Abstract
Purpose
To evaluate the incidence and recovery of persistent sensory disturbances of the infraorbital (IO) nerve after isolated zygomatic complex fractures with various treatment methods.
Methods and Results
The study was inclusive of isolated unilateral zygomatic complex fractures and fractures of IO rim .Tests performed were Pin prick and Electrical detection threshold test. The evaluation was done preoperatively, after 1 month and after 6 months of surgery. The results suggested that neurosensory disturbance was present in all the patients with zygomatic complex fractures. At 1 month post-operatively some sensory deficit was present in all the patients on the affected side. After 6 months all the patients showed near to normal improvement comparable to normal side.
Conclusion
Study shows that earlier the surgical intervention, more the recovery of the nerve injury is appreciable during the 1 and 6 months follow up period.
Keywords: Infraorbital nerve, Zygomatic fracture, Paresthesia
Introduction
The face, and in particular the oral and peri-oral regions, are among the areas with the highest density of peripheral receptors, presumably because of their remarkable importance in daily life. Pain, temperature, touch, pressure, and proprioception (sense of body position) are transmitted centrally from the peri-oral structures via the inferior alveolar, lingual, infraorbital (IO) and mental nerves. After an injury, each of these sensory modalities must be tested and their recovery must be monitored. Maxillofacial neurosensory deficiencies may be caused by various surgical procedures such as third molar surgery, trauma, osteotomies, preprosthetic procedures, excision of large tumors or cysts, surgery of temporomandibular joint [1].
Due to its prominent position in the face, the zygoma is frequently subjected to fracture and dislocation [2].Fractures of the zygoma are always caused by direct violence and clinically often accompanied by a considerable degree of periorbital and subconjunctival ecchymosis. Additionally flattening of the cheek, diplopia, sensory disturbance of the IO region and a palpable fracture displacement of the IO margins are the typical clinical findings [2].
The sensory disturbances of the IO nerve are frequently present in zygomatic complex fractures [3]. The nerve can be damaged by a secondary mechanism through a blunt, crush type of injury or by a bony compression of the nerve at the fracture site as it leaves the IO foramen. The regenerative capacity of IO nerve is a controversial topic in the literature. The recovery rate of sensation depends on several factors, including the nature of injury to the nerve, the time between the injury and surgical intervention and method of treatment [4].
In most cases fracture lines involve the IO foramen, canal, or fissure. Therefore, fractures of the zygomatic complex are characterized by sensory neuropathy (specifically hypoesthesia) in the area of innervations of the IO nerve, both as a presenting symptom, and as a postoperative complication [5].
In the acute stage of non- displaced fractures, at least some degree of hypoesthesia is often encountered as well. Thus, the post traumatic paresthesia over the distribution of the IO nerve has even been considered as indicative of fractures [6]. This sensory deficiency ranges from 18 to 56% [7].
The IO nerve can be damaged also during fracture exposure/manipulation or plate/screw application.
The aim of the study was to evaluate the incidence of persistent sensory disturbances of the IO nerve after recovery from isolated zygomatic complex fractures.
Materials and Methods
This study was conducted on patients with isolated unilateral zygomatic complex fractures and fractures of IO rim. Patients with comminuted zygomatic fractures, combined Le fort fractures, bilateral zygomatic complex fractures and non- displaced fractures were excluded in this study.
Tests performed to know the recovery of IO nerve:
Pin prick
Electrical detection threshold
Procedures for performing these tests:
Pin prick-The tip of a 0.2-mm diameter blunted acupuncture needle (Fig. 1) was pushed against the patients skin until the needle slightly bends (the skin will be dimpled but not penetrated). The specific sites included mid way of the dimensions of lower eye lid, middle of the lateral part of the nose, middle portion of the upper lip and middle of zygoma (Fig. 2).The patients graded sensation was recorded in 100 mm visual analogue scale. Results were recorded as the difference in the VAS values between the control and injured sides.
Electrical detection threshold-Continuous trains of constant-current electrical stimuli were delivered through a pen electrode (active) from an electrical stimulator device (Vectrostim) Passive electrode was placed behind the neck (Fig. 3). Stimulus frequency was 100 Hz with a 50% duty cycle. Polarity of the electrodes was randomized. Detection threshold was assessed by an ascending method of limits. Stimulating current was increased at a fixed rate until the subject indicated detection. The specific sites included mid way of the dimensions of lower eye lid, middle of the lateral part of the nose, middle portion of the upper lip and middle portion of zygoma (Fig. 2) The detection threshold value at each location was noted. Results will be expressed in ratios between the injured side and the control side.
Fig. 1.
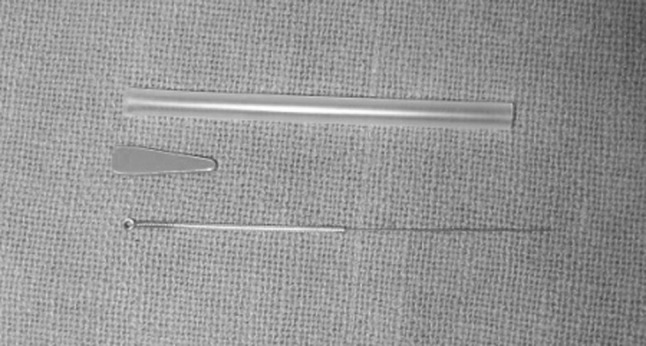
1–0.2-mm diameter blunted acupuncture needle used for pin prick test
Fig. 2.
Specific sites for pin prick and electrical detection threshold tests
Fig. 3.

Electrical stimulator device (Vectrostim) used for electrical detection threshold
The evaluation of the patients using these methods was done preoperatively, 1 and 6 months after surgery.
Statistical Analysis
The statistical analysis was done using SPSS (Statistical Package for Social Sciences) Version 15.0 statistical Analysis Software. The values were represented in number (%) and mean ± SD.
Results
Results of electrical conduction test at different sites in control and affected sites at baseline were observed. It was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site. However, the difference was significant statistically only at lower eyelid (p = 0.016) (Bar diagram 1). After 1 month it was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site (Bar diagram 2). The differences between two sites were found to be significant statistically (p < 0.05) for all the locations except cheek (p = 0.053). On comparing the mean values at control site with that of affected site, statistically no significant difference could be seen (p > 0.05) (Bar diagram 3) after 6 months. Results of pin prick test at different sites in control and affected sites at baseline showed, statistically significant difference (p < 0.05) (Bar diagram 4) at lower eyelid and cheek only though the mean scores at control site were higher as compared to affected site at all the four locations. After 1 month on comparing the mean values at control site with that of affected site, statistically significant difference could be seen (p < 0.05) (Bar diagram 5) at lower eyelid and lateral side of nose though the mean scores at control site were higher as compared to affected site at all the four locations. On comparing the mean values at control site with that of affected site, no statistically significant difference could be seen (p < 0.05) (Bar diagram 6) at any of the four locations after 6 months.
Bar Diagram 1.
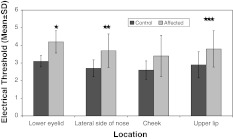
Results of electrical conduction test at different sites in control and affected sides at baseline. *Statistically significant site (p = 0.016). At baseline the mean scores for electrical threshold at different locations at control site were 2.90 ± 0.74, 3.40 ± 1.07, 3.10 ± 0.88 and 3.60 ± 1.43, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 4.60 ± 1.58, 4.70 ± 1.42, 4.20 ± 1.23 and 4.90 ± 1.10, respectively. It was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site. However, the difference was significant statistically only at lower eyelid
Bar Diagram 2.
Results of electrical conduction test at different sites in control and affected sites at 1 month. *Statistically significant site (p = 0.00). **Statistically significant site (p = 0.004). ***Statistically significant site (p = 0.019). At this time interval the mean scores for electrical threshold at different locations at control site were 3.10 ± 0.32, 2.70 ± 0.48, 2.60 ± 0.52 and 2.90 ± 0.74, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 4.20 ± 0.63, 3.70 ± 0.95, 3.40 ± 1.17 and 3.80 ± 1.03, respectively. It was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site. The differences between two sites were found to be significant statistically (p < 0.05) for all the locations except cheek (p = 0.053)
Bar Diagram 3.
Results of electrical conduction test at different sites in control and affected sites at 6 months. At this time interval the mean scores for electrical threshold at different locations at control site were 2.60 ± 0.52, 2.50 ± 0.71, 2.80 ± 0.63 and 2.90 ± 0.32, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 2.70 ± 0.67, 2.50 ± 0.71, 3.00 ± 0.47 and 3.00 ± 0.47, respectively. On comparing the mean values at control site with that of affected site, statistically no significant difference could be seen (p > 0.05)
Bar Diagram 4.
Results of pin prick test at different sites in control and affected sites at baseline. *Statistically significant site (p = 0.030). **Statistically significant site (p = 0.045). At baseline the mean scores for pin prick test at different locations at control site were 4.30 ± 1.25, 4.50 ± 1.35, 4.30 ± 1.77 and 4.60 ± 1.43, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 2.80 ± 1.03, 3.30 ± 1.25, 2.90 ± 1.37 and 3.20 ± 1.69, respectively. On comparing the mean values at control site with that of affected site, statistically significant difference could be seen (p < 0.05) at lower eyelid and cheek only though the mean scores at control site were higher as compared to affected site at all the four locations
Bar Diagram 5.
Results of pin prick test at different sites in control and affected sites at 1 month. *Statistically significant site (p = 0.029). **Statistically significant site (p = 0.010). At 1 month, the mean scores for pin prick test at different locations at control site were 3.70 ± 1.06, 4.10 ± 1.20, 4.00 ± 0.82 and 3.90 ± 1.10, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 2.80 ± 0.79, 3.40 ± 1.07, 3.40 ± 0.97 and 3.30 ± 0.82, respectively. On comparing the mean values at control site with that of affected site, statistically significant difference could be seen (p < 0.05) at lower eyelid and lateral side of nose though the mean scores at control site were higher as compared to affected site at all the four locations
Bar Diagram 6.
Results of pin prick test at different sites in control and affected sites at 6 months. At 6 months, the mean scores for pin prick test at different locations at control site were 3.70 ± 0.48, 3.50 ± 0.71, 4.10 ± 0.74 and 3.60 ± 0.97, respectively, at lower eyelid, lateral side of nose, cheek and upper lip whereas those at affected site were 3.60 ± 0.52, 3.50 ± 0.71, 3.90 ± 0.57 and 3.70 ± 1.42, respectively. On comparing the mean values at control site with that of affected site, no statistically significant difference could be seen (p < 0.05) at any of the four locations
Discussion
The IO nerve is often involved in trauma to the zygomatic complex at the site of the IO fissure, IO canal, or foramen. This results in sensory disturbances including all kinds of dysaesthesia and neuralgiform pain to the skin of the lower eyelid, cheek, lateral side of the nose, and upper lip and to the labial mucosa, gingival and teeth [4].
The reported incidence of initial sensory disturbance in patients ranges from 58 to 94% following orbitozygomatic complex fracture [8]. It was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site. However, the difference was significant statistically only at lower eyelid (p = 0.016). On comparing the mean values at control site with that of affected site, statistically significant difference could be seen (p < 0.05) at lower eyelid and cheek only though the mean scores at control site were higher as compared to affected site at all the four locations. Present study shows statistically significant difference only at the lower eyelid when evaluated with electrical detection threshold test but when pin prick test was performed statistically significant difference was seen on the lower eye lid as well as the cheek but according to Benoliel et al. [5] relatively reduced sensation to pin prick was detected which was not statistically significant.
In the acute stage of non displaced fractures, at least some degree of hypoesthesia is often encountered as well. Thus post-traumatic paresthesia over the IO nerve has even been considered indicative of fracture [9].
The neurological symptoms arise from the fact that the fracture line runs through or in the immediate vicinity of the IO canal and foramen, affecting the IO nerve. This results in dysaesthesia of the skin of the lower eyelid, cheek and nose, the skin and mucosa of the upper lip, gingival and/or teeth on the affected side. Complete impairment of sensation seldom occurs; hypoesthesia is most frequently present followed by paresthesia and hyperesthesia [10]. Benoliel et al. [5] reported prominent pattern of electrical hypoesthesia immediate post injury in 25 patients which were taken in account in their study. In this study preoperative evaluation of the results of skin of the lower eyelid, lateral side of nose, cheek and skin of the upper lip and results with electrical detection threshold test show hypoesthesia in 80% of patients and hyperesthesia was reported in 20% of the cases on the lower eye lid. The nature of the nerve injury in the zygomatic fractures is however unclear and may involve traction, pressure, ischemia, inflammation, and physical damage [5].
Routine use of miniplates for midface and mandibular fractures have resulted in low levels of residual sensory dysfunction and has been recommended as the treatment of choice of fixation [11, 12]. This is in accordance with the present study where almost full regression of symptoms was achieved when anatomical repositioning was achieved with adequate fixation.
De Man and Bax [12] and Zingg et al. [3] stated that reduction and fixation were important factors in the recovery of sensory disturbances of the IO nerve. In the present study optimal fixation was achieved in patients where fixation was done at the frontozygomatic suture.
In the present study the recovery of the IO nerve function was evaluated with two different procedures which included electrical detection threshold and pin prick method. At 1 month time, post operatively Benoliel et al. [5] reported, 50% patients having electrical hyperesthesia when test with electrical detection threshold. It was seen that at all the locations the mean electrical threshold was higher at affected site as compared to control site representing hypoesthesia. The differences between two sites were found to be significant statistically (p < 0.05) for all the locations except cheek (p = 0.053). On comparing the mean values at control site with that of affected site, statistically significant difference could be seen (p < 0.05) at lower eyelid and lateral side of nose though the mean scores at control site were higher as compared to affected site at all the four locations. No statistically significant difference is reported by Benoliel et al. [5].
At the 6 months time point Benoliel et al. [5] reported, 31.6% of patients having electrical hyperesthesia. On comparing the mean values at control site with that of affected site, statistically no significant difference could be seen (p > 0.05).
Benoliel et al. [5] reported almost all cases returned to similar values as on the control side. On comparing the mean values at control site with that of affected site, no statistically significant difference could be seen (p < 0.05) at any of the four locations.
However it is extremely difficult to compare across studies that have employed diverse methodologies to assess nerve function. Two-point discrimination, pressure thresholds, pinprick test, masseter silent period, gross assessment with sharp and blunt instruments and thermography, and gross temperature assessments with ethyl chloride, ice, or warmed gutta and, have all been adapted to the study of IO nerve recovery following trauma [5, 12, 14–16].
Physiological studies have confirmed the Lewis theory, stating that when a nerve is compressed, the fibers are affected differently: the bigger the fiber, the more likely to be affected by trauma. Fibers are therefore affected in the order of their size [17].
The early surgical intervention from the time of injury and the time of surgical intervention will help in preventing post treatment sensory deficit of the IO nerve. Taicher et al. [4], De Man and Bax [12], Zingg M et al. [13], Champy [5], have advocated the similar treatment plan to prevent the sensory deficit of the IO nerve.
Most cases of IO nerve dysfunction following zygomatic fractures will recover by 6 months. The incidence of residual sensory dysfunction varies with the testing modality and was most commonly detected with electrical stimuli. A highly significant beneficial effect on nerve function was noted when plates were used to stabilize fractures [5, 10–12].
The superiority of reduction and the use of miniplates for the fixation of zygomatic fractures in preventing sensory deficit of the IO nerve is supported by our findings.
Summary and Conclusion
This study was undertaken to evaluate the incidence and recovery of persistent sensory disturbances of the IO nerve after isolated zygomatic complex fractures. The main objective was to evaluate the nature of sensory impairment and regeneration of sensation and find out the factors of value in predicting regeneration of nerve function.
The results suggested that neurosensory disturbance in IO nerve was present in all the patients with zygomatic complex fractures.
Our study shows that earlier the surgical intervention, more the recovery of the nerve injury is appreciable during the 1 and 6 months follow up period. This study also states that the patients underwent open reduction with internal fixation had a good recovery of the nerve injury as compared with patients without it.
References
- 1.Akal UK, Sayan NB, Aydogan S, Yaman Z. Evaluation of neurosensory deficiencies of oral and maxillofacial region following surgery. Int J Oral Maxillofac Surg. 2000;29:331–336. doi: 10.1016/S0901-5027(00)80046-6. [DOI] [PubMed] [Google Scholar]
- 2.Kristensen S, Teveteras K. Zygomatic fractures: classification and complications. Clin Otolaryngol. 1986;11:123–129. doi: 10.1111/j.1365-2273.1986.tb00117.x. [DOI] [PubMed] [Google Scholar]
- 3.Rowe NL, Williams JL (1994) Row and William’s maxillofacial injuries, 2nd edn, vol 1. Churchil Livingstone, p 512
- 4.Taicher S, Ardekian L, Samet N, Shoshani Y, Kaffe I. Recovery of the infraorbital nerve after zygomatic complex fractures: a preliminary study of different treatment methods. Int J Oral Maxillofac Surg. 1993;22:339–341. doi: 10.1016/S0901-5027(05)80662-9. [DOI] [PubMed] [Google Scholar]
- 5.Benoliel R, Birenboim R, Regev E, Eliav E. Neurosensory changes in the infraorbital nerve following zygomatic fractures. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;99:657–665. doi: 10.1016/j.tripleo.2004.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Afzelius LE, Rosén C. Facial fractures. A review of 368 cases. Int J Oral Surg. 1980;9(1):25–32. doi: 10.1016/S0300-9785(80)80003-2. [DOI] [PubMed] [Google Scholar]
- 7.Bailey JS, Goldwasser MS, Ed Miloro M, Ghali GE, Larsen PE, Waite PD (2004) Peterson’s principles of oral and maxillofacial surgery, 2nd edn, vol 1. BC Decker Inc., Hamilton, p 457
- 8.Vriens JPM, Glas HW, Bosman F, Moos KF, Koole R. Information on infraorbital nerve damage from multi testing of sensory function. Int J Oral Maxillofac Surg. 1998;27:20–26. doi: 10.1016/S0901-5027(98)80090-8. [DOI] [PubMed] [Google Scholar]
- 9.Jungell P, Lindqvist C. Paraesthesia of the infraorbital nerve following fracture of the zygomatic complex. Int J Oral Maxillofac Surgery. 1987;16:363–367. doi: 10.1016/S0901-5027(87)80160-1. [DOI] [PubMed] [Google Scholar]
- 10.Banovetz JD, Duvall AJ. Zygomatic fractures. Otolaryngol Clin N Am. 1976;9:499–506. [PubMed] [Google Scholar]
- 11.Finlay PM, Ward-Booth RP, Moos KF. Morbidity associated with the use of antral packs and external pins in the treatment of the unstable fracture of the zygomatic complex. Br J Oral Maxillofac Surg. 1984;22:18–23. doi: 10.1016/0266-4356(84)90003-2. [DOI] [PubMed] [Google Scholar]
- 12.Man K, Bax WA. The influence of the mode of treatment of zygomatic bone fractures on the healing process of the infraorbital nerve. Br J Oral Maxillofac Surgery. 1988;26:419–425. doi: 10.1016/0266-4356(88)90095-2. [DOI] [PubMed] [Google Scholar]
- 13.Zingg M, Chowdhury K, Ladrach K, Vuillemin T, Sutter F. Treatment of 813 zygoma-lateral orbital complex fractures. New aspects. Arch Otolaryngol Head Neck Surg. 1991;117(6):611–620. doi: 10.1001/archotol.1991.01870180047010. [DOI] [PubMed] [Google Scholar]
- 14.Schultze-Mosgau S, Erbe M, Rudolph D, Ott R, Neukam FW. Prospective study on post-traumatic and postoperative sensory disturbances of the inferior alveolar nerve and infraorbital nerve in mandibular and midfacial fractures. J Craniomaxillofac Surg. 1999;27:86–93. doi: 10.1016/S1010-5182(99)80019-5. [DOI] [PubMed] [Google Scholar]
- 15.Fogaca WC, Fereirra MC, Dellon AL. Infraorbital nerve injury associated with zygoma fractures: documentation with neurosensory testing. Plast Reconstr Surg. 2004;113:834–838. doi: 10.1097/01.PRS.0000105335.41930.41. [DOI] [PubMed] [Google Scholar]
- 16.Vriens JPM, Moos KF. Morbidity of the infraorbital nerve following orbitozygomatic complex fractures. J Craniomaxillofac Surg. 1995;23:363–368. doi: 10.1016/S1010-5182(05)80131-3. [DOI] [PubMed] [Google Scholar]
- 17.Pedemontet TC, Basili EA. Predictive factors in infraorbital sensitivity disturbances following zygomaticomaxillary fractures Int. J Oral Maxillofac Surg. 2005;34:503–506. doi: 10.1016/j.ijom.2004.10.026. [DOI] [PubMed] [Google Scholar]