;20:470–88. doi: 10.1111/j.1365-2826.2008.01669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Witter FR, Zimmerman AW, Reichmann JP, Connors SL. In utero beta 2 adrenergic agonist exposure and adverse neurophysiologic and behavioral outcomes. Am J Obstet Gynecol. 2009;201:553–9. doi: 10.1016/j.ajog.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 78.Zerrate MC, Pletnikov M, Connors SL, et al. Neuroinflammation and behavioral abnormalities after neonatal terbutaline treatment in rats: implications for autism. J Pharmacol Exp Ther. 2007;322:16–22. doi: 10.1124/jpet.107.121483. [DOI] [PubMed] [Google Scholar]
- 79.Elimian A, Figueroa R, Spitzer AR, Ogburn PL, Wiencek V, Quirk JG. Antenatal corticosteroids: are incomplete courses beneficial? Obstet Gynecol. 2003;102:352–5. doi: 10.1016/s0029-7844(03)00485-x. [DOI] [PubMed] [Google Scholar]
- 80.Costa S, Zecca E, De Luca D, De Carolis MP, Romagnoli C. Efficacy of a single dose of antenatal corticosteroids on morbidity and mortality of preterm infants. Eur J Obstet Gynecol Reprod Biol. 2007;131:154–7. doi: 10.1016/j.ejogrb.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 81.ACOG Committee Opinion No. 402: Antenatal corticosteroid therapy for fetal maturation. Obstet Gynecol. 2008;111:805–7. doi: 10.1097/AOG.0b013e318169f722. [DOI] [PubMed] [Google Scholar]
- 82.Newnham JP, Jobe AH. Should we be prescribing repeated courses of antenatal corticosteroids? Semin Fetal Neonatal Med. 2009;14:157–63. doi: 10.1016/j.siny.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 83.Bevilacqua E, Brunelli R, Anceschi MM. Review and meta-analysis: Benefits and risks of multiple courses of antenatal corticosteroids. J Matern Fetal Neonatal Med. 2010;23:244–60. doi: 10.1080/14767050903165222. [DOI] [PubMed] [Google Scholar]
- 84.Peltoniemi OM, Kari MA, Hallman M. Repeated antenatal corticosteroid treatment: a systematic review and meta-analysis. Acta obstetricia et gynecologica Scandinavica. 2011;90:719–27. doi: 10.1111/j.1600-0412.2011.01132.x. [DOI] [PubMed] [Google Scholar]
- 85.Wapner RJ, Sorokin Y, Mele L, et al. Long-term outcomes after repeat doses of antenatal corticosteroids. New Engl J Med. 2007;357:1190–98. doi: 10.1056/NEJMoa071453. [DOI] [PubMed] [Google Scholar]
- 86.Sandman CA, Davis EP, Buss C, Glynn LM. Prenatal programming of human neurological function. Int J Pept. 2011;2011:837596. doi: 10.1155/2011/837596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garite TJ, Kurtzman J, Maurel K, Clark R. Impact of a 'rescue course' of antenatal corticosteroids: a multicenter randomized placebo-controlled trial. Am J Obstet Gynecol. 2009;200:248, e1–9. doi: 10.1016/j.ajog.2009.01.021. [DOI] [PubMed] [Google Scholar]
- 88.Wapner R, Jobe AH. Controversy: antenatal steroids. Clin Perinatol. 2011;38:529–45. doi: 10.1016/j.clp.2011.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lee BH, Stoll BJ, Mcdonald SA, Higgins RD. Adverse neonatal outcomes associated with antenatal dexamethasone versus antenatal betamethasone. Pediatrics. 2006;117:1503–10. doi: 10.1542/peds.2005-1749. [DOI] [PubMed] [Google Scholar]
- 90.Lee BH, Stoll BJ, Mcdonald SA, Higgins RD. Neurodevelopmental outcomes of extremely low birth weight infants exposed prenatally to dexamethasone versus betamethasone. Pediatrics. 2008;121:289–96. doi: 10.1542/peds.2007-1103. [DOI] [PubMed] [Google Scholar]
- 91.Feldman DM, Carbone J, Belden L, Borgida AF, Herson V. Betamethasone vs dexamethasone for the prevention of morbidity in very-low-birthweight neonates. Am J Obstet Gynecol. 2007;197:284, e1–4. doi: 10.1016/j.ajog.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 92.Elimian A, Garry D, Figueroa R, Spitzer A, Wiencek V, Quirk JG. Antenatal Betamethasone compared with dexamethasone (betacode trial) Obstet Gynecol. 2007;110:26–30. doi: 10.1097/01.AOG.0000268281.36788.81. [DOI] [PubMed] [Google Scholar]
- 93.Honest H, Bachmann LM, Gupta JK, Kleijnen J, Khan KS. Accuracy of cervicovaginal fetal fibronectin test in predicting risk of spontaneous preterm birth: systematic review. BMJ. 2002;325:301. doi: 10.1136/bmj.325.7359.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Leitich H, Kaider A. Fetal fibronectin--how useful is it in the prediction of preterm birth? Brit J Obstet Gynaec. 2003;110 (Suppl 20):66–70. [PubMed] [Google Scholar]
- 95.Iams JD, Goldenberg RL, Meis PJ, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. The New Engl J Med. 1996;334:567–72. doi: 10.1056/NEJM199602293340904. [DOI] [PubMed] [Google Scholar]
- 96.Norwitz ER, Caughey AB. Progesterone supplementation and the prevention of preterm birth. Rev Obstet Gynecol. 2011;4:60–72. [PMC free article] [PubMed] [Google Scholar]
- 97.Hassan SS, Romero R, Vidyadhari D, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double-blind, placebo-controlled trial. Ultrasound Obst Gyn. 2011;38:18–31. doi: 10.1002/uog.9017. [DOI] [PMC free article] [PubMed] [Google Scholar]