Abstract
Purpose
The Age-Related Eye Disease Study (AREDS) demonstrated beneficial effects of oral supplementation with antioxidant vitamins and minerals on the development of advanced age-related macular degeneration (AMD) in persons with at least intermediate AMD (bilateral large drusen with or without pigment changes). Observational data suggest that other oral nutrient supplements might further reduce the risk of progression to advanced AMD.
The primary purpose of Age-Related Eye Disease Study 2 (AREDS2) is to evaluate the efficacy and safety of lutein+zeaxanthin (L+Z) and/or omega-3 long-chain polyunsaturated fatty acid (LCPUFA) supplementation in reducing the risk of developing advanced AMD. The study will also assess the reduction in zinc and the omission of beta-carotene from original AREDS formulation.
Design
Multicenter phase 3 randomized controlled clinical trial
Participants
Persons age 50 to 85 with bilateral intermediate AMD or advanced AMD in one eye
Methods
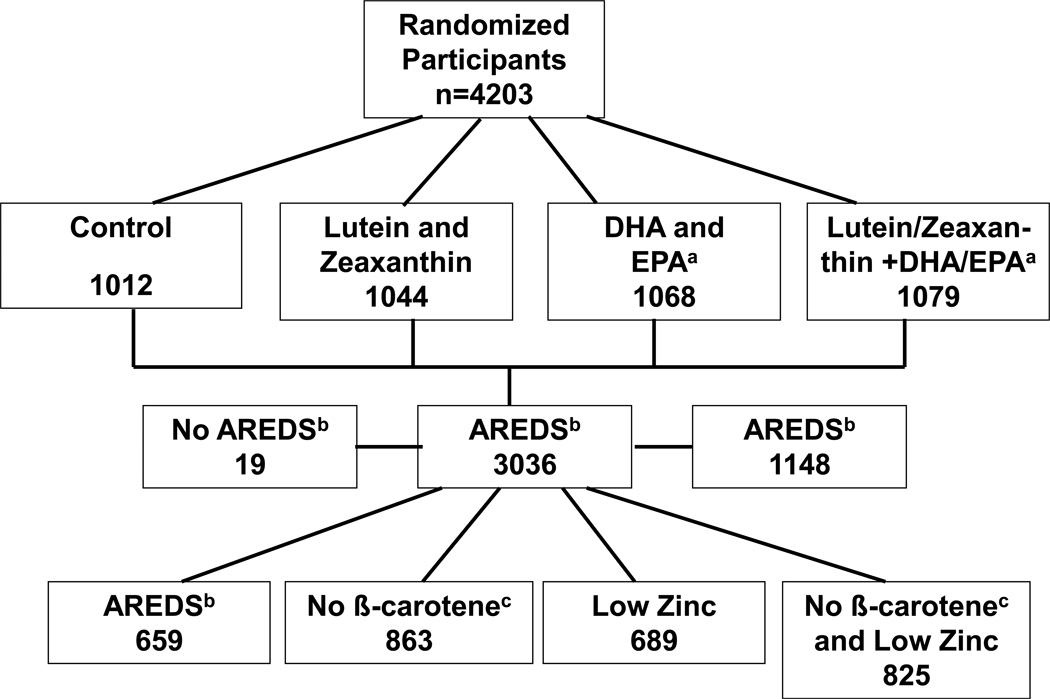
All participants were randomly assigned to: 1) placebo (n=1012); 2) L+Z (10 mg/2 mg, n=1044); 3) ω-3 LCPUFAs (eicosapentaenoic acid (EPA) + docosahexaenoic acid (DHA) [650gmg/350 mg] n=1069); or 4) the combination of L+Z and ω -3 LCPUFAs (n=1078). All participants were offered a secondary randomization to 1 of 4 variations of the original AREDS formulation keeping vitamins C (500 mg), E (400 IU), and copper (2 mg) unchanged while varying zinc and beta-carotene as follows: zinc remains at the original level (80mg), lower only zinc to 25mg, omit beta-carotene only, or lower zinc to 25 mg and omit beta-carotene.
Main Outcome Measures
Progression to advanced AMD determined by centralized grading of annual fundus photographs.
Results
4,203 participants were enrolled at 82 clinical centers located in the U.S. Population characteristics at baseline were as follows: mean age 74 years, 57% female, 97% white, 7% current smokers, 19% with prior cardiovascular disease and 44% and 50% taking statin-class cholesterol lowering drugs and aspirin, respectively. Ocular characteristics include 59% with bilateral large drusen, 32% with advanced AMD in one eye and mean visual acuity of 20/32 in eyes without advanced AMD.
Conclusion
This report presents the AREDS2 study design and the participants’ baseline demographic and ocular characteristics.
Keywords: Age-related macular degeneration, cataract, vision loss, clinical trial, lutein, zeaxanthin, xanthophylls, omega-3 polyunsaturated fatty acids, DHA, EPA, beta-carotene, zinc, cognitive function, cardiovascular disease
INTRODUCTION
Age-related macular degeneration (AMD) and cataract contribute a substantial burden to society as major causes of visual impairment. The Eye Disease Prevalence Research Group estimates that, of the approximately 937,000 legally blind people residing in the United States (US) in 2000, approximately 841,000 (90%) are aged 60 years or older.1 Demographic shifts in longevity will continue to increase the prevalence of age-related eye diseases in the US and other economically developed nations. A 50% increase in the number of US residents aged 65 years and older is projected to occur in the next 20 years, by the year 2020.1
Effective preventive therapies could reduce the enormous burden of age-related eye diseases. AMD and cataract are the major causes of visual disability in the United States, and AMD accounts for more than 50% of all blindness. Approximately 1.2 million persons in 2000 in the U.S. are estimated to have neovascular AMD and 970,000 to have geographic atrophy, while over 8 million have at least large drusen in one eye and 3.6 million of these have bilateral large drusen.2 Without methods to slow AMD progression over the next 20 years, these prevalence figures are expected to increase by approximately 50%. Currently, cataract surgery is one of the most common surgeries conducted in the US.3 The prevalence of age-related cataract is rising, with a prediction that 30 million Americans will be affected by 2020, increasing the public health and economic burden of this disease.4
The Age-Related Eye Disease Study (AREDS), an NEI-sponsored, multi-center, controlled, randomized clinical trial, demonstrated that the combination of oral supplements consisting of antioxidant vitamins C (500 mg), E (400 international units), and β-carotene (15 mg) and minerals, zinc (80 mg of zinc oxide) with copper (2mg cupric oxide), reduced the 5-year risk of developing advanced AMD in eyes with intermediate AMD by 25% (estimated probability of progression was 28% for placebo versus 20% for antioxidants plus zinc).5 Therapy with the AREDS formulation reduced the risk of moderate vision loss (≥15 letters on the logarithmic visual acuity chart) by 19% at 5 years (estimated probability of progression was 29% for placebo versus 23% for antioxidants plus zinc). The AREDS formulation had neither a harmful nor beneficial effect on the development or progression of cataract.6
Observational data from AREDS, other epidemiologic studies and animal studies, provide a rationale for examining the potential impact of other nutrients on the treatment of AMD. Results of various observational studies suggest that higher intake of lutein+zeaxanthin is associated with a decreased risk of cataract development7–12 and a decreased risk of progressing to advanced AMD.13–19 Epidemiologic studies and observational data suggest that increased dietary intake of the ω-3 LCPUFAs, and fish products, is associated with a reduced risk of advanced AMD, both in prevalent and incident cases of advanced AMD.20–28
Lutein and zeaxanthin are components of human macular pigment, and they are found throughout the retina.29 Macular pigment is hypothesized to enhance retinal membrane stability, act as a short-wavelength light filter, modulate intra- and extracellular reduction-oxidation (redox) balance, and possibly modulate signal transduction pathways.30, 31
Lutein and zeaxanthin are the only carotenoids detected in the human lens.32 Lutein has been also identified in the lens of quail33 and appears to have an effect on cataract development in a diabetic rat model.34 In vitro testing with lutein and zeaxanthin appear to have also a protective effect for lens fibers.35
Docosahexaenoic acid (DHA) is the major structural omega-3 LCPUFA of retinal photoreceptor outer segment membranes.36, 37 DHA, its precursor eicosapentaenoic acid (EPA), and their metabolites may have the capacity to help regulate gene expression, cell signaling and survival pathways, as well as modulate immune and inflammatory processes implicated in the pathogenesis of retinal vascular and neural cell disease.38
The Age-Related Eye Disease Study 2 (AREDS2) is designed primarily to evaluate the effects of these nutrients on the progression of AMD and, secondarily, age-related cataracts. This report presents the study design and the baseline characteristics of the cohort.
METHODS
The primary objective of AREDS2 is to evaluate the effect of dietary xanthophylls (lutein+zeaxanthin) with and without omega-3 LCPUFAs (DHA+EPA) on progression to advanced AMD. The study enrolled 4,203 participants aged 50 to 85 years with sufficiently clear media for quality fundus photographs and either: 1) bilateral large drusen or 2) large drusen in one eye and advanced AMD (neovascular AMD or central geographic atrophy) in the fellow eye. Participants will be followed for an average of 5 years. In addition to this primary objective, AREDS2 is also assessing modifications of the original AREDS formulation. Treatment with the AREDS formulation was recommended for AREDS2 participants because they all have intermediate AMD (bilateral large drusen) or advanced AMD in one eye. Since beta-carotene might increase the risk of lung cancer in cigarette smokers,39, 40 and beta-carotene was included in the formulation because lutein and zeaxanthin were not commercially available at the start of the original AREDS, we developed a version of the AREDS formulation without beta-carotene. The amount of zinc included in the original AREDS formulation was 80 mg. The tolerable upper level of daily zinc intake is 40 mg.41 At the AREDS Nutritional Workshop meeting conducted prior to the start of AREDS, experts preferred a lower dose of zinc, but the higher dose was chosen by the National Eye Institute because it was the dose shown to be effective in the only then existing trial demonstrating efficacy.42 In an attempt to evaluate a lower dose of zinc we developed a version of the AREDS formulation with 25 mg of zinc.43 AREDS2 is studying these two different formulations through a factorial design (Table 1) for those who were willing to participate in a secondary randomization. Some participants opted out of the secondary randomization to the AREDS formulation comparisons and continued to take the original AREDS formulation. Participants, investigators, study coordinators, and all other study personnel are masked to treatment assignment in both the primary and secondary randomizations.
Table 1.
Secondary Randomization Agents (Age-Related Eye Disease Study (AREDS)-Type Supplement)
| Formulations | Vitamin C (mg) |
Vitamin E (IU) |
Beta Carotene (mg) |
Zinc Oxide (mg) |
Cupric Oxide (mg) |
|---|---|---|---|---|---|
| 1 | 500 | 400 | 15 | 80 | 2 |
| 2 | 500 | 400 | 0 | 80 | 2 |
| 3 | 500 | 400 | 15 | 25 | 2 |
| 4 | 500 | 400 | 0 | 25 | 2 |
The components and the manufacturing details of the study medications including the placebos for both randomization schemes are described in Appendix 2 (available at http://aaojournal.org).
STUDY POPULATION
Participant eligibility
Enrollment was restricted to people determined to be at high risk of progression to advanced AMD with either bilateral large drusen or non-foveal geographic atrophy (no advanced AMD) or large drusen or non-foveal geographic atrophy in one eye and advanced AMD in the fellow eye (AREDS Simple Scale Score of 2, 3 or 4).44 Potential participants between the ages of 50 and 85 years were evaluated for eligibility at a qualification visit. Run-in medication (study placebo) was administered to all potential subjects to determine the likelihood of adherence to the study regimen. Participants were then randomized to the study medications at the randomization visit (within 3 months of the qualification visit) if they took at least 75% of study medication during the run-in phase.
Eligible participants: 1) were able and willing to consent to both the qualification and the randomization/follow-up phases of the study; 2) were likely, willing and able to undergo yearly examinations for at least five years; 3) agreed to stop current use of supplements containing lutein, zeaxanthin, omega-3 LCPUFAs (specifically DHA+EPA), vitamin C, vitamin E, beta-carotene, zinc or copper, other than those supplied by AREDS2; 4) demonstrated adherence to the run-in regimen (consumption of at least 75 percent of run-in medication as determined by pill count or pill weight); 5) had fundus photographs of adequate quality as assessed with a standardized protocol by the Reading Center (University of Wisconsin Fundus Photograph Reading Center); and 6) were randomized within three months following the qualification visit. Exclusion criteria included: 1) the presence of ocular disease in either eye that may have confounded evaluation of the retina; 2) previous retinal or other ocular surgical procedures (other than cataract extraction) that may have complicated assessment of the progression of AMD; 3) a chronic requirement for any systemic or ocular medication administered for other diseases and known to be toxic to the retina or optic nerve; 4) previous daily supplementation with 2mg or more of lutein and/or 500 mg or more of omega-3 LCPUFAs for a period of 1 year or more prior to the date of randomization (A participant was eligible for the study if he/she agreed to stop taking these supplements during the study run-in period); 5) intraocular pressure of 26 mm Hg or higher or some reason to believe that the participant might have glaucoma; and 6) cataract surgery within 3 months or capsulotomy within 6 weeks prior to the qualification visit. Other exclusion criteria included: history of lung cancer; any systemic disease with a poor five-year survival prognosis; hemochromatosis; Wilson’s disease, or recent diagnosis of oxalate kidney stones; any condition that would make adherence or follow-up difficult or unlikely; current participation in other studies that might affect adherence to the AREDS2 follow-up schedule; or use of systemic anti-angiogenic therapy for treatment of choroidal neovascularization or cancer. At study inception, fundus photographs were not reviewed prior to randomization; however, after approximately 2000 subjects were randomized, the Reading Center determined that just over 300 enrolled participants did not meet ocular eligibility requirements. Thereafter, study procedures included confirmation of eligibility by the Reading Center prior to randomization.
Study supplements
Study participants were randomly assigned to take one of the following study supplements daily: 1) placebo; 2) lutein+zeaxanthin (10 mg/2mg); 3) DHA+EPA (350 mg/650 mg); or 4) lutein+zeaxanthin (10mg/2mg) and DHA+EPA (350mg/650mg). (Figure 1) All participants were offered the AREDS formulation. Those who agreed to take the AREDS formulation and consented to a second randomization were randomly assigned to receive one of four alternative AREDS formulations (see Table 1). Note that this second-tier randomization does not include placebo because for these participants AREDS supplements are considered standard of care. Participants who were current smokers or former smokers who had stopped smoking within the year prior to enrollment were randomly assigned to one of the two arms without beta-carotene (Formulations 2 or 4). AREDS supplements were provided for those participants who were not current or former smokers within the past year and did not consent to this secondary randomization but elected to take the original AREDS supplements. All participants taking a daily multivitamin supplement were required to replace it with Centrum Silver®, which was provided to them.
Figure 1.
Age-Related Eye Disease Study 2 (AREDS2) Study Design
aDHA and EPA: docosahexanoic acid and eicosapentaneoic acid
bAREDS Supplements: vitamins C (500 mg), E (400 international units), beta carotene (15 mg), zinc (oxide 80 mg) and copper (cupric oxide 2 mg)
cβ-Carotene: beta-carotene (smokers were randomly assigned to one of these two groups with no beta-carotene in the formulation)
Enrollment, randomization and follow-up
A random block design was implemented using the AREDS2 Advantage Electronic Data Capture system (AdvantageEDCSM) by the AREDS2 Coordinating Center (The EMMES Corporation, Rockville, Maryland) and stratified by clinical center and AMD status (large drusen both eyes or large drusen in one eye and advanced AMD in the fellow eye) to assure approximate balance across centers over time. In-clinic follow-up visits are scheduled annually post-randomization. Telephone contacts are scheduled at three- and six-months post-randomization and annually thereafter, starting at 18 months post-randomization. Telephone contacts are primarily used to collect information about adverse events.
At baseline and at each annual study visit, participants received a comprehensive eye exam including best-corrected visual acuity obtained with the electronic version of the ETDRS visual acuity charts. Certified photographers obtain stereoscopic fundus photographs of the macula and optic nerve and masked graders use a standard protocol to grade the photographs.
All enrolled participants will be followed until November 2012 allowing for a median of 5 years of follow-up or until the Data and Safety Monitoring Committee (DSMC) has recommended earlier trial termination.
OUTCOMES
The primary purpose of the study is a comparison of the three active treatment arms to placebo on progression to advanced AMD based on reading center grading of color stereoscopic fundus photographs in study eyes. Advanced AMD is defined as atrophic or neovascular changes of AMD that include one or more of the following: 1) definite geographic atrophy involving the center of the macula (minimum diameter for a patch of atrophy to be classified as geographic is 360 µM), 2) evidence suggesting choroidal neovascularization, defined as at least two of the following characteristics of CNV lesions: serous detachment of the sensory retina, hemorrhage, retinal pigment epithelial detachment (non-drusenoid type), fibrous tissue, or hard exudate. In addition, an eye is defined to have developed advanced AMD if the reading center identifies a definite disciform scar, or if there is a history of treatments for AMD (e.g., anti-angiogenic therapy, photodynamic therapy, laser photocoagulation, etc.). Although the primary outcome was determined on color fundus photography, when available, fluorescein angiography and OCT images are sent to the Reading Center to confirm the presence of neovascular AMD.
Secondary analyses include comparison of the three active treatment arms to placebo on 1) the progression to moderate vision loss (a 15-letter or greater loss); 2) the progression of lens opacity based on photography or incidence of cataract surgery; and 3) moderate vision loss or improvement in participants with advanced AMD. The secondary randomization allows the assessment of: 4) the effect of eliminating beta-carotene in the original AREDS formulation on the progression and development of AMD; 5) the effect of reducing zinc from 80 mg in the original AREDS formulation to 25 mg on the progression and development of AMD; 6) the effect of eliminating beta-carotene in the original AREDS formulation on moderate vision loss; and 7) the effect of reducing zinc in the original AREDS formulation on moderate vision loss.
PRIMARY ANALYSIS
The primary efficacy outcome, time to progression to advanced AMD, will be assessed using a Cox proportional hazards model without adjustment for covariates. The Cox proportional hazards modeling using the Wei, Lin and Weissfeld method for obtaining robust variance estimates allowing for dependence among multiple event times will be used to assess the treatment effect.45 The Cox proportional hazards model will be used to compute hazard ratios and 95% confidence intervals (CIs) of the 3 active treatment arms with respect to the placebo arm. Secondary efficacy variables will be analyzed in the same fashion as the primary efficacy outcome. Sensitivity analysis will be performed with covariate adjustment. Potential covariates include baseline AMD status, clinical site, participant’s age and gender.
SECONDARY OUTCOME ANALYSES
Analyses of secondary efficacy outcomes will be conducted for confirmation of treatment effects. Analyses of each secondary outcomes listed below may be adjusted for baseline levels of covariates.
Effect of the Primary Randomization Supplements on Progression to Moderate Vision Loss
A time-based outcome of losing or not losing at least 15 letters from baseline in study eyes with at least 20/32 vision (74 letters) at baseline will be analyzed. A Cox proportional hazards model will be used to estimate the hazard ratios along with 95% CIs of the three treatment arms with respect to the placebo arm.
Effect of the Primary Randomization Supplements on Progression of Lens Opacity
A time-based outcome of progression of lens opacity [defined as a 10% increase from baseline of cortical opacity or a 5% increase in posterior subcapsular (PSC) or cataract surgery] will be analyzed. The model will be used to estimate the hazard ratios along with 95% CIs of the three treatment arms with respect to the placebo arm.
Effect of the Primary Randomization Supplements on Vision
Figures and tables (e.g., mean changes in visual acuity throughout follow-up with CIs) will summarize the mean visual acuity change at each study visit by treatment arm. The mean change in visual acuity at each study visit will be compared among the four arms using a generalized linear model along with the generalized estimating equation (GEE) methodology.
Time-based binary outcomes of vision loss (e.g., 10-letter loss: 0-no or 1-yes) and moderate vision improvement (e.g., 5-letter improvement: 0-no or 1-yes) will be analyzed. Cox proportional hazards models will be used to estimate the hazard ratios of the three treatment arms with respect to the placebo arm.
Effect of the Variations of the AREDS Supplements on Progression to Advanced AMD
A time-based outcome of progression to advanced AMD will be analyzed using a Cox proportional hazards model. Separate models of the main effects (beta-carotene versus no beta-carotene and zinc versus reduced zinc) will be used. Hazard ratios of the beta-carotene and zinc main effects will be estimated.
Effect of the Variations of the AREDS Supplements on Progression to Moderate Vision Loss
The analysis will be similar to the analyses described above using a time-based outcome of progression to moderate vision loss.
SAMPLE SIZE AND POWER CALCULATIONS
Power for AREDS2 is based on the primary objective: to assess the effect of lutein+zeaxanthin and/or DHA+EPA supplementation on the progression to advanced AMD with an average of 5 years of follow-up. Assumptions for the power calculations are based on the following. The 5-year progression rates to advanced AMD for AREDS participants with either bilateral large drusen or large drusen in one eye and advanced AMD in the second eye were 27.7% and 48.7%, respectively; for participants assigned to placebo and 22.4% and 37.5%, respectively; for participants assigned to the active arms. The AREDS formulation was associated with a 25% reduction to advanced AMD compared with placebo. AREDS2 is powered to assess a similar further reduction in the treatment arms compared with the control arm, which would be considered clinically important. Weighted 5-year rates of progression were computed for the primary AREDS2 outcome assuming that 60% of the enrolled participants will have bilateral large drusen and 40% of the participants will have large drusen in one eye and advanced AMD in the fellow eye. Assuming this patient population, the 5-year weighted progression rate to advanced AMD is estimated to be approximately 36%. Thus assuming a 25% treatment effect in each of the treatment arms, the 5-year weighted progression rates to advanced AMD in these treated groups is estimated to be approximately 28%.
To compute the necessary sample size, adjustments were made for projected deaths and losses to follow-up. In AREDS the mortality rate over the first five years was 6%, which was used as the projected death rate for AREDS2. While only 1.3% of the AREDS population was lost to follow-up, we assumed a conservative estimate of a 15% loss to follow-up rate in AREDS2. 4,000 participants will provide adequate power (at least 90%) to detect a 25-percent reduction in the progression to advanced AMD comparing the placebo group with each of the treatment groups.
Ancillary Studies
Ancillary studies are also conducted to evaluate the role of the AREDS2 nutritional supplements on cardiovascular disease and cognitive function. Blood is drawn from consenting participants for DNA production and storage as part of the AREDS2 genetics biorepository. A number of ancillary studies of imaging including fundus autofluorescence (FAF), wide-angle fundus photographs, and spectral domain optical coherence tomography (OCT) are being collected from a large number of AREDS2 participants. These studies are designed to provide longitudinal data on the progression of AMD using these imaging modalities. The AREDS2 study visits are scheduled for completion by the end of 2012.
RESULTS
Enrollment began in October 2006 and continued through September 2008 at the 82 clinical sites across the US. Each clinical center received Institutional Review Board approval of the protocol prior to initiating the study. The sites were chosen to achieve a balance of academic and community-based practices with wide variation of geographic location in order to obtain greater generalizability of the study results. After signing informed consent forms, 5,178 potential participants underwent qualification visits and 4,203 participants were randomized into the clinical trial, while 975 participants (19%) were found to be ineligible for randomization. The primary reasons for ineligibility were patient refusal for the randomization (25%), patient refusal for the study in general (19%), patient wish to take other oral supplements including the study supplements (8%), and ineligibility as determined by the reading center (25%). Approximately 8% of participants did not qualify for the study because they did not meet the requirement of having at least 75% adherence with the run-in supplements. Of the 4,203 randomized participants, 4,188 were included for the AMD trial (at least one study eye without advanced AMD) and 3,159 were eligible for the cataract trial (phakic in at least one eye). A total of 3,036 participants agreed to the secondary randomization evaluating different AREDS supplements. Of the remaining participants, 1,148 chose to take the commercial AREDS formulation and 19 chose to take no AREDS-type supplements at baseline.
Baseline characteristics of the AREDS2 cohort are provided in Table 2. Of the randomized participants, 4,056 (97%) are Caucasian and 2,383 (57%) are female. The median age is 74 years. Seven percent are current smokers while 49% are former smokers. Just over 65% had more than a high school education. A large percentage of participants (89%) were provided Centrum Silver® at study entry.
Table 2.
Age-Related Eye Disease Study 2 (AREDS2) Participant Baseline Characteristics
| Total | ||
|---|---|---|
| AREDS2 Participant Baseline Characteristics | N=4203 N (%) |
|
| RACE | White | 4059 (96.6) |
| Black or African American | 53 (1.3) | |
| Asian | 34 (0.8) | |
| American Indian | 6 (0.1) | |
| Asian Pacific Islander and other | 51 (1.2) | |
| HISPANIC ORIGIN | Hispanic Origin | 85 (2.0) |
| AGE AT RANDOMIZATION | Median Age (yr) | 74.0 |
| AGE AT RANDOMIZATION (%) |
< 55 yrs | 83 (2.0) |
| 55 to 65 yrs | 601 (14.3) | |
| 65 to 75 yrs | 1542 (36.7) | |
| 75 to 80 yrs | 1113 (26.5) | |
| ≥ 80 yrs | 864 (20.6) | |
| FEMALE GENDER | 2383 (56.7) | |
| MARITAL STATUS | Married | 2753 (65.5) |
| Divorced | 431 (10.3) | |
| Widowed | 846 (20.1) | |
| Never Married | 173 (4.1) | |
| EDUCATION | Grade 11 or Less | 274 (6.5) |
| High School Graduate | 1065 (25.3) | |
| Some College or Associate's Degree | 1142 (27.2) | |
| Bachelor's Degree | 781 (18.6) | |
| Post-graduate Work | 864 (20.6) | |
| Refused to Answer | 77 (1.8) | |
| DIABETIC | 546 (13.0) | |
| INSULIN USE | 79 (14.4) | |
| SMOKING STATUS | Never Smoked | 1858 (44.2) |
| Former Smoker | 2062 (49.1) | |
| Current Smoker | 283 (6.7) | |
| CENTRUM USE | 3726 (88.7) | |
| STATIN CLASS CHOLESTEROL LOWERING DRUG USE | 1850 (44.0) | |
| NSAID* USE | 458 (10.9) | |
| ACETAMINOPHEN USE | 386 (9.2) | |
| ASPIRIN USE | No | 2158 (51.3) |
| Yes, less than 2 per day | 1994 (47.4) | |
| Yes, 2 or more per day | 51 (1.2) | |
NSAID: Non-Steroidal Anti-Inflammatory Drug
The majority of AREDS2 participants were classified as having bilateral large drusen/non-central geographic atrophy (59%). Most of the other participants had advanced AMD and large drusen in the fellow eye (32%), but based upon a detailed grading by the reading center, 367 (9%) did not meet the protocol ocular eligibility criteria (Table 3). During early stages of recruitment, participant eligibility was not based on reading center review. This changed midway through recruitment, but during the period prior to reading center review, 74 participants were entered with less than large drusen in both eyes, 160 participants were entered with unilateral large drusen, 160 participants were entered with advanced AMD in one eye and less than large drusen in the fellow eye, and 15 participants had advanced AMD in both eyes. Most participants (84%) have an AREDS Simple Scale score43 of at least 3 or more at baseline and over 98% had at least AREDS Simple Scale score of 2.
Table 3.
Age-Related Eye Disease Study 2 (AREDS2) Participant Retinal Characteristics
| Retinal Characteristics | ||
|---|---|---|
| Drusen/Age-Related Macular Degeneration Status | ||
| Participants with at least large drusen or advanced AMD | ||
| Large Drusen both eyesa | 2484 (59.1) | |
| Advanced age-related macular degeneration in non-study eye, large drusen in study eyeb | 1360 (32.4) | |
| Large drusen, ungradable photo in fellow eye | 2 (0) | |
| Participants with less than large drusen in both eyes or in study eye | ||
| Less than large drusen in both eyes | 74 (1.8) | |
| Large drusen in one eye, less than large drusen fellow eyec | 160 (3.8) | |
| Advanced age-related macular degeneration in non-study eye, less than large drusen in study eye | 108 (2.6) | |
| Ineligible participants | ||
| Advanced age-related macular degeneration in both eyes | 15 (0.3) | |
| Total participants | 4203 | |
| Age-Related Macular Degeneration Status by patient using Age-Related Eye Disease Study (AREDS) Simple Scale Score | ||
| 0 | 10 (0.2) | |
| 1 | 62 (1.5) | |
| 2 | 632 (15.1) | |
| 3 | 1108 (26.5) | |
| 4 | 2374 (57.7) | |
| Total participants | 4186d | |
24 participants with questionable neovascularization (1 neovascular lesion out of the following potential lesions: serous detachment of the sensory retina, hemorrhage, retinal pigment epithelial detachment (non-drusenoid type), fibrous tissue, or hard exudate) in one eye and 2 participants with bilateral questionable neovascularization
15 participants with questionable neovascularization in one eye
3 participants with questionable neovascularization in one eye
Missing are: 15 participants with bilateral neovascular AMD and 2 with ungradable fundus photographs
For participants with bilateral large drusen, both eyes are eligible as study eyes because both have the potential to develop advanced AMD. In participants with advanced AMD in one eye at baseline, the eye without advanced AMD is the “study” eye. All eyes with advanced AMD are considered “non-study” eyes.
At baseline, 1,050 (25%) participants were pseudophakic/aphakic in both eyes, while 280 (7%) were pseudophakic/aphakic in one eye. Approximately 56% of participants had at least one eye without cortical opacity present, and 71% of eyes had at least one eye without PSC opacity (Table 4).
Table 4.
Age-Related Eye Disease Study 2 (ARED2) Participant Lens Characteristics
| Lens Characteristics | |
|---|---|
| Phakic in both eyes | 2878 (68.5) |
| Pseudophakic/Aphakic in one eye | 281 (6.7) |
| Pseudophakic/Aphakic in both eyes | 1044 (24.8) |
| Cortical Opacities on Photographs | |
| Absent/Questionable in both eyes | 1625 (39) |
| Absent/Questionable in one eyee | 722 (17) |
| Present in one eyee | 138 (3) |
| Present in both eyes | 642 (15) |
| Cannot Grade/Pseudophakic/Aphakic in both eyes | 1076 (26) |
| Posterior Subcapsular Opacities on Photographs | |
| Absent/Questionable in both eyes | 2587 (61) |
| Absent/Questionable in one eye1 | 432 (10) |
| Present in one eye1 | 41 (1) |
| Present in both eyes | 66 (2) |
| Cannot Grade/Pseudophakic/Aphakic in both eyes | 1077 (26) |
Ungradable/Pseudopakic/Aphakic in fellow eye
The mean ± standard deviation (SD) visual acuity for the study eyes without advanced AMD was 78 ± 13 letters (approximately 20/32 Snellen equivalent) and for non-study eyes was 46 ± 28 letters (approximately 20/125 Snellen equivalent). The distribution of the visual acuity for study and non-study eyes by visual acuity category is provided in Table 5.
Table 5.
Age-Related Eye Disease Study 2 (AREDS2) Baseline Visual Acuity by Study and Non-Study Eyes
| Study Eyes | Non-Study Eyes |
||
|---|---|---|---|
| N=7088 N (%) |
N=1300 N (%) |
||
| Visual Acuity Status | 20/20 or better | 2608 (36.8) | 73 (5.6) |
| < 20/20 to 20/40 | 3602 (50.8) | 328 (25.2) | |
| < 20/40 – 20/80 | 606 (8.6) | 236 (18.2) | |
| < 20/80 – 20/160 | 106 (1.5) | 160 (12.3) | |
| 20/200 or worse | 166 (2.3) | 503 (38.7) | |
DISCUSSION
This randomized controlled clinical trial is designed to evaluate the role of macular xanthophylls and omega-3 LCPUFAs on the progression of age-related macular degeneration and, secondarily, progression of cataract. The secondary randomization was successful in enrolling a large fraction of the AREDS2 participants, providing the opportunity to assess the effect of removing beta-carotene from the AREDS formulation and to assess the effect of lowering zinc.
The AREDS2 study population (median age 74 years) is older at baseline than the AREDS population (median age 69 years) was partly because the AREDS2 population includes only persons at high risk for developing advanced AMD. The participants with no or early AMD found in AREDS tended to be younger than those with AMD. In both studies, approximately 96% of the study populations are white. There are similar proportions of women (57%) and smokers (7%) in both studies. In contrast, 44% of the AREDS2 participants are taking a statin-class cholesterol lowering drug while only 9% of the AREDS participants were taking this class of drugs. This reflects the change in clinical practice over time. The rate of diabetes is higher in the AREDS2 participants (13%) compared with the AREDS population (6%). Approximately 45% of the AREDS2 study population and 32% of the AREDS population achieved an educational level of at least a bachelor’s degree. These differences may be related to the older population of the AREDS2 cohort.
How representative is the AREDS2 population of those who are affected with AMD and how generalizable will the study results be? As with all controlled clinical trials, it is important to conduct a trial that will give a valid conclusion and this often includes a population with very specific inclusion and exclusion criteria that ensures good compliance with both the treatments as well as the follow-up. This tends to decrease the generalizability to a broadly defined population. However, these results will be clinically important for most persons who are at risk for developing AMD. The data will likely be reported in 2013.
Supplementary Material
Acknowledgments
Financial Support: This study is supported by the intramural program funds and contracts from the National Eye Institute/National Institutes of Health (NEI/NIH), Department of Health and Human Services, Bethesda, MD. Contract No. HHS-N-260-2005-00007-C. ADB Contract No. N01-EY-5-0007. Funds were generously contributed to these contracts by the following NIH institutes: Office of Dietary Supplements (ODS), National Center for Complementary and Alternative Medicine (NCCAM), National Institute on Aging (NIA), National Heart, Lung and Blood Institute (NHLBI), and National Institute of Neurological Disorders and Stroke (NINDS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Financial Interest: A complete list of all AREDS2 investigator financial disclosures, which were collected for regulatory purposes, pursuant to US FDA regulations in 21 CFR Part 54, can be found at www.areds2.org. The member(s) of the writing committee have made the following disclosure(s): Frederick L. Ferris III; Bausch & Lomb (P) and the remainder had no conflicts of interest.
The following material should appear online only: Appendix 1, Appendix 2
References
- 1.Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485. doi: 10.1001/archopht.122.4.477. [DOI] [PubMed] [Google Scholar]
- 2.Eye Diseases Prevalence Research Group. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 3.Goldman HB, Kiffel S, Weinstock FJ. Cataract surgery and the primary care practitioner. Geriatrics. 2009;64:19–22. 25–26. [PubMed] [Google Scholar]
- 4.Eye Diseases Prevalence Research Group. Prevalence of cataract and pseudophakia/aphakia among adults in the United States. Arch Ophthalmol. 2004;122:487–494. doi: 10.1001/archopht.122.4.487. [DOI] [PubMed] [Google Scholar]
- 5.Age-Related Eye Disease Study Group. A randomized, placebo-controlled clinical trial of high-dose supplementation with vitamins C and E, beta carotene, and zinc for age-related macular degeneration and vision loss: AREDS report no. 8. Arch Ophthalmol. 2001;119:1417–1436. doi: 10.1001/archopht.119.10.1417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Age-Related Eye Disease Study Group. A randomized, placebo-controlled, clinical trial of high-dose supplementation with vitamins C and E and beta carotene for age-related cataract and vision loss: AREDS report no. 9. Arch Ophthalmol. 2001;119:1439–1452. doi: 10.1001/archopht.119.10.1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyle BJ, Mares-Perlman JA, Klein BE, et al. Antioxidant intake and risk of incident age-related nuclear cataracts in the Beaver Dam Eye Study. Am J Epidemiol. 1999;149:801–809. doi: 10.1093/oxfordjournals.aje.a009895. [DOI] [PubMed] [Google Scholar]
- 8.Delcourt C, Carriere I, Delage M, et al. POLA Study Group. Plasma lutein and zeaxanthin and other carotenoids as modifiable risk factors for age-related maculopathy and cataract: the POLA Study. Invest Ophthalmol Vis Sci. 2006;47:2329–2335. doi: 10.1167/iovs.05-1235. [DOI] [PubMed] [Google Scholar]
- 9.Vu HT, Robman L, Hodge A, et al. Lutein and zeaxanthin and the risk of cataract: the Melbourne Visual Impairment Project. Invest Ophthalmol Vis Sci. 2006;47:3783–3786. doi: 10.1167/iovs.05-0587. [DOI] [PubMed] [Google Scholar]
- 10.Renzi LM, Johnson EJ. Lutein and age-related ocular disorders in the older adult: a review. J Nutr Elder. 2007;26:139–157. doi: 10.1300/j052v26n03_07. [DOI] [PubMed] [Google Scholar]
- 11.Dherani M, Murthy GV, Gupta SK, et al. Blood levels of vitamin C, carotenoids and retinol are inversely associated with cataract in a North Indian Population. Invest Ophthalmol Vis Sci. 2008;49:3328–3335. doi: 10.1167/iovs.07-1202. [DOI] [PubMed] [Google Scholar]
- 12.Moeller SM, Voland R, Tinker L, et al. CAREDS Study Group. Associations between age-related nuclear cataract and lutein and zeaxanthin in the diet and serum in the Carotenoids in the Age-Related Eye Disease Study (CAREDS), an ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2008;126:354–364. doi: 10.1001/archopht.126.3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Age-Related Eye Disease Study Research Group. The relationship of dietary carotenoid and vitamin A, E, and C intake with age-related macular degeneration in a case-control study: AREDS report no. 22. Arch Ophthalmol. 2007;125:1225–1232. doi: 10.1001/archopht.125.9.1225. [DOI] [PubMed] [Google Scholar]
- 14.Eye Disease Case-Control Study Group. Antioxidant status and neovascular age related macular degeneration. Arch Ophthalmol. 1993;111:104–109. doi: 10.1001/archopht.1993.01090010108035. [DOI] [PubMed] [Google Scholar]
- 15.Seddon JM, Ajani UA, Sperduto RD, et al. Eye Disease Case-Control Study Group. Dietary carotenoids, vitamins A, C, and E, advanced age-related macular degeneration. JAMA. 1994;272:1413–1420. [PubMed] [Google Scholar]
- 16.Mares-Perlman JA, Fisher AI, Klein R, et al. Lutein and zeaxanthin in the diet and serum and their relation to age-related maculopathy in the third National Health and Nutrition Examination Survey. Am J Epidemiol. 2001;153:424–432. doi: 10.1093/aje/153.5.424. [DOI] [PubMed] [Google Scholar]
- 17.Snellen EL, Verbeek AL, Van Den Hoogen GW, et al. Neovascular age-related macular degeneration and its relationship to antioxidant intake. Acta Ophthalmol Scand. 2002;80:368–371. doi: 10.1034/j.1600-0420.2002.800404.x. [DOI] [PubMed] [Google Scholar]
- 18.Moeller SM, Parekh N, Tinker L, et al. CAREDS Research Study Group. Associations between intermediate age related macular degeneration and lutein and zeaxanthin in the Carotenoids in Age-Related Eye Disease Study (CAREDS): ancillary study of the Women’s Health Initiative. Arch Ophthalmol. 2006;124:1151–1162. doi: 10.1001/archopht.124.8.1151. [DOI] [PubMed] [Google Scholar]
- 19.Tan JS, Wang JJ, Flood V, et al. Dietary antioxidants and the long-term incidence of age-related macular degeneration: the Blue Mountains Eye Study. Ophthalmology. 2008;115:334–341. doi: 10.1016/j.ophtha.2007.03.083. [DOI] [PubMed] [Google Scholar]
- 20.Seddon JM, Rosner B, Sperduto RD, et al. Dietary fat and risk for advanced age-related macular degeneration. Arch Ophthalmol. 2001;119:1191–1199. doi: 10.1001/archopht.119.8.1191. [DOI] [PubMed] [Google Scholar]
- 21.Seddon JM, Cote J, Rosner B. Progression of age-related macular degeneration: association with dietary fat, transunsaturated fat, nuts, and fish intake. Arch Ophthalmol. 2003;121:1728–1737. doi: 10.1001/archopht.121.12.1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seddon JM, George S, Rosner B. Cigarette smoking, fish consumption, omega-3 fatty acid intake, and associations with age-related macular degeneration: the US Twin Study of Age-Related Macular Degeneration. Arch Ophthalmol. 2006;124:995–1001. doi: 10.1001/archopht.124.7.995. [DOI] [PubMed] [Google Scholar]
- 23.Chua B, Flood V, Rochtchina E, et al. Dietary fatty acids and the 5-year incidence of age-related maculopathy. Arch Ophthalmol. 2006;124:981–986. doi: 10.1001/archopht.124.7.981. [DOI] [PubMed] [Google Scholar]
- 24.Augood C, Chakravarthy U, Young I, et al. Oily fish consumption, dietary docosahexaenoic acid and eicosapentaenoic acid intakes, and associations with neovascular age-related macular degeneration. Am J Clin Nutr. 2008;88:398–406. doi: 10.1093/ajcn/88.2.398. [DOI] [PubMed] [Google Scholar]
- 25.Swenor BK, Bressler S, Caulfield L, West SK. The impact of fish and shellfish consumption on age-related macular degeneration. Ophthalmology. 2010;117:2395–2401. doi: 10.1016/j.ophtha.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.SanGiovanni JP, Chew EY, Agron E, et al. Age-Related Eye Disease Study Research Group. The relationship of dietary omega-3 long-chain polyunsaturated fatty acid intake with incident age-related macular degeneration: AREDS report no. 23. Arch Ophthalmol. 2008;126:1274–1279. doi: 10.1001/archopht.126.9.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.SanGiovanni JP, Agron E, Clemons TE, Chew EY. Omega-3 long chain polyunsaturated fatty acid intake inversely associated with 12-year progression to advanced age-related macular degeneration [letter] Arch Ophthalmol. 2009;127:110–112. doi: 10.1001/archophthalmol.2008.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.SanGiovanni JP, Argon E, Meleth AD, et al. ARED Research Group. Omega-3 long-chain polyunsaturated fatty acid intake and 12-y incidence of neovascular age-related macular degeneration and central geographic atrophy: AREDS report 30, a prospective cohort study from the Age-Related Eye Disease Study. Am J Clin Nutr. 2009;90:1601–1607. doi: 10.3945/ajcn.2009.27594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Landrum JT, Bone RA. Lutein, zeaxanthin, and the macular pigment. Arch Biochem Biophys. 2001;385:28–40. doi: 10.1006/abbi.2000.2171. [DOI] [PubMed] [Google Scholar]
- 30.Krinsky NI, Landrum JT, Bone RA. Biologic mechanisms of the protective role of lutein and zeaxanthin in the eye. Annu Rev Nutr. 2003;23:171–201. doi: 10.1146/annurev.nutr.23.011702.073307. [DOI] [PubMed] [Google Scholar]
- 31.SanGiovanni JP, Chew EY, Johnson EJ. Lutein. In: Coates PM, Betz JM, Blackman MR, et al., editors. Encyclopedia of Dietary Supplements. 2nd ed. New York: Informa Healthcare; 2010. pp. 493–503. [Google Scholar]
- 32.Yeum KJ, Taylor A, Tang G, Russell RM. Measurement of carotenoids, retinoids, and tocopherols in human lenses. Invest Ophthalmol Vis Sci. 1995;36:2756–2761. [PubMed] [Google Scholar]
- 33.Dorey CK, Granata L, Nichols CR, et al. Dietary modulation of lens zeaxanthin in quail. Exp Eye Res. 2005;81:464–477. doi: 10.1016/j.exer.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 34.Arnal E, Miranda M, Almansa I, et al. Lutein prevents cataract development and progression in diabetic rats. Graefes Arch Clin Exp Ophthalmol. 2009;247:115–120. doi: 10.1007/s00417-008-0935-z. [DOI] [PubMed] [Google Scholar]
- 35.Chitchumroonchokchai C, Bomser JA, Glamm JE, Failla ML. Xanthophylls and alpha-tocopherol decrease UVB-induced lipid peroxidation and stress signaling in human lens epithelial cells. J Nutr. 2004;134:3225–3232. doi: 10.1093/jn/134.12.3225. [DOI] [PubMed] [Google Scholar]
- 36.Neuringer M. The relationship of fatty acid composition to function in the retina and visual system. In: Dobbing J, Benson JD, editors. Lipids, Learning, and the Brain: Fats in Infant Formulas. Report of the 103rd Ross Conference on Pediatric Research. Columbus, OH: Ross Laboratories; 1993. pp. 134–158. [Google Scholar]
- 37.Fliesler SJ, Anderson RE. Chemistry and metabolism of lipids in the vertebrate retina. Prog Lipid Res. 1983;22:79–131. doi: 10.1016/0163-7827(83)90004-8. [DOI] [PubMed] [Google Scholar]
- 38.SanGiovanni JP, Chew EY. The role of omega-3 long-chain polyunsaturated fatty acids in health and disease of the retina. Prog Retin Eye Res. 2005;24:87–138. doi: 10.1016/j.preteyeres.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 39.Albanes D, Heinonen OP, Huttunen JK, et al. Effects of alpha-tocopherol and beta-carotene supplements on cancer incidence in the Alpha-Tocopherol Beta-Carotene Cancer Prevention Study. Am J Clin Nutr. 1995;62(suppl):1427S–1430S. doi: 10.1093/ajcn/62.6.1427S. [DOI] [PubMed] [Google Scholar]
- 40.Omenn GS, Goodman GE, Thornquist MD, et al. Risk factors for lung cancer and for intervention effects in CARET, the Beta-Carotene and Retinol Efficacy Trial. J Natl Cancer Inst. 1996;88:1550–1559. doi: 10.1093/jnci/88.21.1550. [DOI] [PubMed] [Google Scholar]
- 41.Food & Nutrition Board, Institute of Medicine. Washington D.C.: National Academies Press; 2001. [Accessed May 1, 2012]. Dietary Reference Intakes for Vitamin A, Vitamin K, Arsenic, Boron, Chromium, Copper, Iodine, Iron, Manganese, Molybdenum, Nickel, Silicon, Vanadium, and Zinc; pp. 82–146.pp. 290–378.pp. 442–489. Available at: http://www.nal.usda.gov/fnic/DRI//DRI_Vitamin_A/vitamin_a_full_report.pdf. [PubMed] [Google Scholar]
- 42.Newsome DA, Swartz M, Leone NC, et al. Oral zinc in macular degeneration. Arch Ophthalmol. 1988;106:192–198. doi: 10.1001/archopht.1988.01060130202026. [DOI] [PubMed] [Google Scholar]
- 43.Hambidge M. Underwood Memorial Lecture: human zinc homeostasis: good but not perfect. J Nutr. 2003;113(suppl):1438S–14342S. doi: 10.1093/jn/133.5.1438S. [DOI] [PubMed] [Google Scholar]
- 44.Age-Related Eye Disease Study Research Group. A simplified severity scale for age-related macular degeneration: AREDS report no. 18. Arch Ophthalmol. 2005;123:1570–1574. doi: 10.1001/archopht.123.11.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wei LJ, Lin DY, Weissfeld L. Regression analysis of multivariate incomplete failure time data by modeling marginal distributions. J Am Stat Assoc. 1989;84:1065–1073. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.