Abstract
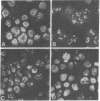
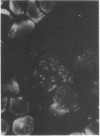
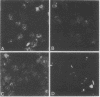
Sera from patients with scleroderma contained several autoantibodies to nuclear antigens which were distinguished by different patterns of nuclear immunofluorescence staining. One of these autoantibodies reacted with centromeric regions of chromosomes. In chromosome spreads, the staining appeared as two small spheres at the centromere, resembling kinetochores. The antigenic determinant appeared to be a protein or polypeptide tightly bound to DNA. The autoantibody was reactive with centromeres of cells derived from humans, mice, and Chinese hamsters. The autoantibody was present in high frequency in the calcinosis/Raynaud's phenomenon/esophageal dysmotility/sclerodactyly/telangiectasia variant (CREST) of scleroderma.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BECK J. S., ANDERSON J. R., McELHINNEY A. J., ROWELL N. R. Antinucleolar antibodies. Lancet. 1962 Sep 22;2(7256):575–577. doi: 10.1016/s0140-6736(62)90446-4. [DOI] [PubMed] [Google Scholar]
- Burnham T. K., Fine G., Neblett T. R. The immunofluorescent tumor imprint technique. II. The frequency of antinuclear factors in connective tissue diseases and dermatoses. Ann Intern Med. 1966 Jul;65(1):9–19. doi: 10.7326/0003-4819-65-1-9. [DOI] [PubMed] [Google Scholar]
- Comings D. E., Harris D. C., Okada T. A., Holmquist G. Nuclear proteins. IV. Deficiency of non-histone proteins in condensed chromatin of Drosophila virilis and mouse. Exp Cell Res. 1977 Mar 15;105(2):349–365. doi: 10.1016/0014-4827(77)90133-1. [DOI] [PubMed] [Google Scholar]
- Douvas A. S., Achten M., Tan E. M. Identification of a nuclear protein (Scl-70) as a unique target of human antinuclear antibodies in scleroderma. J Biol Chem. 1979 Oct 25;254(20):10514–10522. [PubMed] [Google Scholar]
- Eiberg H. New selective Giemsa technique for human chromosomes, Cd staining. Nature. 1974 Mar 1;248(5443):55–55. doi: 10.1038/248055a0. [DOI] [PubMed] [Google Scholar]
- Klein G., Giovanella B., Westman A., Stehlin J. S., Mumford D. An EBV-genome-negative cell line established from an American Burkitt lymphoma; receptor characteristics. EBV infectibility and permanent conversion into EBV-positive sublines by in vitro infection. Intervirology. 1975;5(6):319–334. doi: 10.1159/000149930. [DOI] [PubMed] [Google Scholar]
- Matsukuma S., Utakoji T. Non-histone protein associated with centromeric heterochromatin in the mouse chromosome. Exp Cell Res. 1977 Mar 1;105(1):217–222. doi: 10.1016/0014-4827(77)90168-9. [DOI] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Nakamura R. M., Tan E. M. Recent progress in the study of autoantibodies to nuclear antigens. Hum Pathol. 1978 Jan;9(1):85–91. doi: 10.1016/s0046-8177(78)80010-0. [DOI] [PubMed] [Google Scholar]
- Parker M. D., Kerby G. P. Combined titre and fluorescent pattern of IgG antinuclear antibodies using cultured cell monolayers in evaluating connective tissue diseases. Ann Rheum Dis. 1974 Sep;33(5):465–472. doi: 10.1136/ard.33.5.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinnas J. L., Northway J. D., Tan E. M. Antinucleolar antibodies in human sera. J Immunol. 1973 Oct;111(4):996–1004. [PubMed] [Google Scholar]
- Reedman B. M., Klein G. Cellular localization of an Epstein-Barr virus (EBV)-associated complement-fixing antigen in producer and non-producer lymphoblastoid cell lines. Int J Cancer. 1973 May;11(3):499–520. doi: 10.1002/ijc.2910110302. [DOI] [PubMed] [Google Scholar]
- Rothfield N. F., Rodnan G. P. Serum antinuclear antibodies in progressive systemic sclerosis (scleroderma). Arthritis Rheum. 1968 Oct;11(5):607–617. doi: 10.1002/art.1780110502. [DOI] [PubMed] [Google Scholar]
- Tan E. M., Kunkel H. G. Characteristics of a soluble nuclear antigen precipitating with sera of patients with systemic lupus erythematosus. J Immunol. 1966 Mar;96(3):464–471. [PubMed] [Google Scholar]
- Tan E. M., Lerner R. A. An immunological study of the fates of nuclear and nucleolar macromoleculus during the cell cycle. J Mol Biol. 1972 Jul 14;68(1):107–114. doi: 10.1016/0022-2836(72)90266-5. [DOI] [PubMed] [Google Scholar]
- Tan E. M. Relationship of nuclear staining patterns with precipitating antibodies in systemic lupus erythematosus. J Lab Clin Med. 1967 Nov;70(5):800–812. [PubMed] [Google Scholar]
- Tan E. M., Robinson J., Robitaille P. Studies on antibodies to histones by immunofluorescence. Scand J Immunol. 1976;5(6-7):811–818. doi: 10.1111/j.1365-3083.1976.tb03030.x. [DOI] [PubMed] [Google Scholar]
- Weintraub H. Release of discrete subunits after nuclease and trypsin digestion of chromatin. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1212–1216. doi: 10.1073/pnas.72.3.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weintraub H., Van Lente F. Dissection of chromosome structure with trypsin and nucleases. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4249–4253. doi: 10.1073/pnas.71.10.4249. [DOI] [PMC free article] [PubMed] [Google Scholar]