Abstract
Sialolithiasis accounts for the most common cause of diseases of salivary glands. The majority of sialoliths occur in the submandibular gland or the Wharton’s duct. This article discusses review of literature, predisposing factors, signs and symptoms, diagnostic methods and various modalities available for the management of sialolithiasis. This case report presents a case of sialolith of a large size in the left Wharton’s duct, which was explored and removed via an intra-oral approach.
Keywords: Sialolithiasis, Wharton’s duct, Transoral sialolithotomy
Sialolithiasis, also called salivary calculi or stones, is the occurrence of calcareous concretions in the salivary ducts or glands [1]. Sialolithiasis accounts for more than 50% of the diseases of major salivary glands, and is the most common cause of acute and chronic infections of the salivary glands [2].
Review of Literature
Sialolithiasis usually occurs from the third to fifth decade of life, and slightly more often in males (4:3) [3–5], and is rare in children [4, 5]. The reported incidence of sialolithiasis is 80–94% in submandibular duct or gland, 5–18% in parotid gland, and 2% or less in sublingual gland [4]. The most common site is submandibular gland and Wharton’s duct, probably, as a consequence of mucinous saliva, a tortuous duct, gravity, and the small orifice of the duct [6]. Simultaneous sialolithiasis in more than one salivary gland is rare [2]. Sialolithiasis occurs in the sublingual and minor salivary glands and ducts in the decreasing order of frequency [1]. The right and left glands and ducts are equally affected [3, 4], although bilateral occurrence is rare [4]. However, multiple sialoliths in the same duct or gland are common [4]. Submandibular gland sialoliths have been reported to be radiopaque in 80–94.7% of cases [4, 7, 8].
Although the exact cause of sialolithiasis remains unclear, some sialoliths may be related to dehydration, which increases the viscosity of the saliva; reduced food intake, which decreases the demand for saliva [9]; or certain medications that lower the production of saliva, such as anti-histaminics, anti-hypertensives and anti-psychotics, and antidepressants [9, 10]. The lack of saliva can also be caused by several circumstances, including aging, Sjogren’s syndrome, and radiotherapy of the head and neck region [10].
The predisposing factors are: alkaline pH of saliva, increased calcium concentration in the saliva of submandibular gland, and the tortuous course of the duct [4]. Some other factors inherent to the submandibular gland are: longer and larger caliber of the duct, flow against gravity, slower flow rates and higher mucin content of the saliva [11]. The formation of sialolith is not related to any systemic derangement in calcium or phosphate metabolism [12].
A study conducted by Sheman and McGurk [13] indicated no link between water hardness and sialolithiasis or sialadenitis, suggesting that high calcium intake might not lead to sialolithiasis. Patients with gout and patients on diuretic therapy may be predisposed to sialolithiasis [6].
Calcified concretions in salivary ducts or glands are formed by deposition of calcium salts around a central nidus, which may consist of desquamated epithelial cells, bacteria, foreign bodies or products of bacterial decomposition [1]. The foreign bodies reported in association with sialolith formation include a tooth brush bristle, finger nail sliver, wood splinter, hair, blade of grass, and a fish bone [14]. The annual growth rate of established sialolith has been estimated to be 1 mm per year [15].
Chemically, sialoliths are composed of varying ratios of organic and inorganic substances. The organic substances are glycoproteins, mucopolysaccharides, and cellular debris [16].
The inorganic substances are mainly calcium carbonates and phosphates. Calcium, magnesium, and phosphate ions each comprise between 20 and 25%, with other minerals (manganese, iron, and copper) making up the remainder. The chemical composition consists of microcrystalline apatite (Ca5 [PO4]3OH), as a principal mineral, or whitlockite (Ca3[PO4]2), which is the next common mineral [17, 18]. Apatite is the most prevalent component present throughout the stone, while whitlockite is mainly found in the core [18, 19]. Other crystalline forms include brushite and weddellite, which are present in small amounts, mainly at the periphery of sialoliths [18]. Statherins prevent crystallization of supersaturated calcium phosphate in ductal saliva and oral fluid. Phytate and magnesium are also potent inhibitors of apatite crystallization, by chelating the released ionized calcium [20].
Many patients with sialolithiasis involving a duct of a major salivary gland complain of moderate to severe pain, particularly just before, during and after meals, owing to psychic stimulation of salivary flow, associated with swelling of the salivary gland [1, 21]. In some instances the swelling is diffuse and simulates a cellulitis [1], and presence of swelling of the soft tissues overlying the associated gland or duct [22]. Secondary infection often supervenes with fever, and redness [21]. Tenseness, tenderness, trismus, decreased or absence of salivary flow, or purulent discharge at the orifice of the duct may be present [21, 23]. The obstruction of the duct prevents the free flow of saliva, and the resultant stagnation or accumulation of saliva under pressure produces pain and swelling.
Careful bimanual palpation of the floor of the mouth, along Wharton’s duct on a posterior to anterior direction may reveal the presence of a sialolith [24]. There is inability to milk saliva from the duct orifice [21]. Occasionally the sialolith presents no remarkable symptom, and the only evidence may be a firm mass palpable in the duct or gland, particularly in the more peripheral portion of the duct, and if they are of sufficient size [1].
There are various radiological methods for diagnosis of submandibular sialolithiasis. The conventional methods for detecting obstructions in the salivary ductal system are occlusal films for the sialolith in the Wharton’s duct, lateral oblique mandibular films or panoramic radiographs for sialoliths in the hilum or substance of the submandibular gland.
In the early stages, sialoliths may be too small or insufficiently mineralized to be evident radiographically [25]. Sialoliths in ducts appear as elongated or smooth cylindrical radiopaque structures, and round or oval when located within the gland [4]. The reported incidence of radiolucent submandibular sialoliths is 20–43% [8]. The other advanced imaging modalities also used include: sialography [22], xeroradiography (especially for radiolucent sialoliths) [3], ultrasonography [1], scintigraphy [26] and computerized tomography and magnetic resonance imaging [27].
The treatment depends on the number, size, and location of the sialolith, or whether it is present in the duct or the gland. There are various modalities of treatment employed. If the sialolith is small and single, conservative management may be attempted with local heat, massage and sialogogues [24]. Smaller sialoliths which are located sufficiently peripherally; near the orifice of the duct, are removed by manipulation or milking of the gland. This can be done with the aid of lacrimal probes and dilators for opening the duct [24]. The sialoliths in the anterior part of the duct are treated by sialolithectomy through a ductotomy performed intraorally, usually under local anaesthesia [21]. The sialoliths in the posterior part of the duct are treated by sialolithectomy through a ductotomy performed intraorally, preferably under general anaesthesia [21]. Multiple sialoliths in the duct, or below the posterior edge of mylohyoid muscle [21], or at the hilum of the gland or within the gland, or if the gland has been damaged by recurrent infection and fibrosis, may require surgical removal [28, 29], and those patients who do not respond to conservative therapy [29]. It is performed preferably through an extraoral approach and under general anaesthesia [21].
There are two approaches to submandibular sialoliths that avoid the use of surgery. These are Extracorporeal Shortwave (ESW) lithotripsy [15, 21], and Intracorporeal Endoscopic lithotripsy, or sialendoscopy or sialoendoscopy [23]. ESW lithotripsy refers to a non-invasive method of application of shock waves from an externally applied lithotripter, which causes fragmentation of the sialoliths. These fragments then pass through the duct, as the saliva is stimulated and enhanced by the use of sialogogues; or are removed by normal salivary flow or rinsing with the aid of a catheter [21]. The first report on the use of shock waves to fragment sialoliths was in 1986 by Marmary [30, 31]. In ESW lithotripsy, the average size of fragments produced was about 0.7 mm [32].
Sialendoscopy or Sialoendoscopy is a minimally invasive technique, in which shock waves are delivered directly to the surface of the sialolith lodged within the duct without damaging the adjacent tissues, such as, Nahlieli Sialendoscope (Karl Storz GmbH, Tuttlingen, Germany) [33]. Microendoscopes are introduced into the duct systems, to allow direct visualization and evaluation of the intraductal and intraglandular microanatomy [33].
Case Report
An 84-year-old edentulous female patient, presented with chief complaints of pain of 15 days duration; and pus discharge of 3–4 days duration, from left side of the floor of the mouth in the posterior region. She also complained of inability to eat with the dentures for the previous 2 years. In addition, there was difficulty in swallowing, and low grade intermittent fever for a period of one and half months.
The review of systems was remarkable, as she had hypertension for the previous 5 years and was taking following medications: Tab Aten 25 mg, and Tab Ecosprin 75 mg, both once daily, for the previous 5 years. She was using upper and lower dentures for the last 12–13 years. Extra-oral examination revealed a mildly tender and diffuse swelling in left submandibular region. There was no local rise of temperature and no decrease in the mouth opening. The patient’s vital signs were within normal limits. Intra-oral examination revealed a diffuse swelling of the floor of mouth in the left posterior region. Purulent discharge was visible from the area of the swelling. A firm mass in the posterior region could be felt just below the mucosa of floor of the mouth with bimanual palpation. The salivary flow in both the Stenson’s ducts and the right Wharton’s duct was normal, while the flow in the left Wharton’s duct could not be elicited.
The radiological examination comprised of a panoramic radiograph (OPG) which showed an oval radiopaque shadow superimposing over the superior border of the left ramus of the mandible (Fig. 1), and a RVG picture, which was taken by 2.0 sensor of the left angle of the mandible with an angle of −10°, focussing on the lesion, with long-cone intraoral X-ray tube. It shows an oval radiopaque shadow below the angle of the mandible. The superior margin of the lesion is slightly overlapping the inferior border of the mandible. This is suggestive of an over-sized sialolith in the vicinity of submandibular salivary gland (Fig. 2).
Fig. 1.
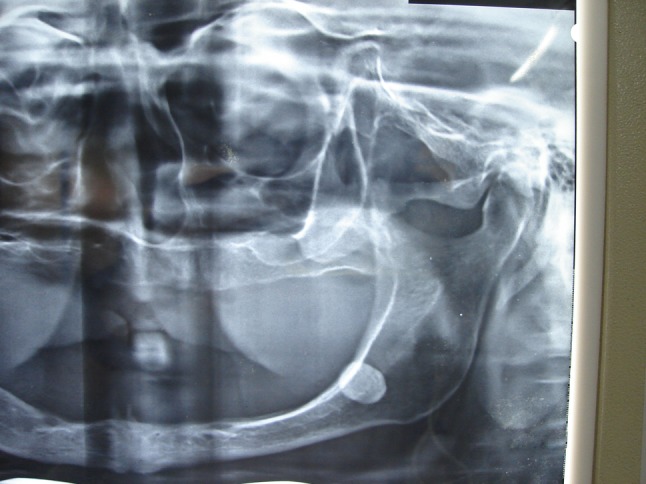
Cropped OPG showing the sialolith superimposed with the angle of the mandible
Fig. 2.

RVG picture showing an oval radiopaque shadow below the angle of the mandible
On the basis of detailed history, clinical findings, and radiological features, the case was diagnosed as chronic suppurative submandibular sialadenitis secondary to sialodocholithiasis in the left Wharton’s duct. Surgical removal of sialolith was planned under general anaesthesia. The results of routine blood and urine investigations, and chest radiograph were within normal limits. Fitness was obtained from the treating physician. The patient was admitted to MH Saboo Siddiq General Hospital, Mumbai. Under GA, with nasoendotracheal intubation, with monitoring, the surgery was performed under routine aseptic precautions via an intra-oral approach. A 3/0 black silk suture was taken posterior to the mass in the floor of the mouth on the left side, with the help of an aneurysmal needle, to prevent posterior displacement of the sialolith, and was removed at the end of the surgery. An incision of 1.5 cm in length was taken in the mucosa of the floor of the mouth. Blunt dissection was carried out in the direction of the sialolith. A small incision was taken over the duct overlying the sialolith. With manipulation, the sialolith was pushed out of the duct and was removed. The area of the surgery and the duct were thoroughly irrigated with betadine saline solution. A thin paediatric cannula (24 gauge, 70 mm) was passed into the duct through the ductotomy site, and was secured in place with 3/0 black silk in the region of the orifice. The duct and the incision in the mucosa were sutured by 5/0 and 3/0 coated vicryl, respectively.
The patient received antibiotics and analgesic drugs, and soft diet from the first post-operative day. She was discharged from the hospital on the third post-operative day, and was advised to continue antibiotics for 5 more days, betadine mouthwash, tab. multivitamin each for 2 weeks, and high protein diet for 4 weeks. She was also advised to take tab. Vitamin C chewable, as a sialogogue, ½ tablet twice a day, from the seventh post-operative day for a period of 1 week. The paediatric cannula (70 mm; 24 G), along with its securing suture was removed on the fifth post-operative day. Two weeks later, it was observed that clear saliva was flowing from the orifice of left Wharton’s duct. Four weeks later, there was absolutely no pain and no pus discharge, and the site was healing satisfactorily.
Discussion
The incidence of sialolithiasis in Wharton’s duct is not uncommon. In many instances, the size of sialolith is so small that it may come out with a normal salivary flow. It is only when the size of the sialolith is much bigger than the size of the duct, then it will cause mechanical obstruction and thereby the symptoms, which need surgical intervention. In this case report, the size of the sialolith, and the position of the stone (posterior), made surgical removal difficult; even under general anaesthesia, due to the mass of the tongue and the surgical anatomy. Hence successful intraoral removal assumes importance.
Surgical intervention is the most appropriate mode of treatment, and depends primarily on the location of the sialolith. Traditionally, sialoliths in Wharton’s duct and gland can be divided into three groups; those in the anterior part of the duct, those in the posterior part of the duct, and those at the hilum of the gland or within the gland. Seward [21, 34] described the principles of surgical management. Sialoliths present at the orifice or in the distal half of the duct, are removed transorally, because of easy accessibility. Initially, a suture is passed around Wharton’s duct posterior to the sialolith and is used as a traction suture to occlude the duct, and for tenting the duct upwards; and for prevention of the sialolith from posterior displacement during surgical procedure. A longitudinal incision is placed over the mucosa of the floor of the mouth directly over the duct over the sialolith. Dissection is carried out down to the duct. The duct is incised in the longitudinal direction and the sialolith is removed. The duct is irrigated and explored to remove microliths, and the edges are sutured to the oral mucosa to form a new orifice. Opening the duct in this manner allows marsupialization of the orifice and prevents stricture [21, 34].
The sialoliths in the posterior part of the duct are technically more difficult to remove by a transoral approach, as the duct lies deeper; there is poor accessibility and proximity to vital anatomical structures such as lingual nerve [21, 39]. These require good retraction of the tongue, as well as the help by the assistant to push the gland upward into the mouth from extraorally. Hence, these sialoliths are preferably removed under general anaesthesia [21, 35]. The sialoliths at the hilum of the gland or within the gland are best treated by removal of the gland, by conventional submandibular approach [21, 35].
There are three different ways of dealing with the duct: suture the mucosa and not to suture the duct [34], suture the duct and mucosa separately, and suture the duct with the cannula inside the duct and suture the mucosa. In the first instance, there are chances that the sutures will give way and there are chances of fistula formation. The distal (anterior) part of the duct will undergo atrophy and fibrosis. In the second instance, it will give rise to stenosis and fibrosis. The third instance can be an ideal situation, where we have two layer closure, keeping the duct intact and its opening patent. It maintains the anatomical continuity of the duct, maintains the distal part of the duct with natural opening, and prevents stenosis. It restores the function of the entire gland [36].
The paediatric cannula is inserted from the ductotomy site and is first passed into the posterior part of the duct. This part of the duct is dilated and hence passage of the cannula is easy. Then the other end of the cannula is inserted from the ductotomy site and inserted in the anterior part of the duct. This part of the duct is usually atrophied and hence passage of the cannula is sometimes difficult. In such circumstances, sometimes, a small cut is made on the orifice of the duct so as to allow the exit of the cannula. The cannula is then sutured to the adjacent part of the mucosa of the floor of the mouth [36].
Sialoliths are commonly 1–10 mm in size, but giant sialoliths (greater than 3.5 cm) have been reported occasionally [37]. A review of the literature by Ledesma-Montes et al. [37] found only 16 reported cases of sialoliths having a size or 3.5 cm or greater. Giant sialoliths are a rare finding; their sizes vary approximately from 1.5 to 7 cm, and their weight varies in the literature [37]. The submandibular gland hosts the largest stones with the largest reported one being 6 cm in length [38]. The size of the sialolith removed in our case was 16 × 11 × 6 mm (Figs. 3).
Fig. 3.
Sialolith in various dimensions
There are conflicting reports whether the architecture and function of the gland are adversely affected or not secondary to obstructive salivary gland disease. Makdissi et al. [39] after studying glandular function after intraoral removal of sialoliths from the hilum of the submandibular gland concluded that glandular function improves to varying degrees in most patients after the removal of a sialolith.
Long-term obstruction in the absence of infection can lead to atrophy of the gland with resultant lack of secretory function and ultimately fibrosis [40]. Some clinicians advocate removal only if there is severe impairment in function; or if the gland is non-functional. If the gland is functional, periodic follow-up examinations are indicated. An atrophic, fibrotic submandibular gland is of little clinical consequence, and excision may not be necessary, if asymptomatic [26]. In this case report, 2-weeks post-operatively, it was observed that clear saliva was flowing from the orifice of left Wharton’s duct. We believe, in this case, the gland had started functioning, and therefore, excision was not indicated. Stimulation of salivary gland with sialogogues, however, is advised.
The intraoral surgical intervention can lead to complications. One such complication is lingual nerve anaesthesia. Occasionally, ranulas can form due to the disruption of the sublingual salivary duct. Excision of the submandibular gland by an external approach carries a 0–8% risk of permanent or temporary facial (marginal mandibular branch) nerve palsy [41]. Sialo-cutaneous fistula is also a possibility with megaliths because of the obstruction of the duct resulting in stasis, infection and subsequent rupture through the skin [38].
Conclusion
Sialolithiasis is not an uncommon disorder of the ducts and parenchyma of salivary glands. In this case report, the authors have presented a case which was treated by sialolithectomy in the posterior part of Wharton’s duct under general anaesthesia. Surgical excision of the gland is undertaken, if there are multiple sialoliths in the duct; or if the sialolith is in the substance of the gland, or in cases of recurrent sialadenitis secondary to sialolithiasis. The future holds great promise due to the development of non-invasive techniques such as extracorporeal shock wave lithotripsy and endoscopic lithotripsy.
References
- 1.Shafer WG, Hine MK, Levy BM. A textbook of oral pathology. 4. Philadelphia: WB Saunders; 1983. pp. 561–562. [Google Scholar]
- 2.Zenk J, Benzel W, Iro H. New modalities in the management of human sialolithiasis. Minim Invasive Ther. 1994;3:275–284. doi: 10.3109/13645709409153022. [DOI] [Google Scholar]
- 3.Haug RH, Bradrick JP, Indresano A. Xeroradiography in the diagnosis of non-radiopaque sialoliths. Oral Surg. 1989;67:146–148. doi: 10.1016/0030-4220(89)90319-8. [DOI] [PubMed] [Google Scholar]
- 4.Lustman J, Regev E, Melamed Y. Sialolithiasis. A survey on 245 patients and a review of the literature. Int J Oral Maxillofacial Surg. 1990;19:135–138. doi: 10.1016/S0901-5027(05)80127-4. [DOI] [PubMed] [Google Scholar]
- 5.Akker HP, Busemann-Sokole E. Submandibular gland function following transoral sialolithectomy. Oral Surg. 1983;56:351–356. doi: 10.1016/0030-4220(83)90341-9. [DOI] [PubMed] [Google Scholar]
- 6.Pollack CV, Jr, Severance HW. Sialolithiasis: case studies and reviews. J Emerg Med. 1990;8:561–565. doi: 10.1016/0736-4679(90)90450-A. [DOI] [PubMed] [Google Scholar]
- 7.Perrotta RJ, Williams JR, Selfe RW. Simultaneous bilateral parotid and submandibular gland calculi. Arch Otolaryngol. 1978;104:469–476. doi: 10.1001/archotol.1978.00790080051014. [DOI] [PubMed] [Google Scholar]
- 8.Isacsson G, Isberg A, Haverling M, Lundquist PG. Salivary calculi and chronic sialoadenitis of the submandibular gland: a radiographic and histologic study. Oral Surg Oral Med Oral Path. 1984;58:622–627. doi: 10.1016/0030-4220(84)90090-2. [DOI] [PubMed] [Google Scholar]
- 9.Schenkels LCP, Veerman ECI, Amerongen AVN. Biochemical composition of human saliva in relation to other mucosal fluids. Crit Rev Oral Biol Med. 1995;6(2):161–175. doi: 10.1177/10454411950060020501. [DOI] [PubMed] [Google Scholar]
- 10.Valdez lH, Fox PC. Diagnosis and management of salivary dysfunction. Crit Rev Oral Med Biol. 1993;4:271–277. doi: 10.1177/10454411930040030301. [DOI] [PubMed] [Google Scholar]
- 11.Work WP, Hecht DW. Inflammatory disease of major salivary glands. In: Paparella MM, Shumrick DA, editors. Otolaryngology vol 13. Philadelphia: WB Saunders; 1980. pp. 2235–2243. [Google Scholar]
- 12.Rajendran R, Sivapathasundharam B. Shafer’s textbook of oral pathology. 5. New Delhi: Elsevier; 2007. p. 754. [Google Scholar]
- 13.Sheman JA, McGurk M. Lack of correlation between water hardness and salivary calculi in England. Br J Oral Maxillofac Surg. 2000;38:50–53. doi: 10.1054/bjom.1999.0074. [DOI] [PubMed] [Google Scholar]
- 14.Abe K, Higuchi T, Kubo S, Oka M. Submandibular sialadenitis due to a foreign body. Br J Oral Maxillofac Surg. 1990;28:50–52. doi: 10.1016/0266-4356(90)90012-A. [DOI] [PubMed] [Google Scholar]
- 15.Rauch S, Gorlin RJ. Disease of the salivary glands. In: Gorlin RJ, Goldmann HM, editors. Thomas’ oral pathology. St Louis: CV Mosby; 1970. pp. 997–1003. [Google Scholar]
- 16.Ashby RA. The chemistry of sialoliths: stones and their homes. In: Norman JED, McGurk M, editors. Color atlas and text of the salivary glands: diseases, disorders, and surgery. London: Mosby-Wolfe; 1995. [Google Scholar]
- 17.Escudier MP, McGurk M. Symptomatic sialoadenitis and sialolithiasis in the English population: an estimate of the cost of hospital treatment. Br Dent J. 1999;186:463–466. doi: 10.1038/sj.bdj.4800141. [DOI] [PubMed] [Google Scholar]
- 18.Yamamoto H, Sakae T, Takagi M, Otake S. Scanning electron microscopic and X-ray microdiffractometeric studies on sialolith-crystals in human submandibular glands. Acta Pathol Jpn. 1984;34:47–53. doi: 10.1111/j.1440-1827.1984.tb02181.x. [DOI] [PubMed] [Google Scholar]
- 19.Anneroth G, Eneroth CM, Isacsson G. Crystalline structure of salivary calculi: a microradiographic and microdiffractometric study. J Oral Pathol. 1975;4:266–272. doi: 10.1111/j.1600-0714.1975.tb01866.x. [DOI] [PubMed] [Google Scholar]
- 20.Grases F, Santiago C, Simonet BM, Costa-Bauza A. Sialolithiasis: mechanism of calculi formation and etiologic factors. Clin Chim Acta. 2003;334:131–136. doi: 10.1016/S0009-8981(03)00227-4. [DOI] [PubMed] [Google Scholar]
- 21.Miloro M, Ghali GE, Larsen PE, Waite PD. Peterson’s principles of oral and maxillofacial surgery vol 1. 2. Canada: BC Decker Inc; 2004. [Google Scholar]
- 22.Ord RA (2000) Salivary gland disease. In: Fonseca RJ (Chief editor). Williams TP, Stewart JCB (eds) Oral and maxillofacial surgery: surgical pathology, vol 5, 1st edn. WB Saunders Company, Philadelphia
- 23.Yoshimura Y, Inoue Y, Odagawa T. Sonographic examination of sialolithiasis. J Oral Maxillofac Surg. 1989;47:907–912. doi: 10.1016/0278-2391(89)90372-8. [DOI] [PubMed] [Google Scholar]
- 24.Williams MF. Sialolithiasis. Otolaryngol Clin North Am. 1999;32(5):819–834. doi: 10.1016/S0030-6665(05)70175-4. [DOI] [PubMed] [Google Scholar]
- 25.Wood NK, Goaz PW. Differential diagnosis of oral lesions. 4. St. Louis: Mosby Year Book; 1991. pp. 565–566. [Google Scholar]
- 26.Yoshimura Y, Morishita T, Sugihara T. Salivary gland function after sialolithiasis: scintigraphic examination of submandibular glands with 99 m-Tc-Pertechnetate. J Oral Maxillofac Surg. 1989;47:704–710. doi: 10.1016/S0278-2391(89)80009-6. [DOI] [PubMed] [Google Scholar]
- 27.Topazian RG, Goldberg MH. Oral and maxillofacial infections. 2. Philadelphia: WB Saunders; 1987. p. 248. [Google Scholar]
- 28.Seward GR. Anatomic surgery for salivary calculi. IV. Calculi in the intraglandular part of the submandibular duct. Oral Surg Oral Med Oral Pathol. 1968;25:670. doi: 10.1016/0030-4220(68)90032-7. [DOI] [PubMed] [Google Scholar]
- 29.Novotny GM. Submandibular sialolithiasis: transoral excision. J Otolaryngol. 1989;18:354–356. [PubMed] [Google Scholar]
- 30.Marmary Y. A novel and non-invasive method for the removal of salivary gland stones. Int J Oral Maxillofac Surg. 1986;15:585–587. doi: 10.1016/S0300-9785(86)80063-1. [DOI] [PubMed] [Google Scholar]
- 31.Iro H, Schneider H, Fodra C, et al. Shockwave lithotripsy of salivary duct stones. Lancet. 1992;339:1333–1336. doi: 10.1016/0140-6736(92)91968-E. [DOI] [PubMed] [Google Scholar]
- 32.Zenk J, Werner G, Hosemann MD, Iro H. Diameters of the main excretory ducts of the adult human submandibular and parotid gland—a histological study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:576–580. doi: 10.1016/S1079-2104(98)90294-3. [DOI] [PubMed] [Google Scholar]
- 33.Nahlieli O, Baruchin AM. Sialendoscopy: three year experience as a diagnostic and treatment modality. J Oral Maxillofac Surg. 1997;55:912–918. doi: 10.1016/S0278-2391(97)90056-2. [DOI] [PubMed] [Google Scholar]
- 34.Seward GR. Anatomic surgery for salivary calculi. II. Calculi in the anterior part of the submandibular duct. Oral Surg Oral Med Oral Pathol. 1968;25:287–293. doi: 10.1016/0030-4220(68)90002-9. [DOI] [PubMed] [Google Scholar]
- 35.Seward GR. Anatomic surgery for salivary calculi. III: calculi in the posterior part of the submandibular duct. Oral Surg Oral Med Oral Pathol. 1968;25:525–531. doi: 10.1016/0030-4220(68)90294-6. [DOI] [PubMed] [Google Scholar]
- 36.Chitre AP. Personal communication
- 37.Ledesma-Montes C, Garces-Ortiz M, Salcido-Garcia JF, Hernandez-Flores F, Hernandez-Guerrero JC. Giant sialolith: case report and review of the literature. J Oral Maxillofac Surg. 2007;65(1):128–130. doi: 10.1016/j.joms.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 38.Vittal U, Shetty SC, Manvikar P, Kini U, Gupta S. Giant (2002) Sialolith (megalith) of submandibular salivary gland. Aus J Otolaryngol. April 2002
- 39.Makdissi J, Escudier MP, Brown JE, Osailan S, Drage N, McGurk M. Glandular function after intraoral removal of salivary calculi from the hilum of the submandibular gland. B J Oral Maxillofac Surg. 2004;42(6):538–541. doi: 10.1016/j.bjoms.2004.08.006. [DOI] [PubMed] [Google Scholar]
- 40.Marchal F, Kurt A, Dulguerov P, Becker, Oedman M, Lehmann W. Histopathology of submandibular glands removed for sialolithiasis. Ann Otol Rhinol Laryngol. 2001;110:464–469. doi: 10.1177/000348940111000513. [DOI] [PubMed] [Google Scholar]
- 41.Ellies M, Laskawi R, Arglebe C, Schott A. Surgical management of nonneoplastic diseases of the submandibular gland. A follow up study. Int J Oral Maxillofac Surg. 1996;25:285–289. doi: 10.1016/S0901-5027(06)80058-5. [DOI] [PubMed] [Google Scholar]



