Abstract
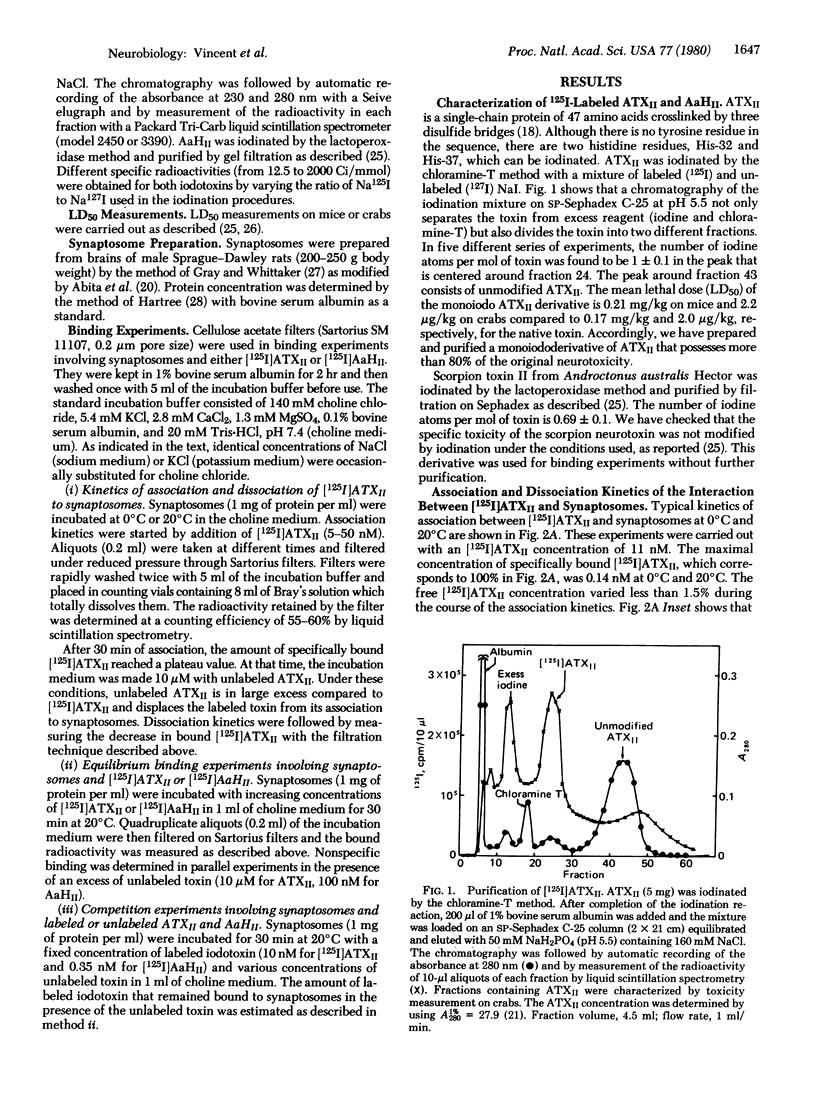
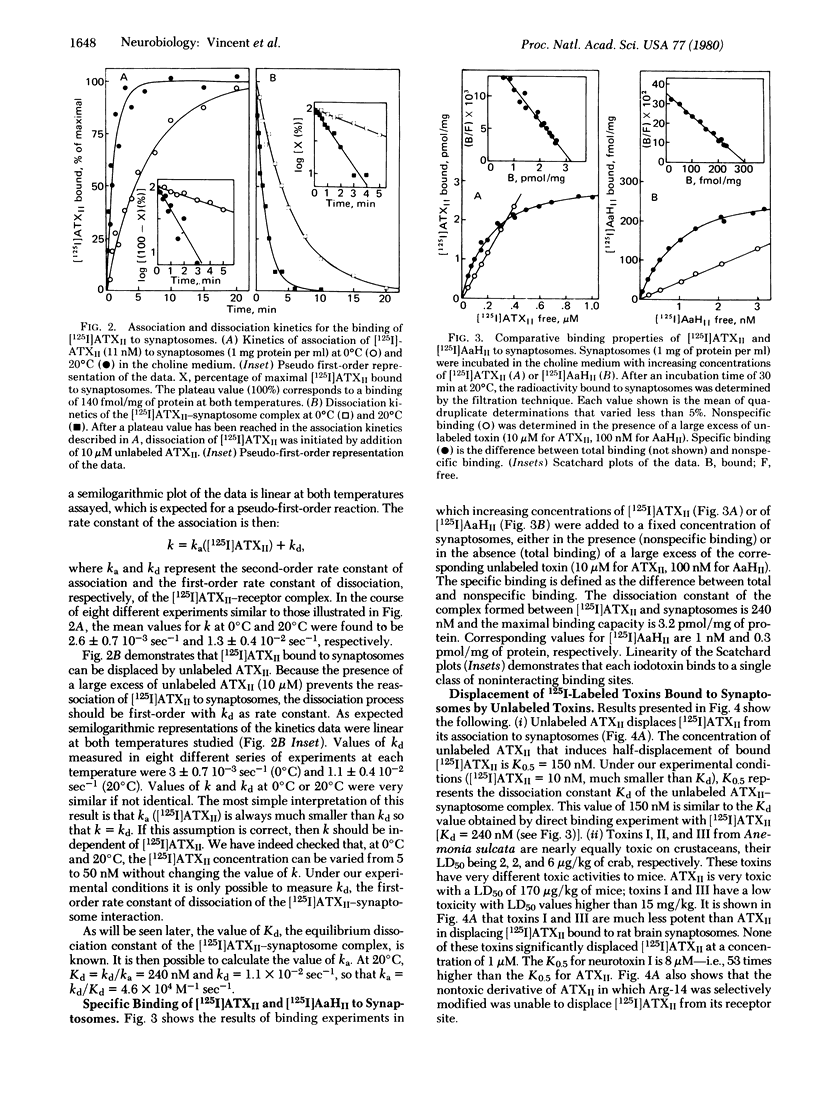
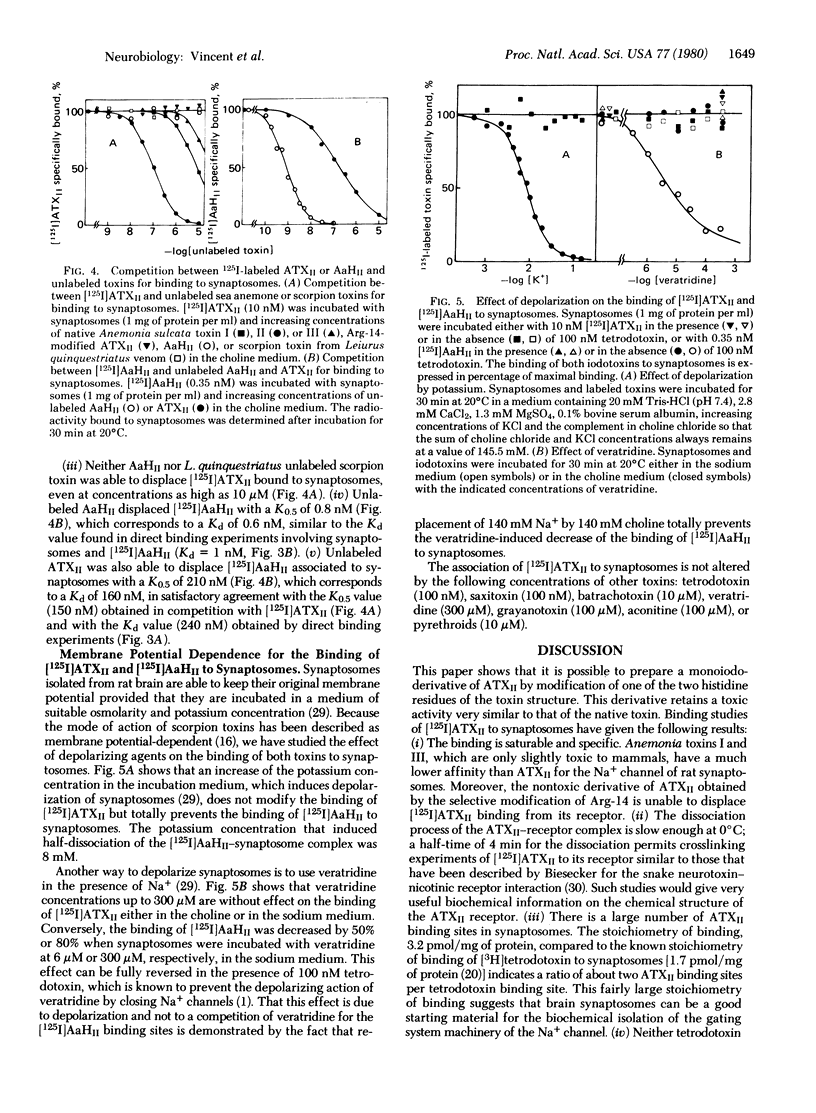
Iodination of toxin II from the sea anemone Anemonia sulcata gives a labeled monoiododerivative that retains 80% of the original neurotoxicity. This derivative binds specifically to rat brain synaptosomes at 20 degrees C and pH 7.4 with a second-order rate constant of association ka = 4.6 x 10(4) M-1 sec-1 and a first-order rate constant of dissociation kd = 1.1 x 10(-2) sec-1. The binding occurs on the Na+ channel at a binding site distinct from that of other gating system toxins like batrachotoxin, veratridine, grayanotoxin, aconitine, and pyrethroids. The maximal binding capacity Bmax is 3.2 pmol/mg of protein (i.e., about two sea anemone toxin binding sites per tetrodotoxin binding site) and the Kd is 240 nM for the monoiododerivative and 150 nM for the native toxin. Corresponding binding parameters for the association of a 125I-labeled derivative of toxin II from the scorpion Androctonus australis Hector are Bmax = 0.3 pmol/mg of protein and Kd = 1 nM, whereas the Kd of the unmodified scorpion toxin is 0.6 nM. Competition experiments involving scorpion toxins, sea anemone toxins, and synaptosomes demonstrate that, although the sea anemone toxin is able to displace the scorpion toxin bound to synaptosomes, the scorpion toxin does not displace the sea anemone toxin. The sea anemone toxin but not the scorpion toxin binds to depolarized synaptosomes. Differences between binding properties of the two polypeptide toxins are analyzed in the discussion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abita J. P., Chicheportiche R., Schweitz H., Lazdunski M. Effects of neurotoxins (veratridine, sea anemone toxin, tetrodotoxin) on transmitter accumulation and release by nerve terminals in vitro. Biochemistry. 1977 May 3;16(9):1838–1844. doi: 10.1021/bi00628a012. [DOI] [PubMed] [Google Scholar]
- Agnew W. S., Levinson S. R., Brabson J. S., Raftery M. A. Purification of the tetrodotoxin-binding component associated with the voltage-sensitive sodium channel from Electrophorus electricus electroplax membranes. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2606–2610. doi: 10.1073/pnas.75.6.2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman C., Dubois J. M., Rojas E., Rathmayer W. Decreased rate of sodium conductance inactivation in the node of Ranvier induced by a polypeptide toxin from sea anemone. Biochim Biophys Acta. 1976 Nov 11;455(1):173–184. doi: 10.1016/0005-2736(76)90162-0. [DOI] [PubMed] [Google Scholar]
- Bernard P., Couraud F., Lissitzky S. Effects of a scorpion toxin from Androctonus australis venom on action potential of neuroblastoma cells in culture. Biochem Biophys Res Commun. 1977 Jul 25;77(2):782–788. doi: 10.1016/s0006-291x(77)80046-6. [DOI] [PubMed] [Google Scholar]
- Biesecker G. Molecular properties of the cholinergic receptor purified from Electrophorus electricus. Biochemistry. 1973 Oct 23;12(22):4403–4409. doi: 10.1021/bi00746a017. [DOI] [PubMed] [Google Scholar]
- Blaustein M. P., Goldring J. M. Membrane potentials in pinched-off presynaptic nerve ternimals monitored with a fluorescent probe: evidence that synaptosomes have potassium diffusion potentials. J Physiol. 1975 Jun;247(3):589–615. doi: 10.1113/jphysiol.1975.sp010949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béress L., Béress R., Wunderer G. Isolation and characterisation of three polypeptides with neurotoxic activity from Anemonia sulcata. FEBS Lett. 1975 Feb 15;50(3):311–314. doi: 10.1016/0014-5793(75)80517-5. [DOI] [PubMed] [Google Scholar]
- Catterall W. A., Beress L. Sea anemone toxin and scorpion toxin share a common receptor site associated with the action potential sodium ionophore. J Biol Chem. 1978 Oct 25;253(20):7393–7396. [PubMed] [Google Scholar]
- Catterall W. A. Membrane potential-dependent binding of scorpion toxin to the action potential Na+ ionophore. Studies with a toxin derivative prepared by lactoperoxidase-catalyzed iodination. J Biol Chem. 1977 Dec 10;252(23):8660–8668. [PubMed] [Google Scholar]
- Catterall W. A. Purification of a toxic protein from scorpion venom which activates the action potential Na+ ionophore. J Biol Chem. 1976 Sep 25;251(18):5528–5536. [PubMed] [Google Scholar]
- Chicheportiche R., Balerna M., Lombet A., Romey G., Lazdunski M. Synthesis and mode of action on axonal membranes of photoactivable derivatives of tetrodotoxin. J Biol Chem. 1979 Mar 10;254(5):1552–1557. [PubMed] [Google Scholar]
- GRAY E. G., WHITTAKER V. P. The isolation of nerve endings from brain: an electron-microscopic study of cell fragments derived by homogenization and centrifugation. J Anat. 1962 Jan;96:79–88. [PMC free article] [PubMed] [Google Scholar]
- HUNTER W. M., GREENWOOD F. C. Preparation of iodine-131 labelled human growth hormone of high specific activity. Nature. 1962 May 5;194:495–496. doi: 10.1038/194495a0. [DOI] [PubMed] [Google Scholar]
- Hartree E. F. Determination of protein: a modification of the Lowry method that gives a linear photometric response. Anal Biochem. 1972 Aug;48(2):422–427. doi: 10.1016/0003-2697(72)90094-2. [DOI] [PubMed] [Google Scholar]
- Jacques Y., Fosset M., Lazdunski M. Molecular properties of the action potential Na+ ionophore in neuroblastoma cells. Interactions with neurotoxins. J Biol Chem. 1978 Oct 25;253(20):7383–7392. [PubMed] [Google Scholar]
- Jover E., Martin-Moutot N., Couraud F., Rochat H. Scorpion toxin: specific binding to rat synaptosomes. Biochem Biophys Res Commun. 1978 Nov 14;85(1):377–382. doi: 10.1016/s0006-291x(78)80053-9. [DOI] [PubMed] [Google Scholar]
- Martinez G., Kopeyan C. Toxin III from Anemonia sulcata: primary structure. FEBS Lett. 1977 Dec 15;84(2):247–252. doi: 10.1016/0014-5793(77)80699-6. [DOI] [PubMed] [Google Scholar]
- Narahashi T. Chemicals as tools in the study of excitable membranes. Physiol Rev. 1974 Oct;54(4):813–889. doi: 10.1152/physrev.1974.54.4.813. [DOI] [PubMed] [Google Scholar]
- Ray R., Morrow C. S., Catterall W. A. Binding of scorpion toxin to receptor sites associated with voltage-sensitive sodium channels in synaptic nerve ending particles. J Biol Chem. 1978 Oct 25;253(20):7307–7313. [PubMed] [Google Scholar]
- Ritchie J. M., Rogart R. B. The binding of saxitoxin and tetrodotoxin to excitable tissue. Rev Physiol Biochem Pharmacol. 1977;79:1–50. doi: 10.1007/BFb0037088. [DOI] [PubMed] [Google Scholar]
- Rochat H., Rochat C., Sampieri F., Miranda F., Lissitzky S. The amino-acid sequence of neurotoxin II of Androctonus australis Hector. Eur J Biochem. 1972 Jul 24;28(3):381–388. doi: 10.1111/j.1432-1033.1972.tb01924.x. [DOI] [PubMed] [Google Scholar]
- Rochat H., Tessier M., Miranda F., Lissitzky S. Radioiodination of scorpion and snake toxins. Anal Biochem. 1977 Oct;82(2):532–548. doi: 10.1016/0003-2697(77)90192-0. [DOI] [PubMed] [Google Scholar]
- Romey G., Abita J. P., Chicheportiche R., Rochat H., Lazdunski M. Scorpion neurotoxin. Mode of action on neuromuscular junctions and synaptosomes. Biochim Biophys Acta. 1976 Nov 2;448(4):607–619. doi: 10.1016/0005-2736(76)90114-0. [DOI] [PubMed] [Google Scholar]
- Romey G., Abita J. P., Schweitz H., Wunderer G., Lazdunski Sea anemone toxin:a tool to study molecular mechanisms of nerve conduction and excitation-secretion coupling. Proc Natl Acad Sci U S A. 1976 Nov;73(11):4055–4059. doi: 10.1073/pnas.73.11.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romey G., Chicheportiche R., Lazdunski M., Rochat H., Miranda F., Lissitzky S. Scorpion neurotoxin - a presynaptic toxin which affects both Na+ and K+ channels in axons. Biochem Biophys Res Commun. 1975 May 5;64(1):115–121. doi: 10.1016/0006-291x(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Romey G., Jacques Y., Schweitz H., Fosset M., Lazdunski M. The sodium channel in non-impulsive cells. Interaction with specific neurotoxins. Biochim Biophys Acta. 1979 Sep 21;556(2):344–353. doi: 10.1016/0005-2736(79)90053-1. [DOI] [PubMed] [Google Scholar]
- Toi K., Bynum E., Norris E., Itano H. A. Studies on the chemical modification of arginine. I. The reaction of 1,2-cyclohexanedione with arginine and arginyl residues of proteins. J Biol Chem. 1967 Mar 10;242(5):1036–1043. [PubMed] [Google Scholar]
- Vincent J. P., Schweitz H., Lazdunski M. Structure-function relationships and site of action of apamin, a neurotoxic polypeptide of bee venom with an action on the central nervous system. Biochemistry. 1975 Jun 3;14(11):2521–2525. doi: 10.1021/bi00682a035. [DOI] [PubMed] [Google Scholar]
- Wunderer G., Eulitz M. Amino-acid sequence of toxin I from Anemonia sulcata. Eur J Biochem. 1978 Aug 15;89(1):11–17. doi: 10.1111/j.1432-1033.1978.tb20890.x. [DOI] [PubMed] [Google Scholar]
- Wunderer G., Fritz H., Wachter E., Machleidt W. Amino-acid sequence of a coelenterate toxin: toxin II from Anemonia sulcata. Eur J Biochem. 1976 Sep;68(1):193–198. doi: 10.1111/j.1432-1033.1976.tb10778.x. [DOI] [PubMed] [Google Scholar]
- de Barry J., Fosset M., Lazdunski M. Molecular mechanism of the cardiotoxic action of a polypeptide neurotoxin from sea anemone on cultured embryonic cardiac cells. Biochemistry. 1977 Aug 23;16(17):3850–3855. doi: 10.1021/bi00636a021. [DOI] [PubMed] [Google Scholar]



