Abstract
To evaluate the efficacy of autologous platelet rich fibrin in soft tissue healing and bone regeneration in mandibular third molar extraction sockets.
Method
The study was conducted in 20 patients visiting in outpatient department of Oral and Maxillofacial Surgery, requiring extraction of bilateral mandibular third molar, following extraction platelet rich fibrin (PRF) was placed in one extraction sockets, the other socket was studied as the control sites with no PRF. The patient were assessed for post operative pain, soft tissue healing and trabecular pattern in healing bone. Radiological assessment of the extraction site was done for period of 3 months to evaluate the change in bone density.
Results
Pain was less in study side compare to control site soft tissue was healing was better in study site. Evaluation of trabecular bone formation started earlier in PRF site compare to control site. The evaluation of bone density by radiological assessment showed the grey level value calculated after 3 months at the PRF site well comparatively higher than the average baseline value of the bone density at the extraction site in control site.
Conclusion
The study showed that autologous PRF is biocompatible and has significantly improved soft tissue healing. Bone regeneration and increase in bone density in extraction sockets. However, a more elaborate study with a large number of clinical cases is essential to be more conclusive regarding its efficacy.
Keywords: Platelet rich fibrin, Bone density, Soft tissue healing, Pain
Introduction
Maxillofacial reconstructions, oral implants, regenerative procedures etc. are highly dependent on successful regeneration and healing, and one of the great challenges faced in clinical research in development of bioactive surgical additives regulating inflammation and increasing healing. Bone regenerative techniques including graft materials, protein and barrier membrane are often used to improve the bone quality. Healing in tissues is mediated by variety of signalling proteins. Understanding of this process at microcellular level is still not complete, but it is proven fact that platelets do play an important role in wound healing [1]. Platelet rich plasma is an autologous concentration of human platelets in small volume of plasma and it contains seven fundamental protein growth factors [2].
Platelet rich fibrin belongs to a next generation of platelet concentrate geared to simplified preparation without biochemical blood handling [3]. Platelet rich fibrin has come up as second generation platelet concentrate with cicatricial properties. Its production protocol attempts to accumulate platelets and release cytokines in a fibrin clot [4].
Physiology of Platelet Rich Fibrin (PRF)
Platelet rich fibrin is an immune and platelet concentrate collecting on a single fibrin membrane, containing all the constituents of a blood favourable for healing and immunity. Through platelet and leukocyte, cytokines play an important part in the biology of this biomaterial; the fibrin matrix supporting them certainly constitutes the determining element responsible for the real therapeutic potential of PRF. To understand the biologic effect of this fibrin matrix, it is important to divide clinical observations into four highly specific aspects of healing angiogenesis, immune control, harnessing the circulating stem cells, and wound protection by epithelial cover. Angiogenesis, immunity and epithelial cover are the three keys to healing and soft tissue maturation. PRF is able to simultaneously support the development of these three phenomena. The angiogenesis property of fibrin matrix is explained by the 3 dimensional structures of the fibrin gel and by the simultaneous action of cytokines trapped in the meshes [5]. The structure and mechanical properties of the fibrin matrix are important factors. The rigidity of the matrix considerably influences the capillary formation by endothelial cells in response to fibroblast growth factor (FGFb) or vascular endothelial growth factor (VEGF) stimulation [6].
Furthermore main angiogenesis soluble factors such as FGFb, VEGF and platelet derived growth factor (PDGF) are included in fibrin gels [7]. Fibrin and fibrinogen degradation products (FDP) stimulate the migration of neutrophil and increase the membrane’s expression of CD11c/CD18 receptor. This receptor permits adhesion of the neutrophil to endothelium and fibrinogen as well as the transmigration of neutrophils. Moreover, the phagocytosis of neutrophils and the enzymatic degradation process are modulated by FDP [8] and hence fibrin also plays an important role in immunity control. Studies have shown that PRF appears to be superior to collagen as a scaffold for human periosteal cell proliferation [9] and, rapid healing of the wound is observed without pain, dryness or purulent complications [7].
Technical Aspect of PRF Preparation
Preparation of PRF is simple and require quite minimal armamentarium i.e sterilized test tubes or vacutainers, 5 cc disposable syringes and a centrifuge.
Approximately 5–10 ml of venous blood is drawn from patient and transferred to test tubes or vacutainers. These vacutainers/test tubes are then kept in centrifuge and the machine is set to work on 3,000 revolutions per minute for 10 min. This procedure separates blood into three fractions i.e. lower fraction containing red blood cells, middle fraction containing fibrin clot and upper fraction containing straw coloured cellular plasma (Fig. 1), so the middle fraction containing PRF is separated from blood (Fig. 2).
Fig. 1.

Lower fraction (LF) containing red blood cells, middle fraction (MF) containing fibrin clot and upper fraction (UF) containing straw coloured cellular plasma
Fig. 2.
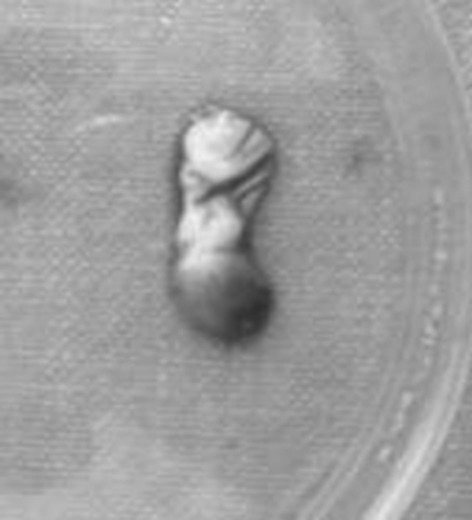
PRF—separated from blood
Important aspect in this procedure is the efficient and quick handling of the blood as there is no anticoagulant used in the procedure and delay can cause the blood sample to coagulate before generation of PRF clot.
This study was undertaken at the Department of Oral and Maxillofacial Surgery, after obtaining ethical committee clearance. This study involved both male and female patients, who were referred to the department of oral and maxillofacial surgery for removal of mandibular 3rd molars.
Inclusion Criteria
Patient aged between 18 and 50 years
Patients requiring bilateral mandibular 3rd molar extraction
ASA grade 1
Patient was non-smoker and non-alcoholic
After obtaining the complete history patient was examined clinically and were explained about the procedure, its complications and the follow up period involved in the study. Informed consent was taken; study sample included 20 patients requiring bilateral 3rd molar extraction. All patients were operated by a single operator. All patients underwent bilateral removal of 3rd molar in single appointment and autologous PRF of that patient was prepared as per the above mentioned protocol. PRF was then placed in the extracted socket on one side and other side was taken as control group. Both sockets were closed primarily by resorbable sutures.
All patients were recalled on day 1, 3, 7, 1st, 2nd and 3rd month post-operatively for follow up study.
Clinical evaluation included assessment of pain, soft tissue healing. Pain was evaluated by visual analogue scale. Evaluation of soft tissue healing by Landry and Turnbull [10].
IOPA radiograph was taken pre-operatively and 4, 8, 12 week post-operatively to assess and compared radiographic bone density between PRF sites and non PRF sites. All data was statistically analyzed with the help of student t test and ANOVA test.
Results
Following the completion of clinical study on the patients, the measurement and data taken from all the patients were tabulated for statistical studies and after the analysis of data following observation was made.
There were 10 (50%) male subject and 10 (50%) female subject who participated in the study. The patients who participated in the studies were of 18–50 years with mean age of 32 years.
Results of Clinical Assessment
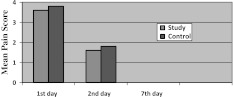
Assessment of Pain (Chart I)
Chart I.
Assessment of pain using VAS post operative
Assessment of pain was visual analogues scale of the 1st day showed mean pain score of 3.6 in study site and 3.8 in control site. On 3rd day mean pain score was 1.6 in the study site and 1.8 in control site. On seventh day pain was 0 in both control and study site. Though pain was less in study site compared to control side there was no statistically significant difference between study and control group and 1st, 3rd day (P = 0.005) and 7th day (P = 0.035).
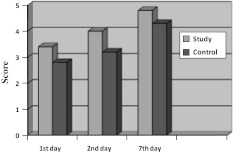
Assessment of Healing Index of Soft Tissue (Chart II)
Chart II.
Assessment of healing index post operative
Assessment of soft tissue healing index by Landry, Turnbell and Howley showed mean scores on 1st day of 3.4 in study site, 2.9 in control site. On 3rd day 4 in study site, 3.2 in control site and on 7th day mean score of 4.8 in a study site and 4.3 in control site. By doing repeated ANNOVA measure test for study and control group healing was better in study site compared to control site. Between 3rd and 7th day P value for 3rd day was P = 0.022 and 7th day P = 0.015. There was significant difference between study and control site in all 20 patients.
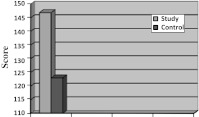
Radiographic Assessment (Chart III)
Chart III.
Assessment of bone density on post operative radiographs
Radiographic assessment at 4 week showed trabecular bone formation in 8 patients at study site but absent in all 20 control site. At 8 week trabecular bone formation seen in 18 patients in study site but only in 10 patients in control site (P = 0.063). There was no significant difference between study and control site. At 12 week trabecular bone formation was seen in all 20 patients including test and control site. Assessment of bone density (Gray level value) at 12 week showed that average gray scale value for PRF (study) site (146.9) was comparatively higher than non-PRF (control) site (123).
Discussion
There are several allografts, xenograft or alloplastic graft materials commonly used for bone regeneration procedures like freezed dried bone grafts, demineralised freezed dried bone grafts, hydroxyapatite, bioactive glass etc. which have shown to possess good osteoinductive and osteoconductive properties, but risk of disease transmission and unpredictable outcome many a times, led to search of materials which can independently produce predictable regeneration or can improve properties of these graft materials [11].
Platelet rich fibrin was first described by Choukroun et al. in France. It has been referred as second generation platelet concentrate. A report of clinical trails comparing the growth factors content of PRF and PRP was presented by Dohan and Diss at the second international Symposium on growth factors held in May 2005. Combining the growth factors has been shown to accelerate bone repair and promote fibroblast proliferation, and increase tissue vascularity, rate of collagen formation, mitosis of mesenchymal stem cells and endothelial cells, as well as osteoblasts, playing key roles in the rate and extent of bone formation. This activity, together with increased vessel ingrowth, is mediated by PDGF and TGF [12]. Its chief advantages include ease of preparation and lack of biochemical handling of blood which makes this preparation strictly autologous. It provides adhesiveness and tensile strength for clot stabilization. Fibrin is natural guide of angiogenesis, traps the circulating stem cells, and provides wound protection by epithelial cover. Several authors have demonstrated that a fibrin matrix provide an optimal support to mesenchymal stem cells [13, 14] which contribute to regeneration of bone cells and many other tissues and helps in osseous defect healing. Because of all of these powerful effects on tissue regeneration, a growing number of human clinical studies have detailed the use of growth factors in reconstructive oral and maxillofacial surgery, periodontal surgery, implants, and sinus grafting [15].
Its preparation is technically easy and cost effective as the steps of addition of thrombin or anticoagulant are eliminated. Few studies have shown that PRP releases growth factors before there is cell outgrowth in surrounding tissues, thereby limiting its regeneration potential which is not the issue seen with PRF due to slow polymerisation in its formation [16, 17].
Our results regarding enhanced soft tissue healing and increased rate of bone formation are due to above mentioned properties of PRF.
Conclusion
In this study, autologous PRF was used as an adjunct to promote healing and osseous regeneration in human mandibular 3rd molar extraction site. The improvement of wound healing, decrease in pain and increase in bone density signifies and highlights the use of PRF as a valid method in promoting and accelerating soft and hard tissue regeneration. The procedure of PRF preparation is simple, cost effective and demonstrated good results. The present study was done with follow up of 3 months; further clinical trials with longer duration of follow up with larger sample size should be done to get more affirmative and conclusive results.
References
- 1.Gassling VL, Acil Y, Springer IN, et al. Platelet-rich plasma and platelet-rich fibrin in human cell culture. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108:48–55. doi: 10.1016/j.tripleo.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 2.Vivek Gk, Sripathi Rao BH. Potential for osseous regeneration of platelet rich plasma: a comparative study in mandibular third molar sockets. J Maxillofac Oral Surg. 2009;8(4):308–311. doi: 10.1007/s12663-009-0075-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dohan DM, Choukroun J, Diss A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E37–E44. doi: 10.1016/j.tripleo.2005.07.008. [DOI] [PubMed] [Google Scholar]
- 4.Brown LF, Lanir N, McDonagh J. Fibroblast migration in fibrin gel matrices. Am J Pathol. 1993;142:273–283. [PMC free article] [PubMed] [Google Scholar]
- 5.Hinsbergh VW, Collen A, Koolwijk P. Role of fibrin matrix in angiogenesis. Ann NY Acad Sci. 2001;936:426–437. doi: 10.1111/j.1749-6632.2001.tb03526.x. [DOI] [PubMed] [Google Scholar]
- 6.Nehls V, Herrmann R. The configuration of fibrin clots determines capillary morphogenesis and endothelial cell migration. Microvasc Res. 1996;51:347–364. doi: 10.1006/mvre.1996.0032. [DOI] [PubMed] [Google Scholar]
- 7.Choukroun J, Diss A, Simonpieri A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E56–E60. doi: 10.1016/j.tripleo.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Kazura JW, Wenger JD, Salata RA, Budzynski AZ, Goldsmith GH. Modulation of polymorphonuclear leukocyte microbicidal activity and oxidative metabolism by fibrinogen degradation products D and E. J Clin Invest. 1989;83:1916–1924. doi: 10.1172/JCI114098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gassling V, Douglas T, Warnke PH. Platelet-rich fibrin membranes as scaffolds for periosteal tissue engineering. Clin Oral Implant Res. 2010;21:543–549. doi: 10.1111/j.1600-0501.2009.01900.x. [DOI] [PubMed] [Google Scholar]
- 10.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HC1 in the treatment of periodontal post-surgical patients. Res Clin Forum. 1988;10:105–118. [Google Scholar]
- 11.Sunitha Raja V, Munirathnam Naidu E. Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res. 2008;19(1):42–46. doi: 10.4103/0970-9290.38931. [DOI] [PubMed] [Google Scholar]
- 12.Anil Kumar K, Geetha A, Unasudhakar Platelet-rich fibrin: a novel root coverage approach. J Indian Soc Periodontol. 2009;13(1):50–54. doi: 10.4103/0972-124X.51897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bensaid W, Triffitt JT, Blanchat C. A biodegradable fibrin scaffold for mesenchymal stem cells transplantation. Biomaterials. 2003;24:2497–2502. doi: 10.1016/S0142-9612(02)00618-X. [DOI] [PubMed] [Google Scholar]
- 14.Boo JS, Yamada Y, Okazaki Y, Hibino Y, Okada K, Hata K, et al. Tissue-engineered bone using mesenchymal stem cells an a biodegradable scaffold. J Craniofac Surg. 2002;13:231–239. doi: 10.1097/00001665-200203000-00009. [DOI] [PubMed] [Google Scholar]
- 15.Lozada JL, Caplanis N, Proussaefs P, Willardsen J, Kammeyer G. Platelet-rich plasma application in sinus graft surgery: part I-background and processing techniques. J Oral Implantol. 2001;27:38–42. doi: 10.1563/1548-1336(2001)027<0038:PPAISG>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 16.Vinazzer H. Fibrin sealing: physiologic and biochemical background. Facial Plast Surg. 1985;2:291–295. doi: 10.1055/s-0028-1085288. [DOI] [PubMed] [Google Scholar]
- 17.Dohan DM, Choukroun J, Diss A, Dohan SL, et al. Platelet-rich fibrin (PRF): a second generation platelet concentrate, part II: platelet related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2006;101:E45–E50. doi: 10.1016/j.tripleo.2005.07.009. [DOI] [PubMed] [Google Scholar]