Abstract
Purpose
The purpose of this case–control study was to draw attention to the possibility that poor oral hygiene resulting from infrequent and inadequate use of chewing sticks might be the sole cause of oral cancer in 60 patients investigated in the maxillofacial units of two specialist hospitals in eastern Nigeria.
Methods
Sixty cases and 60 controls made up the study population. We matched them for age, gender, period of admission and study site. The interview of all the participants contained data on demographic factors, family history of cancer, tobacco habits, oral hygiene, dietary habits and use of alcohol. We took biopsies of the lesions for histo-pathological examination. We entered the collected data into Microsoft excel package and transported it to Stata for generation of statistical test.
Result
Poor oral hygiene due to infrequent tooth brushing was associated with primary oral cancer in this patient sample. On the other hand, frequent tooth brushing was related to healthy status.
Conclusion
In the absence of other known carcinogens, poor oral hygiene may be the single factor that caused oral cancer in these subjects. Research is needed to investigate the pathological mechanism that is associated with this risk factor.
Electronic supplementary material
The online version of this article (doi:10.1007/s12663-012-0359-5) contains supplementary material, which is available to authorized users.
Keywords: Poor oral hygiene, Chewing sticks, Sole cause, Oral cancer, Nigeria
Introduction
The oral cavity plays many important functional roles in humans. Some examples are the preparation and formation of bolus, taste and swallowing. In addition, it is the main site of verbal human communication and the primary entrance for two systems vital to human function, namely, the gastrointestinal tract and respiratory systems. Poor oral hygiene and consequent oral cancer can destroy the integrity of this area. Oral cavity cancers are more common in males than in females, with a ratio of 3:1–4:1 [1]. The factors believed to cause oral cancer vary both geographically and culturally [2]. In India, cancer of the floor of the mouth accounts for 50 % of all cancers and 5 % of all cancers in the United States. Cultural variations and habits (e.g. good oral hygiene in the United States and betel chewing in India) are responsible for the difference [2]. Scully [3] and Nielsen et al. [4] have proposed a viral aetiology in the form of human herpes and human papilloma viruses (HPV). Wynder et al. [5] and Enwonwu and Meeks [6] have implicated malnutrition, dietary deficiency of iron and vitamins A and C as well as occupational exposure to nickel, textiles, and wood dust as cofactors in the genesis of oral cancer. In their study, Marshall et al. [7] made efforts to isolate these factors from tobacco and alcohol. However, whether these entities are cofactors or simply examples of poor compliance with overall healthcare is difficult to assess and has never been clearly elucidated [8].
Poor oral hygiene is one of the many factors, which together with tobacco and alcohol, have additive effects on oral cancer. In Nigeria, some people in the lower economic class, with little or no formal education, have poor oral hygiene. This group and probably similar groups in the developing world use chewing sticks for the purpose of dental and oral hygiene. Chewing sticks are fibrous logs, branches, struts or roots that the people in these regions produce from certain trees and shrubs. Depending on the species, chewing sticks contain trimethylamin, salt, silicates, resin, triterpenoids, diterpenoids, terpenoids, tannins, essential oils, alkaloids, amides, fluorides, silicons, saponins and cathadines. These components are known to be antibacterial, antiphlogistic, anticariogenous, astringent and haemolitic [9]. The producers tie them in bundles and offer them in markets and streets for a small amount of money. The user takes an end of the freshly prepared wood directly in his mouth and chews it until it fuzzes out like a brush. The cleaning process begins by scrubbing movements (up and down movements) along the anterior teeth only (Fig. 1). The problem is not with the chewing sticks per se, but lies in the fact that this group of users does not clean the posterior and the lingual areas of the teeth but only the front teeth for a short period and infrequently.
Fig. 1.
Cleaning the front teeth with chewing stick
We undertook this 5-year prospective study to investigate the potential role of poor oral hygiene as a probable single causative factor of oral cancer in a group that neglected or were ignorant of the importance of good oral hygiene. We would like to sensitize the stakeholders in Nigeria and in countries with similar cultural settings of the need to emphasize the importance of oral health through the print and electronic media.
Materials and Methods
We conducted this case–control study in two specialist hospitals in eastern Nigeria. The period of study was from January 2004 to December 2008. Patients with newly diagnosed carcinoma of the gum, tongue, floor of the mouth and other parts of the oral cavity (International Classification of Diseases [ICD], 9th revision, rubrics 141, 143–145) made up the oral cancer case group. These patients were late presentations in stages III and IV of the TNM system of classification (T = primary tumour; N = lymph node metastasis; M = distant metastasis). We did not include patients with malignant neoplasms on the lip, the salivary glands, and nasopharynx (ICD 140, 142 and 147). We selected control groups from relatives and friends who were attending patients admitted for diseases other than cancer and matched them by age and gender. We considered patients with other neoplasms (ICD 140–239) or mental diseases (ICD 290–319) ineligible. The control patients were frequent users of chewing sticks. In all, we gathered 60 histopathologically confirmed oral cavity squamous cell cancer cases. Of these, forty-eight (80 %) were men, and 12 (20 %) were women. We gathered 60 control patients. Forty-five of them (75 %) were men and 15 (25 %) were women. All these patients were Nigerians and occupied the lowest rung of the socio–economic ladder. They were mainly subsistent farmers, casual workers, artisans and traders. Their ages ranged from 35 to 65 years.
After obtaining approval from the appropriate ethical committees and the subjects’ informed consent, we used a standardized questionnaire to elicit information from cases and controls on health information, sociodemographic variables, environmental and occupational exposures, oral hygiene (with emphasis on the use of chewing sticks), tobacco and alcohol consumption, and diet. The interviews focused on frequency of tooth cleaning before the onset of the condition leading to hospitalization and lasted on the average 60 min. We carried them out before initiating treatment so that the patient’s ability to communicate or to recall information would not be compromised. We interrupted the interviews if the patient experienced difficulty communicating because of pain or speech problems. We entered the patients into an aggressive combined modality treatment regimen consisting of chemotherapy, surgery, and/or radiotherapy because their disease condition had advanced considerably. In addition to the interviews, we carried out the following investigations: Sillness-Löe plaque index (SLPI) 1964 [10], Sulcus bleeding index (SBI), human immunodeficiency virus (HIV) and HPV screening. We did not carry out genetic mapping because we do not have the technical expertise and the funds to do it.
Statistical Analysis
Data collected was entered into Microsoft excel package and transported to Stata1 for the generation of statistical test. We reported the percentages obtained with 95 % confidence intervals (95 CIs). We used Pearson’s correlation coefficient and Chi-square with Mantel-Haenszel 1-way analysis to assess and establish associations between risk factor variable (infrequent use of chewing stick) and oral cancer. Odds ratios (OR) were also calculated at 95 % CIs and adjusted for age, sex, occupation, diet and oral hygiene according to SLPI, 1964 [10]. Population attributable risk fractions were also estimated for different ages and sexes according to the distribution of risk factors among educated and uneducated cases. The level of significance (P value) was set at 0.05 where P < 0.05 is considered significant and P > 0.05 non-significant.
Results
We established not only erythema of the gingiva in all cancer patients but also gross decay of the occlusal surfaces, which appeared as discoloured cavitations. The Sillness and Löe plaque index [10] had a score of 2–3 for all cancer patients and 0–1 among the controls. The SBI was 4–5 for all cancer patients and 0–2 for controls (Table 1). Malignant tumours were present in the oral cavities of the cancer patients (Fig. 2) and these were in stages III and IV according to the TNM system of classification. Extraoral palpation of the mandibular glands revealed enlargement and tenderness in 12 cancer patients. None of these pathological features was seen in the control group. The HIV and HPV screenings were negative in all cases.
Table 1.
Comparison of the Sillness-Löe plaque index (SLPI) and Sulcus bleeding index (SBI) amongst patients and control group
| Oral cancer patients | Control groups | |
|---|---|---|
| Sillness-Löe plaque index | 2–3 | 0–1 |
| Sulcus bleeding index (SBI) | 4–5 | 0–2 |
Fig. 2.
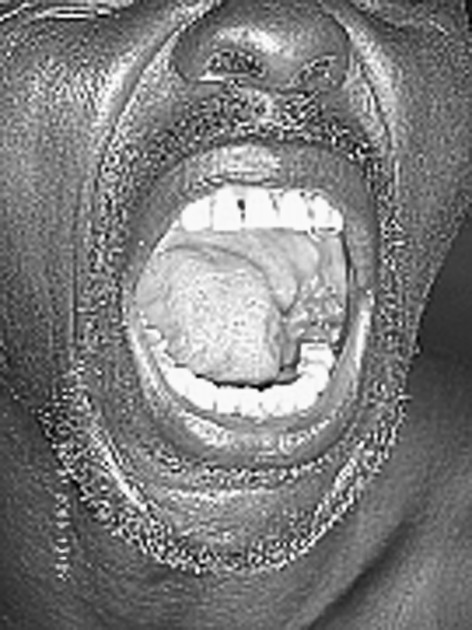
Malignant tumour in the mouth
Table 2 shows the distribution of the main demographic characteristics of the 60 cases and 60 controls included in this study. Majority of cases were male (80 %). More than half were aged 50 years and beyond. Most of them were illiterates (83.3 % of cases and 25 % of controls). Farmers and labourers (66.6 % of cases and 75 % of controls) were mainly involved.
Table 2.
Distribution of subjects according to demographic variables
| Variables | Categories | Cases (%) | Controls (%) |
|---|---|---|---|
| Age (years) | 35–40 | 9 (15) | 10 (16.7) |
| 41–49 | 12 (20) | 15 (25) | |
| 50–60 | 18 (30) | 20 (33.3) | |
| 60–65 | 21 (35) | 15 (20) | |
| Gender | Male | 48 (80) | 42 (70) |
| Female | 12 (20) | 18 (30) | |
| School level | Illiterate | 50 (83.3) | 15 (25) |
| Elementary school | 10 (16.7) | 45 (75) | |
| Occupation | Farmers | 20 (33.3) | 25 (41.7) |
| Labourers | 20 (33.3) | 20 (33.3) | |
| Artisans | 15 (25) | 12 (20) | |
| Traders | 5 (8.4) | 3 (5) |
The distribution of the cancer sites was as follows: other sites of the mouth (retromolar fossa—so-called coffin’s corner, palate and cheek) 25 (41.7 %), tongue 14 (23.3 %), floor of the mouth 12 (20 %), gum 9 (15 %), (Table 3). None of the subjects reported cancer in general and oral cancer in particular in the family history. They consumed neither alcohol nor smoked cigarettes and they did not exhibit any signs of malnutrition. There was also no case of drug abuse. Oral cancer patients, however, reported that they cleaned their teeth and tongues with chewing sticks twice or thrice weekly, and spent only about one min for the procedure. In contrast, the control group cleaned their teeth and tongues with chewing sticks at least twice daily, each cleaning action lasting up to 15 min. Farmers and labourers were at an approximately three fold increased risk compared with artisans in both genders. The number of children did not relate to oral cancer risk in either gender. Neither the cases nor the controls used factory-manufactured toothbrushes and none wore dentures or went for regular dental check-ups. However, knowledge and education played a significant role in the development of oral cancer (P = 0.000*; OR = 38.8) (Table 4). There was no difference in the diet habits of both case and control groups. Their staple diets consisted mainly of gari, yam, cassava (all starchy foods), fruits, vegetables and meat or fish. Control patients had more teeth. Moreover, they cleaned their teeth frequently and extensively. Table 5 shows a statistical difference between the cases and control regarding infrequent use of chewing stick and the risk of developing oral cancer (P = 0.0000*; CI = 95 %).
Table 3.
Distribution of the cancer sites
| Mouth cancer sites | Cases (%) |
|---|---|
| Other sites | 25 (41.7) |
| Tongue | 14 (23.3) |
| Floor of the mouth | 12 (20) |
| Gum | 9 (15) |
| Total | 60 (100) |
Table 4.
Statistics on the level of education and the risk of developing oral cancer
| Patients | Educated | Uneducated | Χ2 | P value |
|---|---|---|---|---|
| Cases | 10 | 50 | 38.8 | 0.000* |
| Control | 45 | 15 |
Confidence interval (CI) = 95 %, odd ratio (OR) = 5.65
Table 5.
Statistics on the use of chewing stick and the risk of developing oral cancer
| Patients | Infrequent use | Frequent use | Χ2 | P value |
|---|---|---|---|---|
| Cases | 60 | 0 | 119 | 0.0000* |
| Control | 0 | 60 |
Confidence interval (CI) = 95 %, odd ratio (OR) = undefined
Discussion
Oral cancer is a multifactorial disease where no single clearly recognizable cause has been found. The precise role of any individual factor or condition is poorly understood. These factors include social habits (tobacco, alcohol, betel chewing), infections (bacterial, fungal, viral), extrinsic factors (poor oral hygiene, actinic radiation, industrial hazards) and intrinsic factors (genetic, nutritional deficiencies, immunodeficiency/suppression) [8].
This case–control study focused on the infrequent and inadequate use of chewing sticks to clean the teeth as a possible sole cause of oral squamous cell carcinoma (OSCC). Our results indicate that infrequent use of chewing sticks is associated with oral cancer. This is consistent with the hypothesis that infrequent tooth brushing was associated with cancer of the oral cavity, particularly of the tongue, gum and other sites of the mouth [11–13]. According to these authors, the effect was independent, persisting after extensive adjustments for cofounders. Zheng et al. [13]. observed a sevenfold elevated risk for men who never brushed their teeth. Gonzalez-Henandez et al. [14] found that daily tooth brushing is a preventive factor against oral cancer. Graham et al. [15] observed that inadequate dentition, measured by the number of missing teeth and the amount of decay, was associated with the risk of oral cancer. This is consistent with our findings because the poor oral hygiene of our patients, which was the result of infrequent and inadequate tooth cleaning, manifested itself in the high SLPI, high SBI (Table 1), the erythema of the gingivae and the gross decay of the occlusal surfaces. In contrast, Marshall et al. [7]. and Talamini et al. [16] observed that poor oral hygiene had only a weak, negligible bearing on the risk of oral cancer. We did not observe any correlation between other known causes of cancer and OSCC in our cases because our patients did not have the habit of consuming alcohol or tobacco and were not exposed to other known causative agents.
The sites involved, in descending order were other sites of the mouth (retromolar fossa—so-called coffin’s corner, palate and cheek) 25 (41.7 %), tongue 14 (23.3 %), floor of the mouth 12 (20 %), gum 9 (15 %), (Table 3). Only 10 (16.7 %) of the cases and 45 (75 %) of the controls had the primary school leaving certificate (Table 2). It is evident from our study that lack of education is related to oral hygiene. This finding is in agreement with those from India [17], Americas [18, 19], China [13] and Europe [16, 20].
All the cases presented late (stages III and IV). It is common in Nigeria that the poorest, illiterate persons amongst whom using chewing sticks as toothbrushes is the usual practice, do go to hospitals for advanced lesions but they seldom attend as outpatients for less severe diseases. In such cases, they consult fake medical practitioners, herbalists and prayer houses [21].
Chewing sticks work as well as toothbrushes if one uses them properly [22, 23]. However, the duration and method of application were incorrect amongst the cases that we studied. Our oral cancer cases cleaned the anterior teeth for about 1–2 min, the posterior teeth not at all and neglected the lingual surfaces. In addition, they performed this procedure only once or twice weekly. The control group, on the other hand, though with similar socio–economic background, was aware of the importance of dental hygiene and used chewing sticks adequately.
To the authors’ knowledge, this is the first single study that investigated the consequences of the infrequent and improper use of chewing sticks amongst members of the lower socio–economic class in the West African sub-region. Although it suggests a relationship between poor oral hygiene due to infrequent and inadequate use of chewing sticks and the genesis of oral cancer, only a large prospective study would provide adequate data to resolve some of the thorny issues associated with this topic. In respect to these issues, Guha et al. [22] described the results of two multicentric case control studies in Latin America and central Europe. These studies comprised of thousands of cases. They are to date the largest and most comprehensive studies of oral health and hygiene in relation to head and neck cancer. The results indicate that periodontal diseases ensuing from inadequate and infrequent tooth brushing as indicated by poor oral condition of the mouth and missing teeth may be independent causes of cancers of the mouth and esophagus.
We are, nonetheless, inclined to agree with the conclusion of Velley et al. [12] and Guha et al. [22] that poor oral hygiene due to infrequent tooth brushing, is a risk factor for mouth cancer and that this association is unlikely to be due to insufficient control of cofoundings. The fact that this risk factor is modifiable emphasizes the need for increasing awareness among the public and policy makers as a first step in the prevention and control of OSCC. In view of the limited financial, material, personnel and organizational situation in developing countries in the area of dentistry and medicine, there are difficulties in achieving widespread behavioral changes in some cultural and economic settings. As ever, primary prevention remains the ideal. In spite of this, inertia and often perversity of human behaviour and custom transcend time and culture, thus remaining a formidable obstacle [24].
Electronic supplementary material
Footnotes
Stata is general-purpose statistical software created in 1985 by StatCorp. The name “Stata” was formed by blending “statistics” and “data”.
References
- 1.Ethem G, Campana J (2007) Malignant tumors of the floor of the mouth. eMedicine (serial online) 1–14. http:/www.emedicine.com/ent/TOPIC258.HTM. Accessed 18 Dec 2007
- 2.McMicheal AJ. Cancers of the head and neck. In: Bourke G, editor. The epidemiology of cancer. Beckenham: Croon Helm; 1983. [Google Scholar]
- 3.Scully C. Viruses and oral squamous carcinoma. Eur J Cancer. 1992;28B:57–59. doi: 10.1016/0964-1955(92)90014-r. [DOI] [PubMed] [Google Scholar]
- 4.Nielsen H, Norrild B, Vedtofte P, Praetorius F, Reibel J, Holmstrup F. Human papilloma virus in oral premalignant lesions. Eur J Cancer. 1996;32B:264–270. doi: 10.1016/0964-1955(96)00011-5. [DOI] [PubMed] [Google Scholar]
- 5.Wynder EL, Bross IJ, Feldman RM. A study of the etiological factors in cancer of the mouth. Cancer. 1957;10:1300–1323. doi: 10.1002/1097-0142(195711/12)10:6<1300::AID-CNCR2820100628>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 6.Enwonwu CO, Meeks VI. Bionutrition and oral cancer in humans. Crit Rev Oral Biol Med. 1995;32:55–62. doi: 10.1177/10454411950060010401. [DOI] [PubMed] [Google Scholar]
- 7.Marshall JR, Graham S, Haughey BP. Smoking, alcohol, dentition and diet in the epidemiology of oral cancer. Eur J Cancer. 1992;28B:9–15. doi: 10.1016/0964-1955(92)90005-l. [DOI] [PubMed] [Google Scholar]
- 8.Lockhart PB, Norris CM, Jr, Pulliam C. Dental factors in the genesis of squamous cell carcinoma of the oral cavity. Oral Oncol. 1998;34:133–139. doi: 10.1016/S1368-8375(97)00086-9. [DOI] [PubMed] [Google Scholar]
- 9.Oji C. Chewing sticks and oral health—review. Niger Dent J. 1997;11:27–32. [Google Scholar]
- 10.Silness J, Löe H. Periodontal disease in pregnancy II. Correlation between oral hygiene and periodontal conditions. Acta Odontol Scand. 1964;22:121–135. doi: 10.3109/00016356408993968. [DOI] [PubMed] [Google Scholar]
- 11.Maier H, Zoller J, Herrman A, Kriess M, Heller WD. Dental status and oral hygiene in patients with head and neck cancer. Otolaryngol Head Neck Surg. 1993;108:655–661. doi: 10.1177/019459989310800606. [DOI] [PubMed] [Google Scholar]
- 12.Velley AM, Franco EL, Schlecht, et al. Relationship between dental factors and risk of upper aerodigestive tract cancer. Oral Oncol. 1998;34:284–291. [PubMed] [Google Scholar]
- 13.Zheng T, Boyle P, Hu H, et al. Dentition, oral hygiene, and risk of oral cancer: a case study in Beijing, People’s Republic of China. Cancer Causes Control. 1990;1:235–241. doi: 10.1007/BF00117475. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Hernandez MJ, Moreno-Lopez LA, Esparza-Gomez GC, Gonzalez-Navaro A, Cerero-Lapeidra R, Dominguez-Rojas V. Risk of oral cancer associated with tobacco smoking, alcohol consumption, and oral hygiene: a case-control study in Madrid, Spain. Oral Oncol. 2000;36:170–174. doi: 10.1016/S1368-8375(99)00084-6. [DOI] [PubMed] [Google Scholar]
- 15.Graham S, Dayal H, Rohrer T. Dentition, diet, tobacco and alcohol in the epidemiology of oral cancer. J Natl Cancer Inst. 1977;59:1611–1616. doi: 10.1093/jnci/59.6.1611. [DOI] [PubMed] [Google Scholar]
- 16.Talamini R, Vaccarella S, Barbone T, et al. Oral hygiene, dentition, sexual habits and risk of oral cancer. Br J Cancer. 2000;83:1238–1242. doi: 10.1054/bjoc.2000.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Balaram P, Sridhar H, Rajkumar T, et al. Oral cancer in southern India: the influence of smoking, drinking, paan-chewing and oral hygiene. Int J Cancer. 2002;98:440–445. doi: 10.1002/ijc.10200. [DOI] [PubMed] [Google Scholar]
- 18.Fernanadez-Garrote L, Herrero R, Ortiz Reyes RM. Risk factors for cancer of the oral cavity and oro-pharynx in Cuba. Br J Cancer. 2001;85:46–54. doi: 10.1054/bjoc.2000.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sankaramarayana R, Duffy SW, Padmakumary G. Tobacco chewing, alcohol and nasal snuff in cancer of the gingiva in Kerala, India. Br J Cancer. 1989;60:638–643. doi: 10.1038/bjc.1989.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bundgaard T, Wildt J, Frydenberg M, Elbrond O, Nielsen JE. Case-control study of squamous cell cancer of the oral cavity in Denmark. Cancer Causes Control. 1995;6:57–67. doi: 10.1007/BF00051681. [DOI] [PubMed] [Google Scholar]
- 21.Oji C. Awareness of oral tumours. Niger J Surg Sci. 1993;3:50. [Google Scholar]
- 22.Guha N, Boffetta P, Filho VW, Neto JE, Shangina O, Zaridze D, Curado PM, Koffman S, et al. Oral health and risk of squamous cell carcinoma of the head and neck and esophagus: results of two multicentric case control studies. Am J Epidemiol. 2007;166(10):1159–1173. doi: 10.1093/aje/kwm193. [DOI] [PubMed] [Google Scholar]
- 23.Hessel B African “chewing sticks” works as well as toothbrush. Public Release on the Internet March 2000. http://www.eurekalert.org/pub_releases/2000-03/ACS-Aswa-0103100.php-6.6KB-PublicPressReleases. Accessed 24 Dec 2007
- 24.Oji C. Late presentation of orofacial tumours. J Craniomaxillofac Surg. 1999;27:1–6. doi: 10.1016/S1010-5182(99)80020-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


