Abstract
Wild-type or phyA, phyB, or hy4 mutant Arabidopsis seedlings lacking phytochrome A (phyA), phytochrome B (phyB), or cryptochrome 1 (cry1), respectively, and the double and triple mutants were used in combination with blue-light treatments given simultaneously with red or far-red light. We investigated the interaction between phytochromes and cry1 in the control of hypocotyl growth and cotyledon unfolding. Under conditions deficient for cry1 (short exposures to blue light) or phyB (far-red background), these photoreceptors acted synergistically: Under short exposures to blue light (3 h/d) added to a red-light background, cry1 activity required phyB (e.g. the hy4 mutant was taller than the wild type but the phyBhy4 mutant was not taller than the phyB mutant). Under prolonged exposures to blue light (24 h/d) added to a far-red light background, phyB activity required cry1 (e.g. the phyAphyB mutant was taller than the phyA mutant but the phyAphyBhy4 mutant was not taller than the phyAhy4 mutant). Under more favorable light inputs, i.e. prolonged exposures to blue light added to a red-light background, the effects of cry1 and phyB were independent. Thus, the synergism between phyB and cry1 is conditional. The effect of cry1 was not reduced by the phyA mutation under any tested light condition. Under continuous blue light the triple mutant phyAphyBhy4 showed reduced hypocotyl growth inhibition and cotyledon unfolding compared with the phyAphyB mutant. The action of cry1 in the phyAphyB double mutant was higher under the red-light than the far-red-light background, indicating a synergistic interaction between cry1 and phytochromes C, D, or E; however, a residual action of cry1 independent of any phytochrome is likely to occur.
When etiolated seedlings emerge from the soil, sunlight activates several photoreceptors that mediate de-etiolation. In Arabidopsis these photoreceptors include phyA, phyB, and cry1 (Sharrock and Quail, 1989; Ahmad and Cashmore, 1993; Clack et al., 1994). Although blue light phototransforms phyA and phyB, phytochromes are not specific blue-light photoreceptors because they absorb maximally in red and far-red light. The effects of blue light on phytochrome status can be avoided if the seedlings are grown under a background of phytochrome-absorbable radiation (Thomas and Dickinson, 1979). In contrast to phytochromes, cry1 is a specific blue-light photoreceptor that operates only in the blue-light/UV-A range and extends its activity to the green region of the spectrum (Ahmad and Cashmore, 1993; Lin et al., 1995).
The interaction between red or far-red light and blue light is not a new subject in plant biology. Many years ago, Meijer and Engelsma (1965) reported that preirradiation of etiolated gherkin seedlings with blue light increased the subsequent effect of continuous red light on hypocotyl growth, whereas red light was not as effective as blue light as a preirradiation treatment. Mohr and coworkers (Oelmüler and Mohr, 1985; Drumm-Herrel and Mohr, 1988) acknowledged that the interactions “should be understood in physiological terms before molecular models are being advanced” (Drumm-Herrel and Mohr, 1988), and their model (see Mohr, 1994) involves phytochrome as the effector proper and cryptochrome as a modulator of the response to the phytochrome signal in photomorphogenesis. Conversely, in this model, phytochrome would modulate the activity of a blue-light photoreceptor in phototropism. The availability of mutants deficient in phyA (phyA; Dehesh et al., 1993; Nagatani et al., 1993; Parks and Quail, 1993; Whitelam et al., 1993), phyB (phyB, formerly hy3; Koornneef et al., 1980; Reed et al., 1993), and cry1 (hy4; Koornneef et al., 1980; Ahmad and Cashmore, 1993) has significantly improved the tools for analyzing the interactions among photoreceptors, particularly because the participation of specific members of the phytochrome and cryptochrome families can be investigated.
Synergistic interactions between phyA in its high-irradiance mode of action and phyB (Casal, 1995), as well as between cry1 and phyB (Casal and Boccalandro, 1995), have been reported for hypocotyl growth and cotyledon unfolding in Arabidopsis. In contrast, phyA in its very-low-fluence mode of action may act antagonistically with phyB (Mazzella et al., 1997; Yanovsky et al., 1997). The synergism between phyB and cry1 is clearly manifested when blue light is added to a background of orange light in seedlings exposed to short photoperiods (3 h); neither the hy4 mutant nor the phyB mutant respond to the supplementary blue light (Casal and Boccalandro, 1995). However, it has frequently been reported in the literature that in the experiments in which continuous blue light is compared with darkness, the interaction between phyB and cry1 is not obvious: the phyB mutant shows almost normal hypocotyl growth inhibition (Koornneef et al., 1980; Liscum and Hangarter, 1991, 1993; Young et al., 1992). The reasons for this apparent discrepancy, to our knowledge, have not yet been analyzed.
The available literature is controversial regarding the interaction between phyA and cry1. According to our previous results, cry1 interacts synergistically with phyB but not with phyA (Casal and Boccalandro, 1995). A similar pattern has recently been reported for blue-light-induced shrinking of protoplasts from Arabidopsis hypocotyls. The effect of blue light was absent in protoplasts from the hy4 mutant or from the phyAphyB double mutant. Separate measurements in the phyA and phyB single mutants indicated that phyB was mainly responsible for the phytochrome dependence of this cry1-mediated response (Wang and Iino, 1997). In contrast, Ahmad and Cashmore (1997) observed reduced responses to blue light compared with darkness in a phyAphyB mutant of Arabidopsis and proposed that phyA or phyB is necessary for cry1 activity.
The aim of this work was to investigate hypocotyl growth and cotyledon unfolding in etiolated Arabidopsis seedlings to determine the following: (a) the conditions that favor a synergistic interaction between cry1 and phyB; (b) whether cry1 is active in the phyAphyB background and, if so, the extent to which this activity depends on the interaction with residual phytochromes; and (c) the interaction between phyA and cry1. Therefore, null phyA, phyB, and hy4 mutants lacking phyA, phyB, and cry1, respectively, and all possible double and triple mutants were used in combination with blue light added to a red-light or a far-red-light background to manipulate cryptochrome and phytochrome status separately.
MATERIALS AND METHODS
Plant Material
The Arabidopsis Heynh ecotype Landsberg erecta was the wild type used in this study. The mutant lacking cry1 was hy4-2.23n (Koornneef et al., 1980; Ahmad and Cashmore, 1993). The mutant lacking phyA was phyA-201 (Nagatani et al., 1993), the mutant lacking phyB was phyB-1 (Koornneef et al., 1980; Reed et al., 1993), and the double mutant lacking phyA and phyB was phyA-201phyB-1 (Mazzella et al., 1997). In some experiments additional alleles, phyA-1 (Whitelam et al., 1993), phyB-5 and phyB-7 (Koornneef et al., 1980; Reed et al., 1993), and phyA-201phyB-5 (Reed et al., 1994), were included as internal standards, but the results with these materials are largely not shown. The phyA-201hy4-2.23n and phyB-5hy4-2.23n were obtained by crossing parental lines and selecting tall plants in the F2 generation grown under far-red light plus blue light or red light plus blue light, respectively. Seeds of the F3 generation obtained from single plants selected in the previous generation were tested under far-red light, far-red light plus blue light, red light, and red light plus blue light. Homozygous lines were used in subsequent experiments. The phyA-201phyB-1hy4-2.23n triple mutant was obtained by crossing the phyAphyB double mutant and hy4. Tall seedlings were selected under fluorescent white light from the F2 generation, and the F3 generation was subjected to the tests described above for double mutants.
Experimental Setting
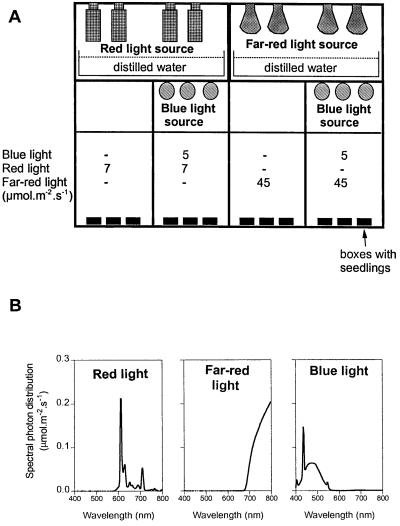
Fifteen seeds of each genotype were sown in clear plastic boxes (40 mm long × 33 mm wide × 15 mm tall) containing 3 mL of 0.8% agar. The boxes were incubated in the dark at 7°C for 3 d, given a red-light pulse, incubated in the dark at 25°C for 24 h, and transferred to light or dark treatments for 3 d (Casal, 1995). The seedlings were exposed to red light (7 μmol m−2 s−1), red light plus blue light (7 and 5 μmol m−2 s−1, respectively), far-red light (45 μmol m−2 s−1), or far-red light plus blue light (45 and 5 μmol m−2 s−1, respectively) (Fig. 1). Red light was provided by a bank of electric lamps (23 W, Philips, Eindhoven, The Netherlands) in combination with water filters and red acetate filters (number 521, 0.2 mm, La Casa del Acetato, Buenos Aires). Far-red light was provided by a bank of 60-W incandescent lamps in combination with a water filter, one red acetate filter, and six blue acrylic filters (no. 2031, 5 mm, Paolini, Buenos Aires). Blue light was provided by fluorescent tubes (L15W/10, Osram, Frankfurt, Germany) in combination with a pale-blue acetate filter (no. 502, 0.2 mm, La Casa del Acetato).
Figure 1.
A, Experimental setting to modify independently the status of phytochromes and cryptochrome. B, Spectral photon distribution of light provided by red, far-red, and blue light sources.
Measurements and Statistics
Hypocotyl length was measured to the nearest 0.5 mm with a ruler and the largest 10 seedlings of each box (i.e. one replicate) were averaged. The angle between the cotyledons was measured with a protractor in the same seedlings used for length measurements, and the 10 values obtained per box were also averaged before statistical analysis. The basic data are presented as means and se values of at least 10 replicate boxes. When different genotypes are compared, hypocotyl length data are expressed relative to dark controls to increase accuracy. The effects of cry1 were calculated as the differences between the average values of plants carrying HY4 versus hy4 alleles (i.e. the wild-type versus the null-mutant allele) for each particular genetic background of the other photoreceptors. For instance, the effect of cry1 on hypocotyl length in the phyAphyB background is the hypocotyl length of the phyAphyBhy4 triple mutant minus the hypocotyl length of the phyAphyB double mutant. A comparable procedure was followed to calculate the effects of phyB (i.e. PHYB versus phyB). These effects of cry1 or phyB (i.e. the differences between the relevant means) are shown with the se values of the difference. The effects of cry1 or phyB in different genetic backgrounds were compared using Student's t test and their significance is indicated.
RESULTS
Conditional Dependence of cry1 Activity on phyB Activity
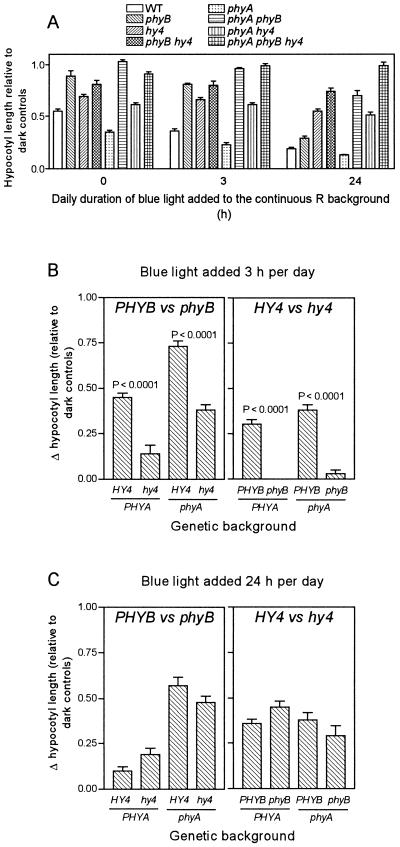
One-day-old seedlings of the wild type and of the phyA, phyB, and hy4 single, double, and triple null mutants were exposed for 3 d to a background of continuous red light with or without the simultaneous addition of blue light (the experimental setting is shown in Fig. 1). Different durations of the blue-light supplement were included to modify the extent of cry1 activation. The basic hypocotyl-length data for seedlings exposed to 0, 3, or 24 h/d supplementary blue light are shown in Figure 2A. For example, under continuous red light plus blue light, both phyB and cry1 were active, because, as expected, the phyB and hy4 mutants were taller than the wild type. The phyBhy4 mutant was taller than any of the parental single mutants. The phyA mutant was not taller than the wild type, but the phyAphyB double mutant was taller than phyB (see also Reed et al., 1994; Mazzella et al., 1997). The triple mutant showed no hypocotyl growth inhibition compared with dark controls.
Figure 2.
cry1 requires phyB when exposures to blue light are short (3 h/d) but becomes independent of phyB when exposures are more extended (24 h/d). The seedlings were grown under continuous red light, continuous red light plus 3 h of blue light per day, or continuous red light plus blue light. A, Hypocotyl length relative to dark controls. B and C, Effects on hypocotyl growth of PHYB versus phyB and HY4 versus hy4 as affected by different genetic backgrounds in seedlings exposed to blue light for only 3 h/d (B) and in seedlings continuously exposed to supplementary blue light (C).
To analyze the interactions among phyA, phyB, and cry1 in detail, the effects of phyB (i.e. the difference between genotypes carrying the wild-type PHYB allele versus the phyB null allele) were calculated for seedlings with or without phyA (i.e. PHYA or phyA background, respectively) and for seedlings with or without cry1 (i.e. HY4 or hy4 background, respectively) in all possible combinations (i.e. PHYAHY4, PHYAhy4, phyAHY4, and phyAhy4). These data are presented in Figure 2B for seedlings exposed to 3 h/d blue light added to continuous red light and in Figure 2C for seedlings exposed to continuous blue light plus red light. For example, the first column in Figure 2B shows the difference between the phyB mutant and the wild type, the second column shows the difference between the phyBhy4 mutant and the hy4 mutant, and so on (taken from Fig. 2A, 3 h of supplementary blue light). A comparable procedure was followed to calculate the effects of cry1 (i.e. the difference between the genotypes carrying the HY4 or the hy4 gene).
For seedlings exposed to blue light for only 3 h/d, the effects of phyB on hypocotyl growth (i.e. the change in hypocotyl length) were larger in the HY4 than in the hy4 background, and the effects of cry1 were larger in the PHYB than in the phyB background (Fig. 2B). In seedlings exposed to continuous supplementary blue light, the effects of phyB were independent of HY4 or hy4 and vice versa (Fig. 2C). Therefore, cry1 and phyB operate synergistically under short (3-h) daily exposures to supplementary blue light but operate independently under continuous supplementary blue light. It is the duration of blue light that is important, because the synergism was observed under short exposures to this wavelength despite the use of continuous red light as a background (Fig. 2B). The effects of cry1 were not reduced by the phyA mutation under short or prolonged exposures to blue light (Fig. 2). The effects of phyB were enhanced by the phyA mutation, as described previously (see Mazzella et al., 1997; Yanovsky et al., 1997).
The pattern of response observed for cotyledon unfolding resembled that described for hypocotyl growth inhibition. Under short exposures to supplementary blue light (3 h/d) the action of cry1 on cotyledon unfolding was significant in the PHYAPHYB background, as shown by the angle between cotyledons (wild type, 152 ± 6°; hy4 mutant, 131 ± 6°; P < 0.05), and in the phyAPHYB background (phyA mutant, 174 ± 4°; phyAhy4 mutant, 151 ± 5°; P < 0.005) but not in the PHYAphyB background (phyB mutant, 33 ± 4°; phyBhy4 mutant, 28 ± 8°). Thus, under short exposures to blue light, cotyledon unfolding depended on phyB but not on phyA. Under continuous supplementary blue light the action of cry1 required neither phyA nor phyB (wild type, 178 ± 1°; hy4 mutant, 154 ± 6°; P < 0.01; phyAphyB mutant, 152 ± 9°; phyAphyBhy4 mutant, 11 ± 5°; P < 0.0001).
The effects of cry1 were not enhanced by phyA under a red-light background (Fig. 2), indicating that cry1 interacts synergistically with phyB and not with phyA. It could be argued, however, that under red light the effect of phyA is smaller than the effect of phyB (see Fig. 2A, 0 h of blue light) and that cry1 could require a minimum effect via phyA or phyB. To test this alternative view the seedlings were also exposed to far-red light or far-red light plus blue light because phyA activity is stronger under far-red light than under red light. The phyB background was used to ensure that phyB was not fulfilling a phytochrome requirement for cry1 activity. A photoperiod of 6 h was chosen because under 6 h of far-red light the reduction of hypocotyl length mediated by phyA (as indicated by the change in hypocotyl length relative to dark controls: phyB, 0.5 ± 0.04; phyBhy4, 0.5 ± 0.04; phyAphyB, 1.0 ± 0.05; phyAphyBhy4, 1.0 ± 0.09) was similar to that mediated by phyB under red light (see Fig. 2). Despite the fact that phyA inhibited hypocotyl growth, cry1 was not active in seedlings exposed daily to 6 h of far-red light plus blue light (phyB, 0.6 ± 0.04; phyBhy4, 0.5 ± 0.04; phyAphyB, 0.9 ± 0.04; phyAphyBhy4, 0.9 ± 0.04).
Conditional Dependence of phyB Activity on cry1 Activity
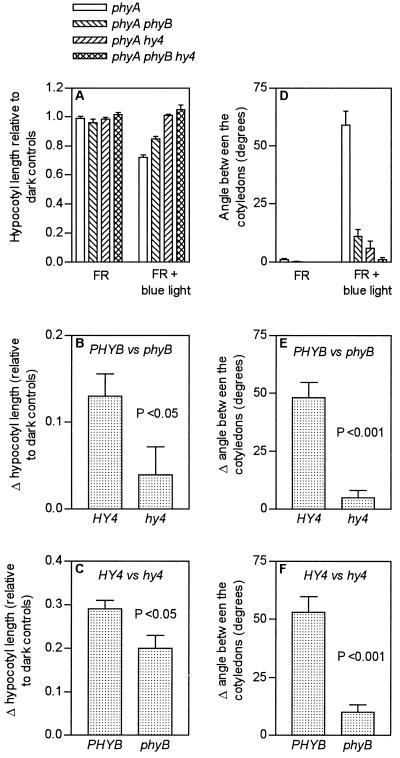
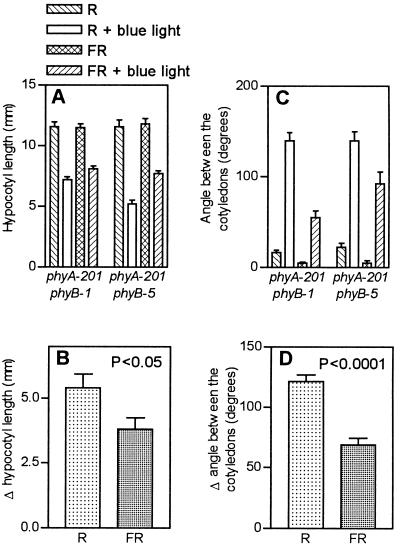
The action of cry1 requires phyB under short but not under prolonged exposures to blue light, i.e. a synergism is observed only under suboptimal conditions. A far-red-light background was used to investigate whether the reciprocal is also true, i.e. whether phyB requires active cry1 if the conditions are suboptimal for phyB activity, because far-red light is predicted to lower the level of active phyB (i.e. phyB Pfr). These experiments were conducted in the phyA background to avoid the strong high-irradiance reactions mediated by phyA under continuous far-red light. The effects of phyB (PHYB versus phyB) were larger in the presence of active cry1 (HY4 background and blue-light supplement) than in the absence of active cry1 (hy4 background and/or no blue-light supplement), and the effects of cry1 (HY4 versus hy4) were larger in the presence of phyB than in its absence (Fig. 3). The results indicate a synergistic interaction between phyB and cry1 when blue light was added continuously to a background of far-red light. The effects of blue light under far-red light were not an artifact involving changes in Pfr because increasing the irradiance of the far-red-light background (from 45 to 90 μmol m−2 s−1) caused no reduction in the effect of supplementary blue light in the phyA and phyAphyB mutants (data not shown).
Figure 3.
Under a background of far-red light (FR) the effects of phyB are significant only if cry1 is active. The seedlings were grown under continuous far-red light or far-red light plus blue light. A, Hypocotyl length relative to dark controls. B, Effects of PHYB versus phyB on hypocotyl growth in the HY4 compared with the hy4 background (all of the seedlings are phyA to avoid a high-irradiance reaction under far-red light). C, Effects of HY4 versus hy4 on hypocotyl growth in the PHYB compared with the phyB background. D, Angle between the cotyledons. E, Effects of PHYB versus phyB on cotyledon unfolding in the HY4 compared with the hy4 background. F, Effects of HY4 versus hy4 on cotyledon unfolding in the PHYB compared with the phyB background.
cry1 Operates in the phyAphyB Background under Continuous Blue Light
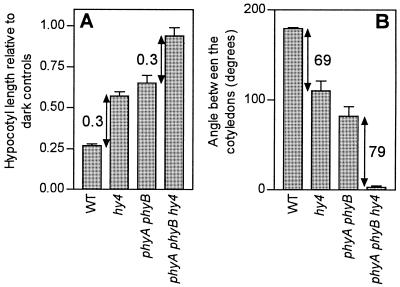
A significant effect of cry1 was observed in the phyAphyB background when blue light was added continuously to red light (Fig. 2) or far-red light (Fig. 3). In contrast, Ahmad and Cashmore (1997) reported reduced responses of the phyAphyB mutant to continuous blue light compared with dark controls (i.e. without using a red- or far-red-light background). One of the possibilities is that, in the absence of red light or far-red light to drive phytochrome photoconversions, the action of blue light had two components, one via phytochrome phototransformation and the other via cry1 phototransformation. Under a red- or far-red-light background, blue light would be important only for cry1 activity. To investigate this hypothesis the double mutant phyAphyB and the triple mutant phyAphyBhy4 were compared under continuous blue light in the absence (for the only occasion in this paper) of a red- or far-red-light background. The response to blue light was reduced in the phyAphyB mutant compared with the wild type, but the effect of cry1 was similar in the PHYAPHYB background (i.e. wild type versus hy4 mutant) and in the phyAphyB background (i.e. phyAphyB mutant versus phyAphyBhy4 mutant) (Fig. 4). This indicates that the reduced response to continuous blue light versus darkness in the phyAphyB mutant was not the result of a cry1 requirement of phyA or phyB but, rather, was due to the mere lack of the response caused by blue light absorbed by phytochromes.
Figure 4.
The effect of cry1 under continuous blue light is not affected by the presence or absence of phyA and phyB. Hypocotyl growth relative to dark controls (A) and cotyledon unfolding (B) in wild-type (WT), hy4, phyAphyB, and phyAphyBhy4 seedlings exposed to blue light (without a red- or a far-red-light background). In dark-grown seedlings the angle between the cotyledons was 0 for all genotypes. The arrows indicate the effect of cry1 in the PHYAPHYB and phyAphyB backgrounds.
cry1 Interacts Synergistically with Phytochrome in the phyAphyB Mutant
To investigate whether phytochromes other than phyA and phyB interact with cry1, the effect of blue light in two phyAphyB double mutants was compared in a red- versus a far-red-light background. Red and far-red light, respectively, establish high or low levels of the Pfr form (predicted to be active) of these phytochromes. The effects of supplementary blue light on hypocotyl growth and cotyledon unfolding were larger in the red- than in the far-red-light background, as indicated by significant interactions (P < 0.05) between blue light and red or far-red light (Fig. 5).
Figure 5.
Evidence for synergism between cry1 and novel phytochrome(s). Seedlings of phyAphyB double mutants were grown under continuous red (R) or far-red light (FR) in factorial combination with or without the simultaneous addition of continuous blue light. A, Hypocotyl length relative to dark controls. B, The effects of blue light on hypocotyl length (i.e. the difference between the seedlings receiving supplementary blue light and those not receiving supplementary blue light) are larger when red light was used as a background than when far-red light was used (the two double-mutant alleles were pooled). C, Angle between the cotyledons. D, Effects of blue light added to red or far-red light on cotyledon unfolding.
Although, compared with red light, the far-red-light background reduced the effect of blue light, this effect was still high (Fig. 5). The effect of supplementary blue light on hypocotyl growth and cotyledon unfolding were noted even for the phyAphyB double mutant under a background of long-wavelength far-red light provided by incandescent lamps in combination with RG9 filters (data not shown). Thus, either the minimal requirement of phytochrome activity for cry1 action is extremely low or the residual effect of cry1 is independent of any phytochrome.
DISCUSSION
phyB and cry1 can interact synergistically to inhibit hypocotyl growth and promote cotyledon unfolding in etiolated Arabidopsis seedlings (Casal and Boccalandro, 1995). Under a red-light background supplemented daily with only 3 h of blue light or under a background of far-red light supplemented continuously with blue light, the effects of phyB (i.e. the difference between PHYB and phyB alleles) were larger in the HY4 than in the hy4 background and the effects of cry1 (i.e. the difference between HY4 and hy4 alleles) were larger in the PHYB than in the phyB background. Under short exposures to blue light cry1 showed almost absolute dependence on phyB (Fig. 2B) and under a background of far-red light phyB showed almost absolute dependence on cry1 (Fig. 3B).
The mutual dependence of cry1 and phyB is conditional. phyB has no absolute requirement for cry1 because it operates under pure red light, i.e. in the absence of active cry1 (Koornneef et al., 1980; Reed et al., 1993) (Fig. 2). cry1 has no absolute requirement for phyB because the responses mediated by cry1 under blue light added continuously to a red-light background were not impaired by the lack of phyB (Fig. 2C). cry1 and phyB acted synergistically only when the light input for cry1 (i.e. blue light) was provided by a relatively short period (3 h/d) or the light input for phyB was deficient because of the presence of a far-red-light background lowering phyB Pfr. No synergism was observed under continuous red light plus blue light, in which phyB and cry1 acted independently.
Part of the effect of cry1 independent of phyB appears to depend on other phytochromes. In the phyAphyB double mutant, the effect of blue light (mediated by cry1) was larger under a background of red light than under a background of far-red light (Fig. 5, B and D). Our interpretation of the latter observation is that phyC, phyD, and/or phyE takes the place of phyB in its synergistic interactions with cry1. Several responses to red-light compared with far-red-light treatments or to different red-/far-red-light ratios have been observed in the phyAphyB mutant (Yang et al., 1995; Devlin et al., 1996; Poppe and Schäfer, 1997), but effects on hypocotyl growth have not been reported before, to our knowledge. Aukerman et al. (1997) recently showed that, particularly in the phyB mutant background, phyD plays a role in the control of hypocotyl growth under red or white light. Thus, phyD is a good candidate to be involved in the residual synergism between phytochrome and cry1 observed in the phyAphyB double mutant.
Finally, part of the effect of cry1 could be independent of any phytochrome. A response to blue light mediated by cry1 was observed even in the phyAphyB double mutant under far-red light (Fig. 5). Thus, either the level of Pfr of phyC, phyD, or phyE required for cry1 activity is extremely low or part of the effect of cry1 may occur independently of any phytochrome. These possibilities cannot be rigorously tested at present. However, the second alternative appears more likely because the residual effect of cry1 was not further reduced by using long-wavelength (RG9) far-red light, which establishes a lower proportion of Pfr than the conventional far-red-light source (data not shown). Effects of a blue-light photoreceptor independent of phytochrome are not apparent in all systems. In the control of hypocotyl growth in pine, simultaneous irradiation with far-red light fully abolishes the effect of blue light (Fernbach and Mohr, 1990). Hypocotyl growth in the lh mutant of cucumber, which lacks phyB (López Juez et al., 1992), shows no response to blue light (Ballaré et al., 1991).
No evidence for synergistic interactions between phyA and cry1 was obvious in either our previous study (Casal and Boccalandro, 1995) or in the present experiments. Ahmad and Cashmore (1997) observed a reduced response to blue light in the phyAphyB mutant exposed to continuous blue light compared with the dark controls (i.e. without a background of phytochrome-absorbable radiation). Such a reduced response would be the result of lack of phytochrome absorption of blue light rather than cry1 dependence on phyA or phyB under continuous blue light. In favor of the latter interpretation, when blue light was provided continuously without a red- or far-red-light background, the absence of phyA and phyB reduced the response to blue light but did not reduce the action of cry1 (Fig. 4).
In summary, for hypocotyl growth and cotyledon unfolding: (a) cry1 interacts synergistically with phyB under suboptimal light conditions, i.e. short exposures to blue light or prolonged blue light added to a far-red-light background; (b) in the absence of phyB, cry1 interacts synergistically with phytochrome(s) other than phyA, but a residual action independent of any phytochrome is likely to occur; and (c) phyA and cry1 show no obvious synergism.
The levels of phyB are not obviously affected by light conditions or by the hy4 or phyA mutations (Somers et al., 1991; Nagatani et al., 1993; Parks and Quail, 1993). The levels of cry1 are not obviously affected by phyA and/or phyB activity (Ahmad and Cashmore, 1997). Thus, the synergism between phyB and cry1 is likely to involve communication between the transduction chains initiated by the respective photoreceptors rather than by changes in the status of the photoreceptors themselves. The model described by Mohr (1994) placed phytochrome as the only terminal effector, cryptochrome as a modulator of the phytochrome response in photomorphogenesis, and phytochrome as a modulator of blue-light photoreceptors in phototropism. The evidence in favor of cry1 activity in the absence of any obvious phytochrome activity would be more easily accommodated by a model in which cry1 and phyB (together with phyC, phyD, and/or phyE) operate via parallel interacting pathways. These interactions should occur at a point at which the transduction chains of phyA and phyB are divergent, because only phyB (not phyA) is able to interact synergistically with cry1.
ACKNOWLEDGMENTS
We thank Pedro Gundel for his technical assistance, Dr. Joanne Chory and Michael Neff (The Salk Institute, University of California, La Jolla) for reading the manuscript and discussing data before publication, and Dr. Maarten Koornneef (Department of Genetics, University of Wageningen, The Netherlands), Dr. Joanne Chory, and the Arabidopsis Biological Research Center (The Ohio State University, Columbus) for their kind provision of original seed batches of single mutants.
Abbreviations:
- cry1
cryptochrome 1
- phyA to phyE
phytochromes A to E
Footnotes
This work was supported by grants from the University of Buenos Aires (no. AG041), Fundación Antorchas (no. A-13434/1), and Consejo Nacional de Investigaciones Científicas y Técnicas (no. PIA 6524).
LITERATURE CITED
- Ahmad M, Cashmore AR. HY4 gene of Arabidopsis thaliana encodes a protein with characteristics of a blue-light photoreceptor. Nature. 1993;366:162–166. doi: 10.1038/366162a0. [DOI] [PubMed] [Google Scholar]
- Ahmad M, Cashmore AR. The blue-light receptor cryptochrome 1 shows functional dependence on phytochrome A or phytochrome B in Arabidopsis thaliana. Plant J. 1997;11:421–427. doi: 10.1046/j.1365-313x.1997.11030421.x. [DOI] [PubMed] [Google Scholar]
- Aukerman MJ, Hirschfeld M, Wester L, Weaver M, Clack T, Amasino RM, Sharrock RA. A deletion in the PHYD gene of the Arabidopsis Wassilewskija ecotype defines a role for phytochrome D in red/far-red light sensing. Plant Cell. 1997;9:1317–1326. doi: 10.1105/tpc.9.8.1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballaré CL, Casal JJ, Kendrick RE. Responses of light-grown wild-type and long-hypocotyl mutant cucumber seedlings to natural and simulated shade light. Photochem Photobiol. 1991;54:819–826. [Google Scholar]
- Casal JJ. Coupling of phytochrome B to the control of hypocotyl growth in Arabidopsis. Planta. 1995;196:23–29. doi: 10.1007/BF00193213. [DOI] [PubMed] [Google Scholar]
- Casal JJ, Boccalandro H. Co-action between phytochrome B and HY4 in Arabidopsis thaliana. Planta. 1995;197:213–218. doi: 10.1007/BF00202639. [DOI] [PubMed] [Google Scholar]
- Clack T, Mathews S, Sharrock RA. The phytochrome apoprotein family in Arabidopsis is encoded by five genes: the sequences and expression of PHYD and PHYE. Plant Mol Biol. 1994;25:413–427. doi: 10.1007/BF00043870. [DOI] [PubMed] [Google Scholar]
- Dehesh K, Franci C, Parks BM, Seeley KA, Short TW, Tepperman JM, Quail PH. Arabidopsis hy8 locus encodes phytochrome A. Plant Cell. 1993;5:1081–1088. doi: 10.1105/tpc.5.9.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin PF, Halliday KJ, Harberd NP, Whitelam GC. The rosette habit of Arabidopsis thaliana is dependent upon phytochrome action: novel phytochromes control internode elongation and flowering time. Plant J. 1996;10:1127–1134. doi: 10.1046/j.1365-313x.1996.10061127.x. [DOI] [PubMed] [Google Scholar]
- Drumm-Herrel H, Mohr H. Mode of coaction between UV-A and light absorbed by phytochrome in control of appearance of ribulose-1,5-bisphosphate carboxylase in the shoot of milo (Sorghum vulgare Pers.) Photochem Photobiol. 1988;47:599–604. [Google Scholar]
- Fernbach E, Mohr H. Coaction of blue/ultraviolet-A light and light absorbed by phytochrome in controlling growth of pine (Pinus sylvestris L.) seedlings. Planta. 1990;180:212–216. doi: 10.1007/BF00193998. [DOI] [PubMed] [Google Scholar]
- Koornneef M, Rolf E, Spruit CJP. Genetic control of light-inhibited hypocotyl elongation in Arabidopsis thaliana (L.) Heynh. Z Pflanzenphysiol. 1980;100:147–160. [Google Scholar]
- Lin C, Ahmad M, Gordon D, Cashmore AR. Expression of an Arabidopsis cryptochrome gene in transgenic tobacco results in hypersensitivity to blue, UV-A and green light. Proc Natl Acad Sci USA. 1995;92:8423–8427. doi: 10.1073/pnas.92.18.8423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Arabidopsis mutants lacking blue light-dependent inhibition of hypocotyl elongation. Plant Cell. 1991;3:685–694. doi: 10.1105/tpc.3.7.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liscum E, Hangarter RP. Genetic evidence that the red-absorbing form of phytochrome-B modulates gravitropism in Arabidopsis thaliana. Plant Physiol. 1993;103:15–19. doi: 10.1104/pp.103.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- López Juez E, Nagatani A, Tomizawa K-I, Deak M, Kern R, Kendrick RE, Furuya M. The cucumber long hypocotyl mutant lacks a light-stable PHYB-like phytochrome. Plant Cell. 1992;4:241–251. [PMC free article] [PubMed] [Google Scholar]
- Mazzella MA, Alconada Magliano TM, Casal JJ. Dual effect of phytochrome A on hypocotyl growth under continuous red light. Plant Cell Environ. 1997;20:261–267. [Google Scholar]
- Meijer G, Engelsma G. The synergistic influence of a pre-irradiation on the photoinhibition of gherkin seedlings. Photochem Photobiol. 1965;4:251–258. [Google Scholar]
- Mohr H. Coaction between pigment systems. In: Kendrick RE, Kronenberg GHM, editors. Photomorphogenesis in Plants, Ed 2. Dordrecht, The Netherlands: Kluwer Academic Publishers; 1994. pp. 353–373. [Google Scholar]
- Nagatani A, Reed JW, Chory J. Isolation and initial characterization of Arabidopsis mutants that are deficient in phytochrome A. Plant Physiol. 1993;102:269–277. doi: 10.1104/pp.102.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oelmüler R, Mohr H. Mode of coaction between blue/UV light and light absorbed by phytochrome in light-mediated anthocyanin formation in the milo (Sorghum vulgare Pers.) seedling. Proc Natl Acad Sci USA. 1985;82:6124–6128. doi: 10.1073/pnas.82.18.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parks BM, Quail PH. hy8, a new class of Arabidopsis long hypocotyl mutants deficient in functional phytochrome A. Plant Cell. 1993;5:39–48. doi: 10.1105/tpc.5.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poppe C, Schäfer E. Seed germination of Arabidopsis thaliana phyA/phyB double mutants is under phytochrome control. Plant Physiol. 1997;114:1487–1492. doi: 10.1104/pp.114.4.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagatani A, Elich TD, Fagan M, Chory J. Phytochrome A and phytochrome B have overlapping but distinct functions in Arabidopsis development. Plant Physiol. 1994;104:1139–1149. doi: 10.1104/pp.104.4.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JW, Nagpal P, Poole DS, Furuya M, Chory J. Mutations in the gene for the red/far-red light receptor phytochrome B alter cell elongation and physiological responses throughout Arabidopsis development. Plant Cell. 1993;5:147–157. doi: 10.1105/tpc.5.2.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharrock RA, Quail PH. Novel phytochrome sequences in Arabidopsis thaliana: structure, evolution and differential expression of a plant regulatory photoreceptor family. Genes Dev. 1989;3:1745–1757. doi: 10.1101/gad.3.11.1745. [DOI] [PubMed] [Google Scholar]
- Somers DE, Sharrock RA, Tepperman JM, Quail PH. The hy3 long hypocotyl mutant of Arabidopsis is deficient in phytochrome B. Plant Cell. 1991;3:1263–1274. doi: 10.1105/tpc.3.12.1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas B, Dickinson HG. Evidence for two photoreceptors controlling growth in de-etiolated seedlings. Planta. 1979;146:545–550. doi: 10.1007/BF00388830. [DOI] [PubMed] [Google Scholar]
- Wang X, Iino M. Blue light-induced shrinking of protoplasts from Arabidopsis hypocotyls: mediation by a blue-light receptor and responsiveness control by phytochrome (abstract no. 1461) Plant Physiol. 1997;114:S-281. [Google Scholar]
- Whitelam GC, Johnson E, Peng J, Carol P, Anderson ML, Cowl JS, Harberd NP. Phytochrome A null mutants of Arabidopsis display a wild-type phenotype in white light. Plant Cell. 1993;5:757–768. doi: 10.1105/tpc.5.7.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y-Y, Nagatani A, Zhao Y-J, Kang B-J, Kendrick RE, Kamiya Y. Effects of gibberellins on seed germination of phytochrome-deficient mutants of Arabidopsis thaliana. Plant Cell Physiol. 1995;36:1205–1211. [PubMed] [Google Scholar]
- Yanovsky MJ, Casal JJ, Luppi JP. The VLF loci, polymorphic between ecotypes Landsberg erecta and Columbia dissect two branches of phytochrome A signalling pathways that correspond to the very-low fluence and high-irradiance responses of phytochrome. Plant J. 1997;12:659–667. doi: 10.1046/j.1365-313x.1997.00659.x. [DOI] [PubMed] [Google Scholar]
- Young JC, Liscum E, Hangarter RP. Spectral-dependence of light-inhibited hypocotyl elongation in photomorphogenic mutants of Arabidopsis: evidence for a UV-A photosensor. Planta. 1992;188:106–114. doi: 10.1007/BF00198946. [DOI] [PubMed] [Google Scholar]