Abstract
To advance our understanding about the emotional and cognitive deficits of patients with frontotemporal dementia with behavioral variant (bvFTD), the current study examined comprehension and expression of emotions from prosodic and facial cues in a 66-year old woman. The patient diagnosed with bvFTD compared to six patients with acute right hemisphere stroke. Recognition of emotion from prosodic cues was assessed using an identification task in four conditions with decreasing verbal demands (neutral sentences, language-like pseudo sentences, monosyllables, and asyllabic vowel sounds). Repetition of utterances with emotional connotations and self-generated conversations were analyzed to measure relative changes in mean fundamental frequency (f0), f0 variance, speech rate, and intensity along with the facial musculature pattern. The patient showed a marked deficit in identifying emotions in all four prosody conditions; and she did not show much variation in modulating mean f0, f0 variance, speech rate and intensity for all emotion categories when compared to neutral utterances. In addition, this patient demonstrated little to no facial expressions during emotionally-provoking tasks, but demonstrated no difficulty recognizing emotions from facial expressions or verbal scenarios. Results show that the patient seems to have selective impairment in recognition of emotions from prosody and expression of emotions using both prosodic and facial features. Impaired processing of emotional prosody and facial expressions could be important for detecting bvFTD with greater right hemisphere atrophy.
Introduction
During human communication, facial expressions and speech prosody (commonly known as “tone of voice”) are particularly important channels to understand emotions. Recent interest in neurocognitive processing of emotions from speaker’s voice and facial expressions indicates that these abilities are governed by a bilaterally distributed neural network, especially the right prefrontal and temporal regions (Adolphs, 2002; Fusar-Poli, Placentino, Carletti, et al., 2009; Pell, 2006; Ross & Monnot, 2008; Schirmer & Kotz, 2006). Patients with behavioral variant of frontotemporal dementia (bvFTD) are characterized by progressive deterioration of behavior and cognition associated with frontal and temporal atrophy (Rascovsky, Hodges, Knopman, et al., 2011), unlike the patients with semantic and agrammatic variant, who exhibit language difficulties. Because bvFTD causes diverse patterns of anatomic damage to the frontal and temporal regions either bilaterally or unilaterally along with deficits in emotion processing (Chaby & Narme, 2009; Kipps, Mioshi, & Hodges, 2009; Mendez, McMurtray, Licht, Shapira, Sau, & Miller, 2006), bvFTD patients are uniquely suited to examine the neural correlates of emotion processing from prosodic and facial cues. Another important motivation to study bvFTD patients is that rather than having unpredictable and varied lesion sites as in stroke patients, dementia patients typically have specific patterns of neurodegeneration consistent with their disease.
To advance our understanding about the deficits of bvFTD patients, the current study investigated comprehension and expression of emotions from prosody and facial expressions and empathy functioning in a bvFTD patient. We were particularly interested in studying this patient, as she exhibited signs of severe emotional blunting. We further compared her performance with that of patients with acute ischemic stroke in the right hemisphere (RHS) on all emotion and empathy tasks.
Methods
Participants
The patient is a 66-year old woman (Ms Y) who had noticed behavioral changes for approximately four years. The clinical profile and the neuroradiologic scans supported a diagnosis of probable bvFTD by consensus criteria (Rascovsky, et al., 2011). PET scan revealed bilateral hypometabolism in frontal and anterior temporal lobes that was more severe on the right. Her MRI is described below. We also examined six patients (five male, one female) with acute right hemisphere ischemic stroke (RHS) with a mean age of 54 years (SD – 13.2). The RHS patients with lesions confined to fronto-temporo-parietal network were examined in this study. All participants provided written consent for participation in the study.
Structural Imaging
To determine the locations of the patient’s atrophy, the volumes as described in greater detail in Fig 1a of each area of the grey and white matter of the patient were compared with four age (mean = 66years) and gender-matched controls. In addition, an overlay of the lesions of all RHS patients was genrated using MRIcro (www.mricro.com).
Fig. 1.
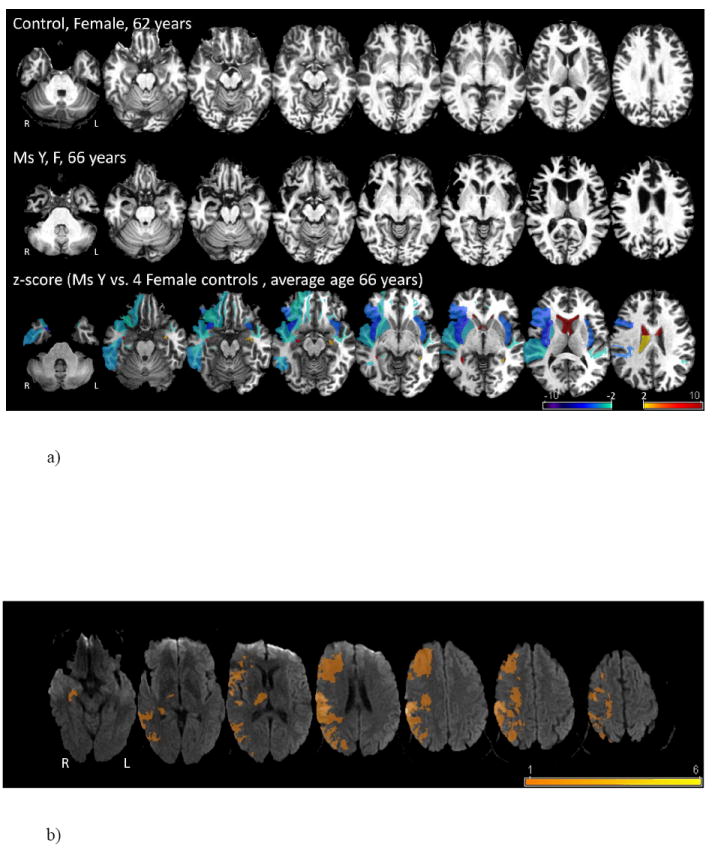
a) Predominant right frontal and temporal atrophy of bvFTD patient (Ms Y) compared to 4 female control subjects with an average age of 66 years.
b) Overlay of the lesion areas of the patients with acute right hemisphere ischemic strokes.
Neuropsychological Battery
As part of the clinical evaluation, a comprehensive neuropsychological battery was conducted. The battery included tests of global cognitive functioning (Mini Mental State Examination, MMSE; Folstein, &. Folstein, 2010; Dementia Rating Scale-2, DRS-2; Mattis, Jurica, & Leitten, 2001), processing speed (Symbol Digit Modality Test, SDMT, Smith, 1982), attention and working memory (Trail Making Test, TMT, Lezak, et al, 2004; Digit Span subtest from the Wechsler Adult Intelligence Test, 4th edition, WAIS-IV, Wechsler, et al., 2008; Spatial span subtest from the Wechsler Memory Scale, 3rd edition, WMS-III, Wechsler, D., 1998); language processing (Boston Naming Test, BNT, Kaplan et al, 2001), verbal memory (Hopkins Verbal Learning Test – Revised, HVLT-R, Brandt, & Benedict, 2001), visual-spatial processing and memory (Rey Complex Figure Test, RCFT, Meyers, &. Meyers, 1996); and executive functioning (Controlled Oral Word Association Test, COWAT, Lezak, et al, 2004; Category fluency, Lezak, et al, 2004; Tower of London – Drexel revision, ToLDX, Culbertson, & Zillmer, 2001).
In addition, the patient completed a functional evaluation of her cognitive skills. The Executive Function Performance Test (EFPT; Baum, Morrison, Hahn, & Edwards, 2003) is a “top down” assessment of executive functioning as opposed to “bottom up” neuropsychological measurement of specific aspects of cognition (e.g. cognitive control, working memory). The EFPT breaks down the task into executive functioning “components” of initiation, organization, sequencing, judgment/safety, and task termination. A standardized cuing system is used to assess the individual’s ability to successfully complete each component of four basic tasks of independent living (simple cooking, telephone use, medication management, and bill payment). The examiner notes the level of cueing required for task to be completed (i.e., 0 = independent performance, 1 = verbal guidance needed, 2 = gestural guidance needed, 3 = verbal direct instruction needed, 4 = physical assistance needed, 5 = examiner must perform component for the participant). The higher the level of cuing indicates a poorer performance and greater degree of cognitive impairment. Component scores are calculated by summing the cuing level required for each executive functioning component (i.e., initiation, organization, sequencing, judgment/safety, and termination) across each of the four tasks.
Experimental Tasks
a) Affective Prosody Tasks
Four central tasks evaluated emotional processing for prosodic features alone and a control task evaluated emotional processing when semantic cues provided emotion information in addition to the emotional meaning from prosody. The four central tasks assessed the ability to categorize emotions from prosody in four conditions with decreasing verbal demands (neutral sentences, language-like pseudo sentences, monosyllables, and asyllabic vowel sounds), adapted from the Aprosodia Battery, which was developed to distinguish between affective-prosodic deficits result from left versus right hemisphere damage (Ross et al., 1997; Ross and Monnot, 2008). In the word identification task, participants were presented utterances that were semantically neutral but communicated specific emotions through the prosody (e.g., I am going to the other movies). In the monosyllabic identification task, participants were presented with monosyllabic utterances that conveyed specific emotions through prosody (e.g., ba ba ba ba ba ba ba). In the asyllabic identification task, participants were presented with voiced asyllabic sounds which conveyed emotions through prosodic cues (e.g., aaaaaaah). In word, monosyllabic and asyllabic identification tasks, participants listened to each utterance and then identified the emotion of the speaker based on the prosodic features in a six forced-choice response format (alternatives - happy, surprise, angry, sad, disinterest, neutral). In pseudo identification task, participants were presented language-like pseudo utterances that were semantically anomalous but which communicated specific emotions through prosody (e.g., Someone migged the pazing). In pseudo identification task, participants listened to each utterance and then identified emotion of the speaker based on the prosodic cues in a seven forced-choice response format (alternatives – happy, surprise, angry, disgust, fear, sad, neutral). Stimuli for each of these tasks were specifically developed to assess comprehension of emotional prosody in patient as well as healthy adults and this type of stimuli has been used successfully in previous studies (Pell, Paulmann, Dara, Alasseri, & Kotz, 2009; Ross & Monnot, 2008; Ross, Thompson, & Yenkosky, 1997). In the control task, participants listened to semantically well-formed sentences and then identified emotions from seven alternatives (happy, surprise, angry, disgust, fear, sad, neutral). The stimuli for the control task were created to provide emotion information through verbal as well as prosodic cues (Pell, et al., 2009).
Three tasks were employed to assess the ability to produce emotional prosody. A repetition task was administered using the utterances from the three identification tasks (word, monosyllable, and asyllabic). The participants listened to the utterances presented in a pseudo random order and then were required to produce the utterances with same emotional intonation. For the second task, prosody expression task, short six – eight syllable semantically neutral sentences were presented to the participants on a computer screen. The sentences were constructed such that each statement was conducive to four emotional interpretations (happy, sad, angry, and neutral). A short verbal scenario was provided for each sentence to bias a response in specific emotion and the patient was required to produce the sentence in each of the four emotion categories. Finally, the participant was encouraged to make self-generated emotional conversations that were events from the participants past life experiences to tap into prosodic modulations initiated by the participant. All prosody expressions were recorded and further analyzed to measure relative changes in mean fundamental frequency (f0), f0 variance, speech rate, and intensity.
b) Facial Expressions Tasks
In addition, recognition of emotions was examined from static facial expressions in an emotion categorization task. In the face identification task, facial expressions were presented centrally on a computer screen and the participants were required to identify the emotion in a seven forced-choice response format (alternatives – happy, surprise, angry, disgust, fear, sad, neutral). The stimuli for facial expressions were selected from a set of perceptually validated pictures (Pell & Leonard, 2005).
Facial musculature pattern was video-taped when the patient performed all the prosody expression tasks. An anatomically based, microanalytic system, Affex (Izard, Doughterty, & Hembree, 1983) was used to code the patient’s facial expressions. A trained coder (KK) assigned predetermined numbers to facial movements that were later converted to affect labels. Twenty-five minutes of video-tape of the patient’s face was coded as she completed the expressive prosody tasks. The proportion of time the target muscles (zygomatic, corrigator, orbicularis oculi) were engaged was calculated.
c) Empathy Tasks
The ability to recognize and respond to affective experiences of another person or empathy functioning was examined using a standardized test, Interpersonal Reactivity Index (IRI; Davis, 1983). IRI test has been designed to test four components of empathy in adults - fantasy, perspective taking, empathic concern, personal distress. The scores of two RHS patients were excluded from the analysis as they were not able to complete the task.
Results
a) Imaging
Bilateral frontal and temporal atrophy was observed by the structural magnetic resonance imaging (MRI) and positron emission tomography (PET) scans. The comparison of the scans of the patient and healthy controls revealed atrophy (right greater than left) in superior and middle temporal gyrus (STG, MTG), anterior prefrontal cortex, pars triangularis of the inferior frontal gyrus (pt IFG), fusiform gyrus, insula, and putamen (see Fig. 1a). An overlay of the lesions of the RHS patients is shown in Fig 1b. (Figure 1a, 1b about here)
b) Neuropsychological Battery
Detailed descriptions of the standardized results of all the tests of cognitive functioning are summarized in Table 1. The patient performed within expected ranges on the majority of tests. The only impairments in her performance were on verbal fluency tasks, and on the initiation/ preservation subscale of the DRS-2. Specifically, her semantic fluency was in the borderline impaired range and her phonemic fluency was in the moderately impaired range, with phonemic fluency .7 of a standard deviation lower than semantic fluency. On the initiation/perseveration subscale, she performed in the borderline impaired range, losing points for motor perseverations (2 points lost) and semantic verbal fluency (4 points lost, I/P total = 31 out of 37).
Table 1.
Performance of bvFTD patient (Ms Y) on neuropsychological tests
| Domain/Test | Standard Score |
|---|---|
| WRAT-4 | SS = 109 |
| DRS-2 Total Score | ss = 9 |
| Attention | ss = 10 |
| Initiation/Perseveration | ss = 6 |
| Construction | ss = 10 |
| Conceptualization | ss = 12 |
| Memory | ss = 13 |
| MMSE | 30/30 |
| BNT (15-item) | 15/15 |
| HVLT-R | |
| Total Immediate recall | z =+1.74 |
| Delayed Recall | z =+1.22 |
| Recognition Discriminability | z =+0.14 |
| Trail Making Test, Part A | z =+0.28 |
| Symbol Digit Modality Test (written) | z =+0.31 |
| WAIS-IV Digit Span | |
| Digits Forward (max digits) | z =+0.38 |
| Digits Backward (max digits) | z = -0.38 |
| Digits Sequencing (max digits) | z =+1.07 |
| WMS-III Spatial span, total score | ss = 8 |
| COWAT (F,A,S) | Total words = 18 |
| T = 28 | |
| Category Fluency: Animals | Total words = 14 |
| T = 35 | |
| T= 51 | |
| Trail Making Test, Part B | |
| Tower of London | |
| Total moves | SS = 122 |
| Total correct | SS = 134 |
| Total time | SS = 104 |
| Rule violations | SS < 60 |
Note: T-scores have a mean of 50 and a standard deviation (SD) of 10, standard scores (SS) have a mean of 100 and a SD of 10, and scaled scores (ss) have a mean of 10 and a SD of 3. WRAT-IV = Wide Range Achievement Test, 4th Edition; DRS-2 = Dementia Rating Scale, 2nd Edition; MMSE = Mini Mental Status Examination; HVLT-R = Hopkins Verbal Learning Test – Revised; WAIS-IV = Wechsler Adult Intelligence Scale, 4th Edition; WMS-III; Wechsler Memory Scale, 3rd Edition. COWAT = Controlled Oral Word Association Test
On the EFPT tasks, the patient was able to complete all tasks. However, she required up to level 2 cuing (i.e., gestural guidance) for two out of four tasks (e.g. accurately measuring ingredients in cooking task, remembering to balance checkbook and correctly subtracting check amount from the balance in the bill paying task, recognizing that she did not have enough money to pay a bill in the bill paying task). These cues were required for the executive functioning components of organization, sequencing, and safety/judgment.
c) Emotional Prosody Tasks
The patient showed marked deficit in identifying emotions in all four central prosody identification tasks (word - 45%, monosyllabic - 38%, asyllabic - 38%, and pseudo - 32% correct). The profile of deficit seen on the word, monosyllabic and asyllabic tasks was consistent with right hemisphere damage. (Ross et al., 1997; Ross and Monnot, 2008). In comparison, the mean accuracy scores of the RHS patients were – word - 33%, monosyllabic - 66%, asyllabic -52%, and pseudo - 59% correct. As shown in Fig. 2, the patient did not show any difficulty in recognizing emotions from verbal scenarios (91% correct) as compared to the RHS patients (50% mean correct). The patient did not show any significant difference in modulating mean f0, f0 variance, speech rate or intensity for any emotion category and neutral utterances in repetition, consistent with the deficit profile observed after right hemisphere damage (Ross et al., 1997, Ross and Monnot, 2008), and expressing verbal scenarios or self-generated conversations about past life experiences.
Fig. 2.
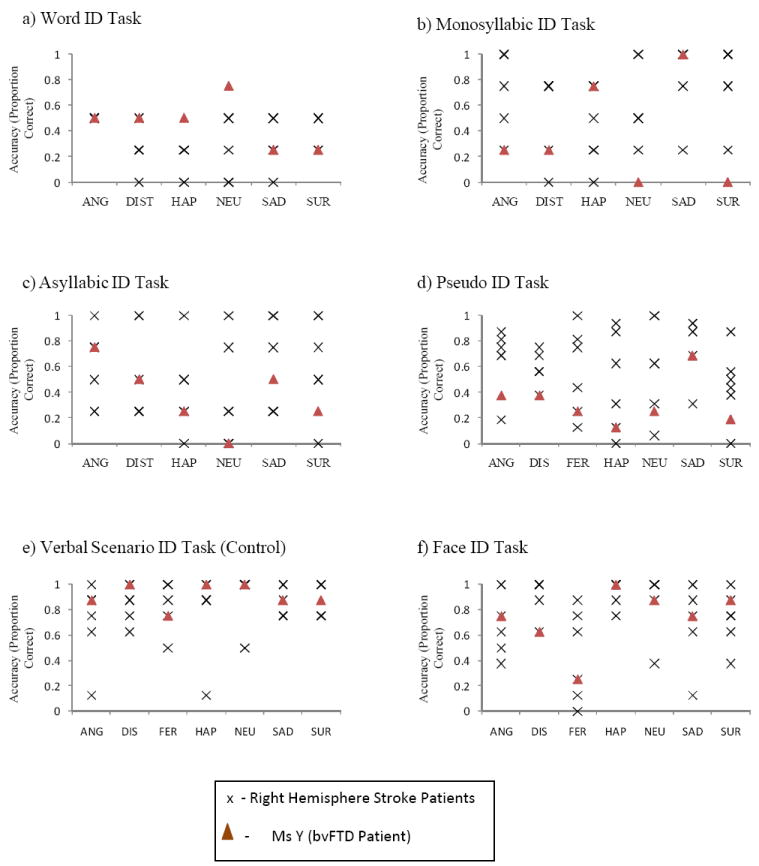
Performance of bvFTD patient compared with patients with right hemisphere stroke on the recognition of emotions (ANG – Angry, DIST – Disinterest, DIS – Disgust, HAP – Happy, SAD – Sad, SUR – Surprise, FER – Fear, NEU - Neutral) from prosodic, verbal and facial cues.
d) Facial Expression Tasks
The patient did not show any difficulty in recognizing emotions from facial expressions (73% correct) when compared to the accuracy scores of the RHS patients (54% mean correct; Fig. 2). However, the patient demonstrated very little facial expressions herself. During the repetition and prosody production tasks, she demonstrated no muscle movements in any of the target muscle groups. She performed similarly for the negative life events self-report task in that she generated no muscle movements. In contrast, during the positive life events self-report task, she demonstrated engagement of the zygomatic muscles for small epochs of time. The corrigator and orbicularis oculi were never engaged. The total proportion of time of visible muscle engagement was less than .035 seconds. Thus, the patient presented with a pattern of emotional processing deficits consistent with the syndrome of Motor Aprosodia with Pure Prosodic Deafness (Ross, 1981; see Case 10).
e) Empathy Tasks
The patient performed similarly to normative group for all the components of empathy. Her scores for the four empathy components were – fantasy = 11(female normative group = 18.8 ±5.17); perspective taking = 23 (female normative group = 18 ±4.9); empathic concern = 28 (female normative group = 21.7 ±3.8); personal distress = 17 (female normative group = 12.3 ±5). The mean and standard deviations of the RHS patients were - fantasy = 22 (4.8); perspective taking = 20 (5.8); empathic concern = 25 (3.3); personal distress = 7 (5.4).
Discussion
To advance our understanding of the cognitive and emotional profile of patients with bvFTD, we examined the performance of a patient diagnosed with bvFTD on various tasks of emotion processing from prosody and facial expressions, along with empathy cognitive functioning. The primary findings of this study were that the patient had a selective impairment in recognition of emotions from prosody, but not facial expressions, and marked reduction in production of emotions through prosody and facial expressions. In addition, the source of the deficits cannot be attributed to a generalized impairment in comprehension of emotions, as she could recognize emotions if utterances provided the information in verbal-semantic form rather than affective prosody alone as well as static facial expressions. Interestingly, the patient did not show any significant deficit on empathy tasks or on various cognitive measures of memory, attention or executive functioning. This case study provides preliminary evidence that patients with bvFTD may have marked deficits in processing emotion information from prosodic and facial cues during speech communication.
The current data provides some novel insight into the impairment of bvFTD patients in communication of emotion using two critical non-verbal channels – prosody and facial expressions. Ghacibeh & Heilman (2003) have previously shown that a patient with significant atrophy in right frontal lobe had severe impairment in expression of emotional prosody and facial expressions with spared comprehension of emotional prosody. Impairment of comprehension in addition to expression of emotional prosody in our patient is most likely due to significant atrophy involving both right frontal and temporal lobes. Also, other reports investigating patients with left frontotemporal atrophy have not observed deficits in either understanding or expressing emotions using prosody and facial expressions (Tsao, Dickey, & Heilman, 2004; Werner, et al., 2007). These findings are in line with the notion that right prefrontal and temporal regions are critical for processing emotional prosody as shown by previous studies in stroke patients (Adolphs, Damasio, & Tranel, 2002; Heilman, Bowers, Speedie, & Coslett, 1984; Pell, 1998; Ross & Monnot, 2008) and functional imaging (Bach, et al., 2008; Beaucousin, et al., 2007; Grandjean, et al., 2005; Kotz, et al., 2003). It can therefore be argued that the impairment in recognition of emotions from prosody as well as producing emotional utterances is a direct consequence of the patients atrophy involving the frontal and temporal regions of her right hemisphere. In fact, the bvFTD patient in our study performed worse than the RHS patients for most of our prosody tasks except the word identification task in which she seemed to perform similar to the RHS patients. As shown in Fig. 1a, the patient had significant atrophy in right prefrontal and STG areas which seems to be more extensive than that of the RHS patients (Fig. 1b). The differences in the performance of the RHS patients and our FTD patient could be a direct consequence of the variation in the region of atrophy.
Interestingly, the patient was able to recognize emotions from facial expressions but she had severe impairment in exploiting facial cues to express emotions. Consistent with other study findings of frontotemporal dementia (Ghacibeh & Heilman, 2003; Perry, et al., 2001), this patient demonstrated little to no facial expressions during emotionally-provoking tasks. When facial expressions were evoked, only the zygomatic muscles were engaged and only during expression of positive emotions. Some previous reports have shown that patients with FTD have difficulty in recognizing emotions from static facial expressions as well (Kipps, et al., 2009; Mendez, et al., 2006). Unlike our face stimuli, Kipps, Mioshi & Hodges (2009) included the faces that were morphed with multiple emotions in each picture and the patients had to identify the best representing emotions. One possible reason for the impairment in emotion recognition from faces in Kipps, Mioshi & Hodges (2009) investigation could be task difficulty. Another probable reason could be disease severity; becasue the FTD group in their study had marked deficits in activities of daily living unlike the patient in our study. Therefore, further investigations with varying task difficulty are warranted to examine the ability of the patients with bvFTD in emotion comprehension from facial expressions.
Also, the patient’s impairment in emotional prosody and facial expressions tasks do not seem to be a consequence of reduced cognitive capacities, as she showed no clinically significant impairments in cognitive functioning based upon neuropsychological testing and her performance on the functional assessment of executive functioning. Consistent with previously reported findings regarding lack of MMSE’s sensitivity to impairments in FTD patients (Giovagnoli, Erbetta, Reati F, & O., 2008; Torralva, Roca, Gleichgerrcht, Bekinschtein, & Manes, 2009), our patient achieved a perfect score on MMSE and scored above cutoffs across most measures used for dementia screening (DRS-2), except for initiation, which was a singular finding. Within the context of estimated at least high average premorbid functioning, her performances fell within expected ranges on measures of attention, working memory, confrontational naming, verbal and visual-spatial learning and memory, and visual-spatial reasoning. Furthermore, the patient required minimal assistance on three components of executive functioning on the EFPT assessment – organization, sequencing and judgment and showed insight into her illness. She did not show any difficulty in initiation or termination for any of the activities of independent living.
In sum, this study provides some novel and important insights into the emotional and cognitive deficits due to bvFTD in this individual. The profound social dysfunction of our patient with bvFTD could be in part due to her difficulty in processing emotional facial and prosodic cues during human communication. Impairment of comprehension and expression of emotional prosody and facial expressions may be included in the clinical profile of patients with greater right frontotemporal atrophy, in addition to hyper-religiosity, visual hallucinations and cross-modal sensory experiences that are associated with right temporal atrophy (Chan, et al., 2008). Impairment in processing emotional prosody should be used as an important clinical parameter for the diagnosis of bvFTD, especially in those patients with greater right hemisphere atrophy.
Acknowledgments
We thank Melissa Newhart and Cameron Davis for helping with testing of patients. This work was supported by NIH (NINDS) grant, R01NS047691 to AH.
Contributor Information
C. Dara, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA, cdara1@jhmi.edu.
L. Kirsch-Darrow, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, MD, USA, lkirschdarrow@jhmi.edu.
E. Ochfeld, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA, eochfeld@gmail.com.
A. Agranovich, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, MD, USA, aagrano1@jhmi.edu.
A.V. Faria, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA, fariaav@gmail.com.
E. Ross, VA Medical Center, Oklahoma City, OK, USA, Elliott-Ross@ouhsc.edu.
A.E. Hillis, Department of Neurology, Johns Hopkins University, Baltimore, MD, USA, ahillis1@jhem.jhmi.edu.
K. Kortte, Department of Physical Medicine and Rehabilitation, Johns Hopkins University, Baltimore, MD, USA, kbechto1@jhmi.edu.
References
- Adolphs R. Neural systems for recognizing emotion. Current Opinion in Neurobiology. 2002;12:169–177. doi: 10.1016/s0959-4388(02)00301-x. [DOI] [PubMed] [Google Scholar]
- Adolphs R, Damasio H, Tranel D. Neural systems for recognition of emotional prosody: a 3-D lesion study. Emotion. 2002;2(1):23–51. doi: 10.1037/1528-3542.2.1.23. [DOI] [PubMed] [Google Scholar]
- Bach DR, Grandjean D, Sander D, Herdener M, Strik WK, Seifritz E. The effect of appraisal level on processing of emotional prosody in meaningless speech. NeuroImage. 2008;42(2):919–927. doi: 10.1016/j.neuroimage.2008.05.034. [DOI] [PubMed] [Google Scholar]
- Baum CM, Morrison T, Hahn M, Edwards DF. Test Manual: Executive Function Performance Test. St Louis, MO: Washington University; 2003. [Google Scholar]
- Beaucousin V, Lacheret A, Turbelin M-R, Morel Ml, Mazoyer B, Tzourio-Mazoyer N. FMRI study of emotional speech comprehension. Cerebral Cortex. 2007;17(2):339–352. doi: 10.1093/cercor/bhj151. [DOI] [PubMed] [Google Scholar]
- Brandt J, Benedict RH. Hopkins Verbal Learning Test-Revised. Lutz, FL: Psychological Assessment Resources; 2001. [Google Scholar]
- Chaby L, Narme P. Processing facial identity and emotional expression in normal aging and neurodegenerative diseases. Psychologie et NeuroPsychiatrie du Vieillissement. 2009;7(1):31–42. doi: 10.1684/pnv.2008.0154. [DOI] [PubMed] [Google Scholar]
- Chan D, Anderson V, Pijnenburg Y, Whitwell J, Barnes J, Scahill R, Stevens JM, Barkhof F, Scheltens P, Rossor MN, Fox NC. The clinical profile of right temporal lobe atrophy. Brain. 2009 May;132(Pt 5):1287–98. doi: 10.1093/brain/awp037. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. Tower of London –Drexel University (ToLDx) Technical Manual. NY: Multi-Health Systems; 2001. [Google Scholar]
- Davis MH. Measuring individual differences in empathy: Evidence for a multidimensional approach. Journal of Personality and Social Psychology. 1983;44(1):113–126. [Google Scholar]
- Folstein MF, Folstein E. Mini-Mental State Examination, 2nd Edition (MMSE -2) Lutz, FL: Psychological Assessment Resources; 2010. Manual. [Google Scholar]
- Fusar-Poli P, Placentino A, Carletti F, Allen P, Landi P, Abbamonte M, et al. Laterality effect on emotional faces processing: ALE meta-analysis of evidence. Neuroscience Letters. 2009;452(3):262–267. doi: 10.1016/j.neulet.2009.01.065. [DOI] [PubMed] [Google Scholar]
- Ghacibeh GA, Heilman KM. Progressive affective aprosodia and prosoplegia. Neurology. 2003;60(7):1192–1194. doi: 10.1212/01.wnl.0000055870.48864.87. [DOI] [PubMed] [Google Scholar]
- Giovagnoli AR, Erbetta A, Reati F, B O. Differential neuropsychological patterns of frontal variant frontotemporal dementia and Alzheimer’s disease in a study of diagnostic concordance. Neuropsychologia. 2008;46(5):1495. doi: 10.1016/j.neuropsychologia.2007.12.023. [DOI] [PubMed] [Google Scholar]
- Grandjean D, Sander D, Pourtois G, Schwartz S, Seghier M, Scherer KR, et al. The voices of wrath: brain responses to angry prosody in meaningless speech. Nature Neuroscience. 2005;8:145–156. doi: 10.1038/nn1392. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Bowers D, Speedie L, Coslett H. Comprehension of affective and nonaffective prosody. Neurology. 1984;34:917–920. doi: 10.1212/wnl.34.7.917. [DOI] [PubMed] [Google Scholar]
- Izard CE, Doughterty LM, Hembree EA. A system for identifying affect expression by holistic judgment (AFFEX) Newark: University of Delaware, Computer Network Services and University Media Services; 1983. [Google Scholar]
- Kaplan E, Goodglass H, Weintraub S. Boston Naming test, 2nd edition (BNT-2) Lippincott Williams & Wilkins; 2001. [Google Scholar]
- Kipps CM, Mioshi E, Hodges JR. Emotion, social functioning and activities of daily living in frontotemporal dementia. Neurocase. 2009;15(3):182–189. doi: 10.1080/13554790802632892. [DOI] [PubMed] [Google Scholar]
- Kotz SA, Meyer M, Alter K, Besson M, von Cramon DY, Friederici AD. On the lateralization of emotional prosody: An event-related functional MR investigation. Brain and language. 2003;86:366–376. doi: 10.1016/s0093-934x(02)00532-1. [DOI] [PubMed] [Google Scholar]
- Kucharska-Pietura K, Phillips ML, Gernand W, David AS. Perception of emotions from faces and voices following unilateral brain damage. Neuropsychhologia. 2003;41:1082–1090. doi: 10.1016/s0028-3932(02)00294-4. [DOI] [PubMed] [Google Scholar]
- Lezak MD, Howieson DB, Loring DW, Hannay HJ, Fischer JS. Neuropsychological assessment. 4. New York, NY US: Oxford University Press; 2004. [Google Scholar]
- Mattis S, Jurica PJ, Leitten CL. Dementia Rating Scale Second Edition (DRS-2) Lutz: Psychological Assessment Resources; 2001. Manual. [Google Scholar]
- Mendez MF, McMurtray A, Licht E, Shapira JS, Sau RE, Miller BL. The scale for emotional blunting in patients with frontotemporal dementia. Neurocase. 2006;12(4):242–246. doi: 10.1080/13554790600910375. [DOI] [PubMed] [Google Scholar]
- Meyers JE, Meyers KR. Rey Complex Figure Test (RCFT) Lutz, FL: Psychological Assessment Resources; 1996. Manual. [Google Scholar]
- Pell MD. Recognition of prosody following unilateral brain lesion: influence of functional and structural attributes of prosodic contours. Neuropsychologia. 1998;36(8):701–715. doi: 10.1016/s0028-3932(98)00008-6. [DOI] [PubMed] [Google Scholar]
- Pell MD. Cerebral mechanisms for understanding emotional prosody in speech. Brain and language. 2006 doi: 10.1016/j.bandl.2005.04.007. In press. [DOI] [PubMed] [Google Scholar]
- Pell MD, Leonard CL. Facial expression decoding in early Parkinson’s disease. Cognitive Brain Research. 2005;23(2-3):327–340. doi: 10.1016/j.cogbrainres.2004.11.004. [DOI] [PubMed] [Google Scholar]
- Pell MD, Paulmann S, Dara C, Alasseri A, Kotz SA. Factors in the recognition of vocally expressed emotions: a comparison of four languages. Journal of Phonetics. 2009;37:417–435. [Google Scholar]
- Perry RJ, Rosen HR, Kramer JH, Beer JS, Levenson RL, Miller BL. Hemispheric dominance for emotions, empathy, and social behaviour: evidence from right and left handers with frontotemporal dementia. Neurocase. 2001;7(2):145–160. doi: 10.1093/neucas/7.2.145. [DOI] [PubMed] [Google Scholar]
- Rascovsky K, Hodges JR, Knopman D, Mendez MF, Kramer JH, Xie S, van Swieten JC, Seelaar H, Dopper EGP, Onyike CU, Hillis AE, Josephs KA, Boeve BF, Kertesz A, Seeley WW, Rankin KP, Johnson JK, Gorno-Tempini ML, Rosen H, Prioleau-Latham CE, Lee A, Kipps CM, Lillo P, Piguet O, Rohrer JD, Rossor MN, Warren JD, Fox NC, Galasko D, Salmon DP, Black SE, Mesulam M, Weintraub S, Dickerson BC, Diehl-Schmid J, Pasquier F, Deramecourt V, Lebert F, Pijnenburg Y, Chow TW, Manes F, Grafman J, Cappa SF, Freedman M, Grossman M, Miller BL. Sensitivity of revised diagnostic criteria for the behavioral variant of Frontotemporal Dementia. Brain. 2011;134:2456–77. doi: 10.1093/brain/awr179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross ED. The approsodias: functional-anatomic organazation of the affective components of language in the right hemisphere. Archives of Neurology. 1981;38:561–569. doi: 10.1001/archneur.1981.00510090055006. [DOI] [PubMed] [Google Scholar]
- Ross ED, Monnot M. Neurology of affective prosody and its functional-anatomic organization in right hemisphere. Brain and Language. 2008;104:51–74. doi: 10.1016/j.bandl.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Ross ED, Thompson RD, Yenkosky J. Lateralization of affective prosody in brain and the callosal integration of hemispheric language functions. Brain and Language. 1997;56:27–54. doi: 10.1006/brln.1997.1731. [DOI] [PubMed] [Google Scholar]
- Schirmer A, Kotz SA. Beyond the right hemisphere: brain mechanisms mediating vocal emotional processing. Trends in Cognitive Sciences. 2006;10(1):24–30. doi: 10.1016/j.tics.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Smith A. Symbol Digit Modalities test (SDMT) Los Angeles: Western Psychological Services; 1982. Manual (revised) [Google Scholar]
- Torralva T, Roca M, Gleichgerrcht R, Bekinschtein T, Manes M. Neuropsychological battery to detect specific executive and social cognitive impairments in early frontotemporal dementia. Brain. 2009;132(5):1299. doi: 10.1093/brain/awp041. [DOI] [PubMed] [Google Scholar]
- Tsao JW, Dickey DW, Heilman KM. Emotional prosody in primary progressive aphasia. Neurology. 2004;63:192–193. doi: 10.1212/01.wnl.0000132836.03040.2d. [DOI] [PubMed] [Google Scholar]
- Wechsler D, Coalson DL, Raiford SE. WAIS-IV technical and interpretive manual. San Antonio, TX: Pearson; 2008. [Google Scholar]
- Wechsler D. WMS-III Manual. San Antonio, TX: Pearson; 1998. [Google Scholar]
- Werner KH, Roberts NA, Rosen HJ, Dean DL, Kramer JH, Weiner MW, Miller BL, Levenson RW. Emotional reactivity and emotion recognition in frontotemporal lobar degeneration. Neurology. 2007 Jul 10;69(2):148–55. doi: 10.1212/01.wnl.0000265589.32060.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]


