Abstract
Leptospirosis is an important zoonosis and has a worldwide impact on public health. This paper will discuss both the role of immunogenic and pathogenic molecules during leptospirosis infection and possible new targets for immunotherapy against leptospira components. Leptospira, possess a wide variety of mechanisms that allow them to evade the host immune system and cause infection. Many molecules contribute to the ability of Leptospira to adhere, invade, and colonize. The recent sequencing of the Leptospira genome has increased our knowledge about this pathogen. Although the virulence factors, molecular targets, mechanisms of inflammation, and signaling pathways triggered by leptospiral antigens have been studied, some questions are still unanswered. Toll-like receptors (TLRs) are the primary sensors of invading pathogens. TLRs recognize conserved microbial pattern molecules and activate signaling pathways that are pivotal to innate and adaptive immune responses. Recently, a new molecular target has emerged—the Na/K-ATPase—which may contribute to inflammatory and metabolic alteration in this syndrome. Na/K-ATPase is a target for specific fatty acids of host origin and for bacterial components such as the glycolipoprotein fraction (GLP) that may lead to inflammasome activation. We propose that in addition to TLRs, Na/K-ATPase may play a role in the innate response to leptospirosis infection.
1. Introduction
Leptospirosis is a zoonosis of global importance caused by several species and more than 200 different serovars of pathogenic Leptospira spp. The disease affects both animals and humans and has veterinary, economic, and medical relevance [1, 2]. Leptospirosis is still a major public health problem in tropical countries, with epidemic outbreaks occurring in the rainy season and after floods [3–5]. The annual incidence of this disease is estimated at 10–100 per 100,000 in tropical regions and 0.1–1.0 per 100,000 in temperate areas [6]. In recent years, leptospirosis outbreaks have occurred all over the world; thus, an adequate disease notification system would be useful to create surveillance networks [7]. Leptospirosis is transmitted to humans primarily by water contaminated with the urine of either wild or domestic mammals that have been chronically colonized by Leptospira spp [8]. It has recently been reported that Leptospira can persist in certain organs, indicating that people themselves can act as hosts [9].
In developed countries, the transmission mechanism is mainly associated with occupational and recreational activities [10–14]. The infection may be nonsymptomatic or may result in different clinical conditions ranging from a mild “flu-like” disease to a severe form known as Weil's disease [15–19]. Icterohemorrhagic syndrome is a severe form of leptospirosis in which symptoms comprise hepatitis, hemorrhage, acute lung injury, and renal failure [3, 18, 20, 21].
The leptospiral genome is greater than that of other spirochetes such as Treponema sp, which may explain the ability of Leptospira to live in several different environments and hosts [22, 23]. Leptospira species were recently grouped according to their genetic homology [24, 25], and studies aimed at the development of an efficacious vaccine are underway [26, 27].
After reaching the blood stream, spirochetes preferentially colonize the liver and kidney [28]. These organs can offer a large lipid supply because fatty acids are an essential requirement for leptospiral growth [29, 30]. There is evidence that leptospiras form a biofilm during kidney colonization in the proximal renal tubule lumen of rabbit novergicus [31]. Leptospiras can, however, also be found in other organs such as the lung and central nervous system [29, 30].
2. Pathogenesis
Toxin production and/or the host immune response seem to be the main pathogenic mechanisms in leptospirosis. Like other spirochetes, leptospiras have a distinctive double membrane architecture that shares characteristics of both Gram-positive and Gram-negative bacteria [32].
A large proportion of the structural and functional outer membrane proteins (OMPs) is either lipoproteins such as LipL 32, LipL 21, and LipL 41 [33] or integral membrane proteins such as the porin OmpL1 [34]. In particular, OMPs may play key roles in pathogenesis by acting as adhesion or antigenic targets for bactericidal antibodies, receptors for various host molecules, and/or porins. Recent studies using five independent experimental methods have identified four novel surface-exposed and membrane-integrated leptospiral proteins (OmpL36, OmpL37, OmpL47, and OmpL54), although no functional roles have been described for them [35]. OmpA70 was identified in L. interrogans serovar Copenhageni [36] and the Lsa66 is a novel OmpA-like protein with dual activity that may promote the attachment of Leptospira to host tissues and may contribute to leptospiral invasion [37], indicating that OmpA-like proteins may have a role in leptospirosis pathogenesis.
Virulence, characterized by mobility and the ability to invade tissues, may be associated with some lipopolysaccharides and adhesins [38–40]. Bacterial mobility likely plays a major role in the disease process of multiple spirochetes [41]. The ability to move rapidly in a sticky environment could contribute to the ability of the spirochete to cross through epithelial cells [38]. In vitro, pathogenic leptospiras penetrate the intercellular junction of endothelial cells while saprophytic L. biflexa do not [39]. The ability of leptospiras to penetrate and disseminate in mammalian tissue also depends on their ability to attach to cells and to the extracellular matrix. In vitro, L. interrogans binds to a variety of cell lines including fibroblasts, endothelial cells, and kidney epithelial cells [42].
Some proteins are potential virulence factors and have a role in bacterial adhesion to host tissues, such as the Lig protein and the leptospiral endostatin-like (Len) outer membrane proteins [43, 44]. Pathogenic leptospiras also express surface-exposed proteins that possess bacterial immunoglobulin-like domains such as LigA, LigB, and LigC, which are adhesin candidates [45]. Recent work has shown that LigB binds fibrinogen and inhibits fibrin formation [46]. Several groups have reported that immunization with the LigA-unique region conferred protection from lethal infection in both a mouse model [47] and a hamster model [48, 49] of leptospirosis. In addition, resistance in hamsters seems to depend on an immunity against a conformational epitope of Lig A that includes domains 11 and 12 and a third flanking domain (either 10 or 13) that may be required for proper conformational folding [50]. Moreover, the endostatin-like protein A (Len A) was shown to bind to the host component laminin [51] and to human plasminogen [52].
Comparative studies of different serovar genomes have suggested that other components such as integrin alpha-like protein (also an adhesin candidate), lipopolysaccharides, cell surface capsular polysaccharides, and exopolysaccharides may also play a role in bacterial survival in specific host organs [22]. The OmpA-like protein Loa22 was reported to be essential for leptospiral virulence [53] and to promote inflammatory responses in cultured rat renal cells [54]. The virulence factor Loa22 is a highly conserved lipoprotein with a peptidoglycan-binding motif similar to OmpA that is upregulated during acute leptospira infection [19]. Hemoxygenase, FliY (flagellar motor switch protein), and LPS are other recognized virulence factors [32].
Other molecules that could play a part in leptospira infection include potential toxins such as the hemolysin SphH, a pore-forming protein without sphingomyelinase or phospholipase activities [55], and the enzyme catalase (KatE), which is produced only by pathogenic strains and is involved in resistance to oxidative killing [22, 56].
3. Leptospira Metabolism and Endotoxins
Leptospiras are strictly aerobic spirochetes. In their culture medium, they require ammonia as the nitrogen source [57] and long chain fatty acids as the sole carbon and fuel sources [58], and they obtain energy through the fatty acid β-oxidation pathway [29]. The most commonly used culture medium is Ellinghausen-McCullough/Johnson-Harris medium, which contains oleic acid, bovine serum-albumin, and polysorbate [19].
The biological activity of the lipopolysaccharide-like substance (LLS) extracted from the L. interrogans serovar canicola was weaker than the lipopolysaccharide (LPS) obtained from other gram-negative bacteria [59]. Lipid A is the active component of LPS and is responsible for its toxic activity. The lipid A of leptospiral LPS has an unusual fatty acid composition and, more strikingly, a unique methylated phosphate residue [60]. Leptospiral lipid A is structurally and functionally different than the lipid A of E. coli [61]. The glycolipoprotein fraction (GLP) is another leptospiral component that has cytotoxic activity [62].
Due to their peculiar metabolism, leptospiras are able to store lipids such as fatty acids [62, 63]. Some lipids are stored associated with GLP (palmitovacenic, linoleic, and oleic acids) [62], while others are stored associated with LPS and LLS (hydroxylauric, palmitic, and oleic acids) [64, 65]. These reports indicate that leptospiras are able to store and associate fatty acids with their endotoxins (LPS and GLP). This ability may have important pathophysiological consequences.
4. Toll-Like Receptors and Immune Response in Leptospirosis
The innate immune response is based on the recognition of pathogen-associated molecular patterns (PAMPs) [66, 67]. Immune cells express proteins called pathogen recognition receptors (PRRs) that allow them to recognize conserved microbial motifs such as peptidoglycans and LPS [68–70].
TLR4 was the first PRR to be described and was identified in 1997 [71]. TLR4 shows a highly orchestrated usage of coreceptors to discriminate between ligands. This receptor signals the presence of LPS in association with the CD14 [72] and MD-2 proteins [73]. This multifaceted receptor system additionally plays a role in triggering several signal transduction pathways [74]. For example, LPS binding to TLR4 activates transcription factors such as the nuclear factor NF-κB, which induces the production of inflammatory interleukins (IL-1β, IL-6, IL-8) and tumor necrosis factor (TNF) [69].
Another TLR, TLR2, is essential for the recognition of Gram-positive bacterium components such as the macrophage-activating lipopeptide 2 (MALP-2) and lipoarabinomannan, the main glycolipid of Mycobacterium tuberculosis [75]. In association with another TLR (TLR6), TLR2 triggers intracellular signaling through the mitogen-activated protein kinases (MAPKs) and NF-κB [70].
During leptospirosis, bacterial recognition by host is under disclosure, but Leptospira presence may be sensed through TLR4 and TLR2 receptors [76].
It is well known that LPS from Gram-negative bacteria activates the TLR4 signaling cascade. Paradoxically, L. interrogans LPS binds both CD14 and TLR2 but does not generate intracellular signaling through TLR4 activation [77]. The lipid A from Leptospira LPS apparently stimulates mouse cells through the TLR4-MD2 complex but does not induce signaling in human cells [61], indicating that there are species-specific aspects of LPS signaling that differ between mouse and human cells.
In recent years, considerable research has been conducted on the outer membrane proteins expressed by Leptospira spp. during infection. LipL32 is the major leptospiral outer membrane lipoprotein expressed during infection and is the immune-dominant antigen recognized in humoral responses against leptospirosis in humans [78, 79]. This lipoprotein is highly conserved among pathogenic Leptospira species [79] and signals through TLR2 [77], as recently confirmed by data showing the LipL32 binding to TLR2 in renal cells [80]. However, LipL32 was not required either for the development of acute leptospirosis in hamsters or for renal colonization in a rat model [81]. LipL21, the second major outer membrane protein of the Leptospira interrogans serovar Lai, exhibits potent immunogenic activity [82].
It has been reported that the Leptospira santarosai serovar Shermani activates the production of proinflammatory chemokines induced by p38 MAPK phosphorylation through TLR2 activation in proximal tubule epithelial cells in mice [83]. These same investigators also observed that OMPs and LipL32 increased TLR2 expression in human embryonic kidney cells (HEK 293). In addition, LipL32 augmented iNOS and CCL2/MCP1 mRNA expression and protein secretion via TLR2 binding [84].
The infection of guinea pigs with the L. interrogans serovar Icterohemorrhagiae increased the levels of IL-6 and TNFα mRNA in the lungs [85], and uveitis of leptospiral origin was associated with an increased production of the cytokines IL-6 and IL-8 [86]. An increase in cytokine production was also linked to a lethal outcome in leptospirosis patients [87].
C3H/HeJ mice have deficient LPS signaling and only respond to high doses of LPS [88]. Animals unable to detect LPS appropriately are susceptible to infection by Gram-negative bacteria [66]. When C3H/HeJ mice were infected with the Leptospira interrogans serovar icterohemorrhagiae, they presented with a lethal infection with morphological changes in the kidney and lungs [89] as well as sustained expression of CCL2/MCP-1 and CXCL1/KC in the lungs, which were correlated to the severity and progression of disease [90]. Another strain of mice, C57BL/10ScCr, carries a null TLR4 mutation, does not express TLR4 protein, and is resistant to high doses of LPS [88]. These animals do not express the receptor to IL-12p40. Both C3H/HeJ and C3H/SCID mice presented with a lethal outcome when infected with the Leptospira interrogans serovar Copenhageni [91]. The C3H/HeJ animals died after an intraperitoneal injection of Leptospira interrogans serovar icterohemorrhagiae, presenting with liver disease and lung hemorrhage [92].
Virulent leptospiras can protect themselves against components of the host's innate immune system, such as phagocytic cells and the complement system. Pathogenic leptospiras escape from phagocytosis and are resistant to intracellular killing mechanisms [93, 94]. To establish a successful leptospirosis infection, the leptospiras must be able to evade the complement system. In contrast, nonpathogenic leptospiras are killed after exposure to the human complement system [95]. It has been shown that the acquisition of factor H (FH) and other complement modulators displayed on the Leptospira surface is crucial for bacterial survival in serum. Leptospiras isolated from patients can bind the complement system inhibitor FH, a regulatory complement protein that prevents complement activation, and can restrict the deposition of the late complement components on their surfaces [96]. Thus, binding of this major alternative complement pathway inhibitor is related to serum resistance in Leptospira spirochetes. Interestingly, FH binding was shown to be dependent upon Lig proteins [97]. The multifunctional LigB protein also binds to C3b and C4b and interferes with complement activation [98]. Lsa30, a novel leptospiral adhesion protein, may help pathogenic Leptospira to escape the immune system by interfering with the complement cascade through interaction with the C4bp regulator [99]. Lsa33 also bind to C4bp and may be important in immune evasion [100]. The recently described LcpA (leptospiral complement regulator-acquiring protein A) also binds to C4bp [101].
Acquired immunity that is protective against reinfection by Leptospira does occur, but this has been shown in animal models to be dependent on the specific Leptospira serovar [102]. Specific antibodies to Leptospira membrane proteins may play a role in host defense [103] in animal vaccination models. Vaccines prepared with the LipL21 antigen protected guinea pigs from leptospiral infection [82], but there is currently no consensus regarding which signaling pathway is involved. Recent work showed that murine B cells were crucial to clearing Leptospira, through both early IgM production against LPS, which depends on TLR4, and protective IFNγ production, which depends on TLR2 and TLR4 activation [104]. It has also been shown that cattle immunized with a killed Leptospira vaccine develop protective immunity associated with CD4+ T cells and γδT cells [105]. Nevertheless, patients who have recovered from leptospirosis do not seem to generate memory T cells that can be activated by in vitro stimulation with Leptospiral protein antigens [106].
5. New Insights
When humans come in contact with contaminated water or soil, pathogenic leptospirasenter the blood stream either via skin lesions or by actively penetrating the mucosa and colonizing organs such as the kidney and liver (Figure 1). Meanwhile, the immune system induces bacterial lysis, releasing many antigens, including the glycolipoprotein GLP and LPS.
Figure 1.
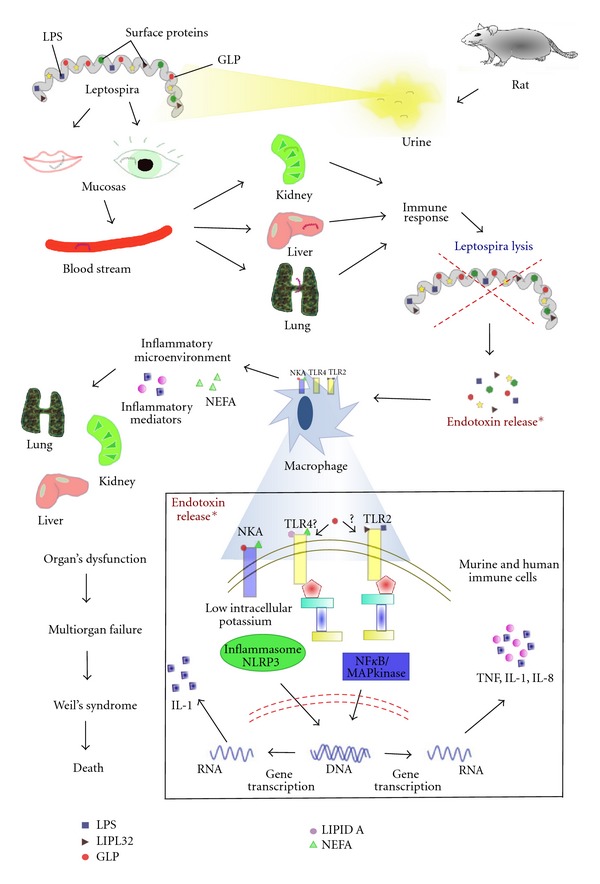
Severe leptospirosis: from the infection to immunological target. Due to their mobility, leptospiras are able to penetrate mucosal tissues and injured skin. Transported by the blood stream, they reach target organs, mainly the kidney and liver. The host immune response kills the bacteria, promoting endotoxin release. The innate immune system of both human and mouse recognizes endotoxins through specific receptors. This immune cell response is mediated by Toll-like receptors and Na/K-ATPase, which sense antigen molecules and trigger intracellular signaling pathways driving the translocation of transcription factors, leading to increased inflammatory mediator production. This scenario creates an inflammatory microenvironment that can lead to organ dysfunction. Another important observation in this disease is the increased NEFA levels in the systemic circulation (mainly oleic acid). Augmented albumin unbound-NEFA may play an important role in multiorgan dysfunction by acting on endothelium and immune cells. TLR2: Toll-like receptor 2; TLR4: Toll-like receptor 4; NKA: Na/K-ATPase; NF-κB: nuclear factor kappa-light-chain-enhancer of activated B cells; NEFA: nonesterified fatty acid; LIPL32: major outer membrane leptospiral lipoprotein; GLP: leptospiral glycolipoprotein.
The hypothesis that Leptospira produces an endotoxin released after bacterial lysis due to the host immune response was investigated and is supported by clinical and histopathological observations [107]. Nevertheless, the severity of Weil's syndrome seems to be related not only to the virulence and toxin liberation from the infective serovar but also to the intensity and the speed of the host immune response [3, 108]. The production of specific antibodies is essential to protect mice from Leptospira infection because macrophages can only efficiently phagocytose leptospiras in the presence of a specific antibody [109]. The L. interrogans GLP is also released by bacterial lysis and can activate inflammatory cells, such as peripheral blood mononuclear cells (PBMC), leading to an increased production of TNFα and IL-6 [16], an increased expression of the adhesion molecule CD69, and an augmented secretion of prostaglandin E2, leukotriene B4, and nitric oxide [110].
Acute lung injury (ALI) is characterized by cytokine release and the loss of epithelium/endothelium integrity. The increased permeability leads to protein extravasation and edema. This is the hallmark of all ALI/ARDS [111]. The presence of leptospiras and leptospiral antigens in lung endothelial cells is thought to be evidence that pulmonary lesions are triggered by bacteria and their toxic products [3, 112, 113]. Patients with fatal leptospirosis generally suffer extensive pulmonary hemorrhage [114]. Leptospira infections in monkeys mimic the features of severe human leptospirosis, including pulmonary hemorrhage [115]. The pulmonary hemorrhage is thought to be linked to the deposition of immunoglobulin and complement in the alveolar septa [116]. Pulmonary hemorrhage is a serious life-threatening disorder and is the major cause of death due to leptospirosis in Brazil [18].
In the lung, the enzyme adenosine triphosphatase is activated by Na+, K+, and Mg++ (Na/K-ATPase) and removes sodium from alveolar fluid, contributing to edema clearance and acting as a homeostatic mechanism to maintain lung integrity [117–119]. Inhibition of the Na/K pump in this organ may contribute significantly to lung failure in severe cases [120]. The kidney is another important leptospiral target, and acute kidney injury is an early manifestation of leptospirosis [121]. Inhibition of the Na/K pump in the kidney leads to loss of potassium and to hypokalemia [122]. Indeed, acute renal failure in leptospirosis is initially characterized by hypokalemia [123, 124]. Dysfunctional Na+ transporters in the kidney and lung have already been observed in the context of this disease [125]. Interestingly, engulfed GLP has been detected in phagocytes in the kidney [126] and, as we have demonstrated, is a specific Na/K-ATPase inhibitor [127].
The liver is another organ that is affected in leptospirosis infections. Inhibition of Na/K-ATPase in liver contributes to liver functional disorder and causes decreased albumin and increased nonesterified fatty acids (NEFA) and bilirubin in the plasma [127]. We also showed that this inhibition may be caused by nonesterified monounsaturated fatty acids (NEUFA) such as oleic and linoleic acids, which are GLP components and are substantially augmented in the plasma of patients with severe leptospirosis [128]. High NEFA levels are characteristic of patients with severe leptospirosis and other inflammatory conditions [128]. Increased circulating levels of NEFA also occur in some respiratory diseases, and as NEFA are known to be immune-stimulatory agents [129], this increase may directly contribute to systemic inflammation and more severe disease by stimulating the production of inflammatory mediators [130]. High levels of circulating NEFA can either inhibit or activate TLR4, triggering the inflammatory response [131]. Similar to LPS, saturated fatty acids can induce inflammatory responses in dendritic cells [132], although polyunsaturated fatty acids negatively modulate TLR4 [133]. Fatty acids such as lauric, palmitic, and oleic acids activate TLR4 in adipocytes and macrophages, leading to augmented IL-6 and TNFα production [130]. Furthermore, NEFA binding to free fatty acid receptors stimulates intracellular responses, augmenting the formation of inflammatory mediators [134, 135] via the activation of NF-κB and AP-1, as demonstrated in human endothelial cells [136].
Recently, Na/K-ATPase has been described as a receptor for intracellular signaling cascades. In this novel role, the enzyme functions as a receptor for nanomolar ouabain concentrations and other cardiac glycosides and triggers intracellular signaling cascades without changing the intracellular Na+ and K+ concentrations [137, 138]. Protein interactions with Na/K-ATPase have an important role in membrane rafts, which are linked to calcium signaling [139], and can be released through IP3 receptor binding [140]. In the presence of ouabain, calcium oscillations lead to NF-κB activation [141] and ERK/MAPK activation, which may lead to the activation of the transcription factor AP-1 [142]. The ouabain effects in signal transduction occur through a pool of Na/K-ATPase without interfering with pump activity [143]. In this respect, it was demonstrated that ouabain acts on lymphocytes without depolarizing the membrane, suggesting a mechanism that is independent of classic pump inhibition [144].
Na/K-ATPase binding triggers intracellular pathways that lead to the production of proinflammatory mediators [136, 137]. The binding of ouabain to Na/K-ATPase induces mononuclear cells to secrete TNF-α and IL-1 [145]. In the context of inflammatory leptospirosis, monocytes stimulated by leptospiras and their extracts respond by activating intracellular pathways, phosphorylating p38, activating NF-κB, and releasing cytokines and nitric oxide [94, 146]. The relevance of inflammatory mediators to the physiopathology of experimental and clinical leptospirosis is well known. Hamsters infected with L. interrogans sorovar Icterohemorrhagiae that exhibit lung injury had increased mRNA levels of TNF and IL-6 [85]. Components of Leptospira are able to induce TNF release [147]. The L. interrogans GLP, a bacterial fraction that inhibits Na/K-ATPase [122, 127, 148], is able to induce inflammatory cell activation and increase TNFα and IL-6 production [16]. Increased TNF production is a predictor of poor clinical outcome in patients with leptospirosis [149]. Furthermore, the uveitis seen in leptospirosis is associated with a rise in IL-6, IL-8, TNF-α, and IL-10 production [86]. Increased cytokine production is associated with increased patient mortality during the disease progression [87]. IL-1β and IL-18 are produced by inflammasome activation [150]. The inflammasome consists of several proteins, of which NLRP3 is involved in the recognition of bacterial RNA, ATP, uric acid, and low intracellular potassium concentrations [151]. A recent report showed that Leptospira induces production of the cytokine IL1β through synergy between LPS signaling via TLRs and leptospiral GLP, which inhibits the Na/K ATPase, triggers a decrease in intracellular potassium levels, and activates the NLRP3 inflammasome [152]. Thus, it is possible that the increased production of inflammatory mediators in leptospirosis is related both to recognition mechanisms involving TLR4 and fatty acid receptors and to a mechanism dependent on Na/K-ATPase signaling. In this way, both GLP and ouabain inhibit Na/K-ATPase and induce the production of inflammatory mediators directly involved in the pathophysiology of leptospirosis.
We cannot dismiss the hypothesis that GLP, also a specific Na/K-ATPase inhibitor, and the increased NEFA concentrations observed in the plasma of leptospirosis patients, represent a novel mechanism of triggering the inflammatory cascade, leading to the exacerbation of the immune response associated with the multiorgan dysfunction observed in this disease.
6. Final Remarks
In summary, the existing data still form an incomplete picture. TLR4 seems to be a crucial effector in the fight against Leptospira and is directly involved in the development of resistance to leptospiral infection. TLR2 also has an important role in leptospiral protein and LPS recognition. Furthermore, both TLR4 and TLR2 seem to be involved in the protection against pathogenic Leptospira antigens. Although TLR4 and TLR2 are directly implicated in the immune response to this disease, other mechanisms could be involved in the recognition of leptospiral molecular patterns. Some candidates are now emerging.
Leptospira components that are directly released after bacterial lysis may be involved in the pathophysiology of this disease either by causing direct injury or by triggering inflammation. In this respect, Na/K-ATPase alterations caused by GLP binding or by increased plasma levels of NEFA can trigger direct or indirect damage through the exacerbation of the inflammatory response.
Conflict of Interests
The authors declare no conflict of interests.
Abbreviations
- TLR:
Toll-like receptor
- LipL:
Leptospiral outer membrane lipoprotein
- OMP:
Outer membrane protein
- GLP:
Glycolipoprotein fraction
- FliY:
Flagellar motor switch protein
- KAtE:
Enzyme catalase
- PRR:
Pathogen recognition receptor
- (LLS):
Lipopolysaccharide-like substance
- (LPS):
Lipopolysaccharide
- IL:
Interleukin
- TNF:
Tumor necrosis factor
- MALP-2:
Macrophage-activating lipopeptide 2
- MAPK:
Mitogen-activated protein kinase
- HEK 293:
Embryonic kidney cells
- (PBMC):
Peripheral blood mononuclear cells
- ALI:
Acute lung injury
- NEFA:
Nonesterified fatty acids
- NEUFA:
Nonesterified monounsaturated fatty acids
- AP-1:
Activator protein
- NF-κB:
Nuclear factor kappa-light-chain-enhancer of activated B cells.
References
- 1.Adler B, de la Peña Moctezuma A. Leptospira and leptospirosis. Veterinary Microbiology. 2010;140(3-4):287–296. doi: 10.1016/j.vetmic.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 2.Bharti AR, Nally JE, Ricaldi JN, et al. Leptospirosis: a zoonotic disease of global importance. The Lancet Infectious Diseases. 2003;3(12):757–771. doi: 10.1016/s1473-3099(03)00830-2. [DOI] [PubMed] [Google Scholar]
- 3.Dolhnikoff M, Mauad T, Bethlem EP, Carvalho CRR. Pathology and pathophysiology of pulmonary manifestations in leptospirosis. Brazilian Journal of Infectious Diseases. 2007;11(1):142–148. doi: 10.1590/s1413-86702007000100029. [DOI] [PubMed] [Google Scholar]
- 4.Reis RB, Ribeiro GS, Felzemburgh RDM, et al. Impact of environment and social gradient on Leptospira infection in urban slums. PLoS Neglected Tropical Diseases. 2008;2(4, article e228) doi: 10.1371/journal.pntd.0000228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ko AI, Galvão Reis M, Ribeiro Dourado CM, Johnson WD, Riley LW. Urban epidemic of severe leptospirosis in Brazil. The Lancet. 1999;354(9181):820–825. doi: 10.1016/s0140-6736(99)80012-9. [DOI] [PubMed] [Google Scholar]
- 6.W.H.O. Human Leptospirosis: Guidance for Diagnosis, Surveillance and Control. WHO library Cataloguing-in-Publication Data Malta; 2003. (W.H. Organization and I.L. Society). [Google Scholar]
- 7.Pappas G, Papadimitriou P, Siozopoulou V, Christou L, Akritidis N. The globalization of leptospirosis: worldwide incidence trends. International Journal of Infectious Diseases. 2008;12(4):351–357. doi: 10.1016/j.ijid.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 8.Faria MTD, Calderwood MS, Athanazio DA, et al. Carriage of Leptospira interrogans among domestic rats from an urban setting highly endemic for leptospirosis in Brazil. Acta Tropica. 2008;108(1):1–5. doi: 10.1016/j.actatropica.2008.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ganoza CA, Matthias MA, Saito M, Cespedes M, Gotuzzo E, Vinetz JM. Asymptomatic renal colonization of humans in the Peruvian Amazon by Leptospira . PLoS Neglected Tropical Diseases. 2010;4(2, article e612) doi: 10.1371/journal.pntd.0000612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Palaniappan RUM, Ramanujam S, Chang YF. Leptospirosis: pathogenesis, immunity, and diagnosis. Current Opinion in Infectious Diseases. 2007;20(3):284–292. doi: 10.1097/QCO.0b013e32814a5729. [DOI] [PubMed] [Google Scholar]
- 11.Songer JG, Thiermann AB. Leptospirosis. Journal of the American Veterinary Medical Association. 1988;193(10):1250–1254. [PubMed] [Google Scholar]
- 12.Levett PN. Leptospirosis: a forgotten zoonosis? Clinical and Applied Immunology Reviews. 2004;4(6):435–448. [Google Scholar]
- 13.Baranton G, Postic D. Trends in leptospirosis epidemiology in France. Sixty-six years of passive serological surveillance from 1920 to 2003. International Journal of Infectious Diseases. 2006;10(2):162–170. doi: 10.1016/j.ijid.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 14.Van Crevel R, Speelman P, Gravekamp C, Terpstra WJ. Leptospirosis in travelers. Clinical Infectious Diseases. 1994;19(1):132–134. doi: 10.1093/clinids/19.1.132. [DOI] [PubMed] [Google Scholar]
- 15.Martínez García MA, De Diego Damiá A, Villanueva RM, López Hontagas JL. Pulmonary involvement in leptospirosis. European Journal of Clinical Microbiology and Infectious Diseases. 2000;19(6):471–474. doi: 10.1007/s100960000294. [DOI] [PubMed] [Google Scholar]
- 16.Dorigatti F, Brunialti MKC, Romero EC, Kallas EG, Salomão R. Leptospira interrogans activation of peripheral blood monocyte glycolipoprotein demonstrated in whole blood by the release of IL-6. Brazilian Journal of Medical and Biological Research. 2005;38(6):909–914. doi: 10.1590/s0100-879x2005000600013. [DOI] [PubMed] [Google Scholar]
- 17.McBride AJA, Athanazio DA, Reis MG, Ko AI. Leptospirosis. Current Opinion in Infectious Diseases. 2005;18(5):376–386. doi: 10.1097/01.qco.0000178824.05715.2c. [DOI] [PubMed] [Google Scholar]
- 18.Gouveia EL, Metcalfe J, De Carvalho ALF, et al. Leptospirosis-associated severe pulmonary hemorrhagic syndrome, Salvador, Brazil. Emerging Infectious Diseases. 2008;14(3):505–508. doi: 10.3201/eid1403.071064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Evangelista KV, Coburn J. Leptospira as an emerging pathogen: a review of its biology, pathogenesis and host immune responses. Future Microbiology. 2010;5(9):1413–1425. doi: 10.2217/fmb.10.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dobrina A, Nardon E, Vecile E, Cinco M, Patriarca P. Leptospira icterohemorrhagiae and leptospire peptidolgycans induce endothelial cell adhesiveness for polymorphonuclear leukocytes. Infection and Immunity. 1995;63(8):2995–2999. doi: 10.1128/iai.63.8.2995-2999.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sekkach Y, Qaçif H, Jira M, El qatni M, El omri N, Ghafir D. Acute respiratory distress revelated asever pulmonary leptospirosis. Revue de Medecine Interne. 2007;28(1):48–51. doi: 10.1016/j.revmed.2006.09.021. [DOI] [PubMed] [Google Scholar]
- 22.Nascimento ALTO, Ko AI, Martins EAL, et al. Comparative genomics of two Leptospira interrogans serovars reveals novel insights into physiology and pathogenesis. Journal of Bacteriology. 2004;186(7):2164–2172. doi: 10.1128/JB.186.7.2164-2172.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ren SX, Fu G, Jiang XG, et al. Unique physiological and pathogenic features of Leptospira interrogans revealed by whole-genome sequencing. Nature. 2003;422(6934):888–893. doi: 10.1038/nature01597. [DOI] [PubMed] [Google Scholar]
- 24.Vijayachari P, Sugunan AP, Shriram AN. Leptospirosis: an emerging global public health problem. Journal of Biosciences. 2008;33(4):557–569. doi: 10.1007/s12038-008-0074-z. [DOI] [PubMed] [Google Scholar]
- 25.Nalam K, Ahmed A, Devi SM, et al. Genetic affinities within a large global collection of pathogenic Leptospira: implications for strain identification and molecular epidemiology. PLoS ONE. 2010;5(8) doi: 10.1371/journal.pone.0012637.e12637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koizumi N, Watanabe H. Identification of a novel antigen of pathogenic Leptospira spp. that reacted with convalescent mice sera. Journal of Medical Microbiology. 2003;52(7):585–589. doi: 10.1099/jmm.0.05148-0. [DOI] [PubMed] [Google Scholar]
- 27.Gamberini M, Gómez RM, Atzingen MV, et al. Whole-genome analysis of Leptospira interrogans to identify potential vaccine candidates against leptospirosis. FEMS Microbiology Letters. 2005;244(2):305–313. doi: 10.1016/j.femsle.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Athanazio DA, Silva EF, Santos CS, et al. Rattus norvegicus as a model for persistent renal colonization by pathogenic Leptospira interrogans . Acta Tropica. 2008;105(2):176–180. doi: 10.1016/j.actatropica.2007.10.012. [DOI] [PubMed] [Google Scholar]
- 29.Baseman JB, Cox CD. Intermediate energy metabolism of Leptospira . Journal of Bacteriology. 1969;97(3):992–1000. doi: 10.1128/jb.97.3.992-1000.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stern N, Shenberg E, Tietz A. Studies on the metabolism of fatty acids in Leptospira: the biosynthesis of delta 9- and delta 11-monounsaturated acids. European Journal of Biochemistry. 1969;8(1):101–108. doi: 10.1111/j.1432-1033.1969.tb00501.x. [DOI] [PubMed] [Google Scholar]
- 31.Ristow P, Bourhy P, Kerneis S, et al. Biofilm formation by saprophytic and pathogenic leptospires. Microbiology. 2008;154(5):1309–1317. doi: 10.1099/mic.0.2007/014746-0. [DOI] [PubMed] [Google Scholar]
- 32.Fraga TR, Barbosa AS, Isaac L. Leptospirosis: aspects of innate immunity, immunopathogenesis and immune evasion from the complement system. Scandinavian Journal of Immunology. 2011;73(5):408–419. doi: 10.1111/j.1365-3083.2010.02505.x. [DOI] [PubMed] [Google Scholar]
- 33.Cullen PA, Xu X, Matsunaga J, et al. Surfaceome of Leptospira spp. Infection and Immunity. 2005;73(8):4853–4863. doi: 10.1128/IAI.73.8.4853-4863.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shang ES, Exner MM, Summers TA, et al. The rare outer membrane protein, OmpL1, of pathogenic Leptospira species is a heat-modifiable porin. Infection and Immunity. 1995;63(8):3174–3181. doi: 10.1128/iai.63.8.3174-3181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinne M, Haake DA. A comprehensive approach to identification of surface-exposed, outer membrane-spanning proteins of Leptospira interrogans . PLoS ONE. 2009;4(6) doi: 10.1371/journal.pone.0006071.e6071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Fraga TR, Chura-Chambi RM, Gonçales AP, et al. Refolding of the recombinant protein OmpA70 from Leptospira interrogans from inclusion bodies using high hydrostatic pressure and partial characterization of its immunological properties. Journal of Biotechnology. 2010;148(2-3):156–162. doi: 10.1016/j.jbiotec.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Oliveira R, de Morais ZM, Gonçales AP, Romero EC, Vasconcellos SA, Nascimento ALTO. Characterization of novel OmpA-like protein of Leptospira interrogans that binds extracellular matrix molecules and plasminogen. PLoS ONE. 2011;6(7) doi: 10.1371/journal.pone.0021962.e21962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito T, Yanagawa R. Leptospiral attachment to extracellular matrix of mouse fibroblast (L929) cells. Veterinary Microbiology. 1987;15(1-2):89–96. doi: 10.1016/0378-1135(87)90133-7. [DOI] [PubMed] [Google Scholar]
- 39.Thomas DD, Higbie LM. In vitro association of leptospires with host cells. Infection and Immunity. 1990;58(3):581–585. doi: 10.1128/iai.58.3.581-585.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Liu Y, Zheng W, Li L, Mao Y, Yan J. Pathogenesis of leptospirosis: interaction of Leptospira interrogans with in vitro cultured mammalian cells. Medical Microbiology and Immunology. 2007;196(4):233–239. doi: 10.1007/s00430-007-0047-0. [DOI] [PubMed] [Google Scholar]
- 41.Charon NW, Goldstein SF. Genetics of motility and chemotaxis of a fascinating group of bacteria: the spirochetes. Annual Review of Genetics. 2002;36:47–73. doi: 10.1146/annurev.genet.36.041602.134359. [DOI] [PubMed] [Google Scholar]
- 42.Breiner DD, Fahey M, Salvador R, Novakova J, Coburn J. Leptospira interrogans binds to human cell surface receptors including proteoglycans. Infection and Immunity. 2009;77(12):5528–5536. doi: 10.1128/IAI.00546-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choy HA, Kelley MM, Chen TL, Møller AK, Matsunaga J, Haake DA. Physiological osmotic induction of Leptospira interrogans adhesion: LigA and LigB bind extracellular matrix proteins and fibrinogen. Infection and Immunity. 2007;75(5):2441–2450. doi: 10.1128/IAI.01635-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barbosa AS, Abreu PAE, Neves FO, et al. A newly identified leptospiral adhesin mediates attachment to laminin. Infection and Immunity. 2006;74(11):6356–6364. doi: 10.1128/IAI.00460-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Matsunaga J, Barocchi MA, Croda J, et al. Pathogenic Leptospira species express surface-exposed proteins belonging to the bacterial immunoglobulin superfamily. Molecular Microbiology. 2003;49(4):929–945. doi: 10.1046/j.1365-2958.2003.03619.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Choy HA, Kelley MM, Croda J, et al. The multifunctional LigB adhesin binds homeostatic proteins with potential roles in cutaneous infection by pathogenic Leptospira interrogans . PLoS ONE. 2011;6(2) doi: 10.1371/journal.pone.0016879.e16879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Koizumi N, Watanabe H. Leptospiral immunoglobulin-like proteins elicit protective immunity. Vaccine. 2004;22(11-12):1545–1552. doi: 10.1016/j.vaccine.2003.10.007. [DOI] [PubMed] [Google Scholar]
- 48.Silva ÉF, Medeiros MA, McBride AJA, et al. The terminal portion of leptospiral immunoglobulin-like protein LigA confers protective immunity against lethal infection in the hamster model of leptospirosis. Vaccine. 2007;25(33):6277–6286. doi: 10.1016/j.vaccine.2007.05.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Faisal SM, Yan W, Chen CS, Palaniappan RUM, McDonough SP, Chang YF. Evaluation of protective immunity of Leptospira immunoglobulin like protein A (LigA) DNA vaccine against challenge in hamsters. Vaccine. 2008;26(2):277–287. doi: 10.1016/j.vaccine.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 50.Coutinho ML, Choy HA, Kelley MM, et al. A ligA three-domain region protects hamsters from lethal infection by Leptospira interrogans . PLoS Neglected Tropical Diseases. 2011;5(12) doi: 10.1371/journal.pntd.0001422.e1422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stevenson B, Choy HA, Pinne M, et al. Leptospira interrogans endostatin-like outer membrane proteins bind host fibronectin, laminin and regulators of complement. PLoS ONE. 2007;2(11) doi: 10.1371/journal.pone.0001188.e1188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Verma A, Brissette CA, Bowman AA, Shah ST, Zipfel PF, Stevenson B. Leptospiral endostatin-like protein a is a bacterial cell surface receptor for human plasminogen. Infection and Immunity. 2010;78(5):2053–2059. doi: 10.1128/IAI.01282-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ristow P, Bourhy P, da Cruz McBride FW, et al. The OmpA-like protein Loa22 is essential for leptospiral virulence. PLoS Pathogens. 2007;3(7):p. e97. doi: 10.1371/journal.ppat.0030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang Y, Bao L, Zhu H, Huang B, Zhang H. OmpA-like protein Loa22 from Leptospira interrogans serovar Lai is cytotoxic to cultured rat renal cells and promotes inflammatory responses. Acta Biochimica et Biophysica Sinica. 2010;42(1):70–79. doi: 10.1093/abbs/gmp109. [DOI] [PubMed] [Google Scholar]
- 55.Lee SH, Kim S, Park SC, Kim MJ. Cytotoxic activities of Leptospira interrogans hemolysin SphH as a pore-forming protein on mammalian cells. Infection and Immunity. 2002;70(1):315–322. doi: 10.1128/IAI.70.1.315-322.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Picardeau M, Bulach DM, Bouchier C, et al. Genome sequence of the saprophyte Leptospira biflexa provides insights into the evolution of Leptospira and the pathogenesis of leptospirosis. PLoS ONE. 2008;3(2) doi: 10.1371/journal.pone.0001607.e1607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Johnson RC, Rogers P. Metabolism of leptospirae. I. Utilization of amino acids and purine, and pyrimidine bases. Archives of Biochemistry and Biophysics. 1964;107(3):459–470. doi: 10.1016/0003-9861(64)90302-9. [DOI] [PubMed] [Google Scholar]
- 58.Johnson RC, Walby JK. Cultivation of leptospires: fatty acid requirements. Applied Microbiology. 1972;23(5):1027–1028. doi: 10.1128/am.23.5.1027-1028.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shimizu T, Matsusaka E, Takayanagi K. Biological activities of lipopolysaccharide-like substance (LLS) extracted from Leptospira interrogans serovar canicola strain Moulton. Microbiology and Immunology. 1987;31(8):727–735. doi: 10.1111/j.1348-0421.1987.tb03134.x. [DOI] [PubMed] [Google Scholar]
- 60.Que-Gewirth NLS, Ribeiro AA, Kalb SR, et al. A methylated phosphate group and four amide-linked acyl chains in Leptospira interrogans lipid A: the membrane anchor of an unusual lipopolysaccharide that activates TLR2. Journal of Biological Chemistry. 2004;279(24):25420–25429. doi: 10.1074/jbc.M400598200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nahori MA, Fournié-Amazouz E, Que-Gewirth NS, et al. Differential TLR recognition of leptospiral lipid A and lipopolysaccharide in murine and human cells. Journal of Immunology. 2005;175(9):6022–6031. doi: 10.4049/jimmunol.175.9.6022. [DOI] [PubMed] [Google Scholar]
- 62.Vinh T, Adler B, Faine S. Glycolipoprotein cytotoxin from Leptospira interrogans serovar copenhageni . Journal of General Microbiology. 1986;132(1):111–123. doi: 10.1099/00221287-132-1-111. [DOI] [PubMed] [Google Scholar]
- 63.Arimitsu Y, Moribayashi A, Goto N. Skin reaction to lipids from avirulent strain Shibaura of Leptospira interrogans serovar copenhageni . Canadian Journal of Microbiology. 1989;35(11):1009–1014. doi: 10.1139/m89-168. [DOI] [PubMed] [Google Scholar]
- 64.Vinh T, Adler B, Faine S. Ultrastructure and chemical composition of lipopolysaccharide extracted from Leptospira interrogans serovar copenhageni . Journal of General Microbiology. 1986;132(1):103–109. doi: 10.1099/00221287-132-1-103. [DOI] [PubMed] [Google Scholar]
- 65.Shimizu T, Matsusaka E, Nagakura N. Chemical properties of lipopolysaccharide-like substance (LLS) extracted from Leptospira interrogans serovar canicola strain Moulton. Microbiology and Immunology. 1987;31(8):717–725. doi: 10.1111/j.1348-0421.1987.tb03133.x. [DOI] [PubMed] [Google Scholar]
- 66.Beutler B. Innate immune sensing of microbial infection: the mechanism and the therapeutic challenge. Wiener Medizinische Wochenschrift. 2002;152(21-22):547–551. doi: 10.1046/j.1563-258x.2002.02097.x. [DOI] [PubMed] [Google Scholar]
- 67.Creagh EM, O’Neill LAJ. TLRs, NLRs and RLRs: a trinity of pathogen sensors that co-operate in innate immunity. Trends in Immunology. 2006;27(8):352–357. doi: 10.1016/j.it.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 68.Kawai T, Akira S. TLR signaling. Seminars in Immunology. 2007;19(1):24–32. doi: 10.1016/j.smim.2006.12.004. [DOI] [PubMed] [Google Scholar]
- 69.Verstak B, Hertzog P, Mansell A. Toll-like receptor signalling and the clinical benefits that lie within. Inflammation Research. 2007;56(1):1–10. doi: 10.1007/s00011-007-6093-7. [DOI] [PubMed] [Google Scholar]
- 70.Underhill DM, Ozinsky A. Toll-like receptors: key mediators of microbe detection. Current Opinion in Immunology. 2002;14(1):103–110. doi: 10.1016/s0952-7915(01)00304-1. [DOI] [PubMed] [Google Scholar]
- 71.Medzhitov R, Preston-Hurlburt P, Janeway CA. A human homologue of the Drosophila toll protein signals activation of adaptive immunity. Nature. 1997;388(6640):394–397. doi: 10.1038/41131. [DOI] [PubMed] [Google Scholar]
- 72.Mollnes TE, Christiansen D, Brekke OL, Espevik T. Hypothesis: combined inhibition of complement and cd14 as treatment regimen to attenuate the inflammatory response. Advances in Experimental Medicine and Biology. 2008;632:253–263. [PubMed] [Google Scholar]
- 73.Akashi S, Shimazu R, Ogata H, et al. Cutting edge: cell surface expression and lipopolysaccharide signaling via the Toll-like receptor 4-MD-2 complex on mouse peritoneal macrophages. Journal of Immunology. 2000;164(7):3471–3475. doi: 10.4049/jimmunol.164.7.3471. [DOI] [PubMed] [Google Scholar]
- 74.Ostuni R, Zanoni I, Granucci F. Deciphering the complexity of Toll-like receptor signaling. Cellular and Molecular Life Sciences. 2010;67(24):4109–4134. doi: 10.1007/s00018-010-0464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Asong J, Wolfert MA, Maiti KK, Miller D, Boons GJ. Binding and cellular activation studies reveal that toll-like receptor 2 can differentially recognize peptidoglycan from gram-positive and gram-negative bacteria. Journal of Biological Chemistry. 2009;284(13):8643–8653. doi: 10.1074/jbc.M806633200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lesur I, Textoris J, Loriod B, et al. Gene expression profiles characterize inflammation stages in the acute lung injury in mice. PLoS ONE. 2010;5(7) doi: 10.1371/journal.pone.0011485.e11485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Werts C, Tapping RI, Mathison JC, et al. Leptospiral lipopolysaccharide activates cells through a TLR2-dependent mechanism. Nature Immunology. 2001;2(4):346–352. doi: 10.1038/86354. [DOI] [PubMed] [Google Scholar]
- 78.Hauk P, Macedo F, Romero EC, et al. In LipL32, the major leptospiral lipoprotein, the C terminus is the primary immunogenic domain and mediates interaction with collagen IV and plasma fibronectin. Infection and Immunity. 2008;76(6):2642–2650. doi: 10.1128/IAI.01639-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Haake DA, Chao G, Zuerner RL, et al. The leptospiral major outer membrane protein LipL32 is a lipoprotein expressed during mammalian infection. Infection and Immunity. 2000;68(4):2276–2285. doi: 10.1128/iai.68.4.2276-2285.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hsu SH, Lo YY, Tung JY, et al. Leptospiral outer membrane lipoprotein LipL32 binding on toll-like receptor 2 of renal cells as determined with an atomic force microscope. Biochemistry. 2010;49(26):5408–5417. doi: 10.1021/bi100058w. [DOI] [PubMed] [Google Scholar]
- 81.Murray GL, Srikram A, Hoke DE, et al. Major surface protein LipL32 is not required for either acute or chronic infection with Leptospira interrogans . Infection and Immunity. 2009;77(3):952–958. doi: 10.1128/IAI.01370-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.He H, Wang W, Wu Z, Lv Z, Li J, Tan L. Protection of guinea pigs against Leptospira interrogans serovar Lai by lipL21 DNA vaccine. Cellular and Molecular Immunology. 2008;5(5):385–391. doi: 10.1038/cmi.2008.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hung CC, Chang CT, Tian YC, et al. Leptospiral membrane proteins stimulate pro-inflammatory chemokines secretion by renal tubule epithelial cells through toll-like receptor 2 and p38 mitogen activated protein kinase. Nephrology Dialysis Transplantation. 2006;21(4):898–910. doi: 10.1093/ndt/gfi316. [DOI] [PubMed] [Google Scholar]
- 84.Yang CW, Hung CC, Wu MS, et al. Toll-like receptor 2 mediates early inflammation by leptospiral outer membrane proteins in proximal tubule cells. Kidney International. 2006;69(5):815–822. doi: 10.1038/sj.ki.5000119. [DOI] [PubMed] [Google Scholar]
- 85.Marinho M, Oliveira-Júnior IS, Monteiro CMR, Perri SH, Salomão R. Pulmonary disease in hamsters infected with Leptospira interrogans: histopathologic findings and cytokine mRNA expressions. American Journal of Tropical Medicine and Hygiene. 2009;80(5):832–836. [PubMed] [Google Scholar]
- 86.Priya CG, Rathinam SR, Muthukkaruppan V. Evidence for endotoxin as a causative factor for leptospiral uveitis in humans. Investigative Ophthalmology and Visual Science. 2008;49(12):5419–5424. doi: 10.1167/iovs.08-2174. [DOI] [PubMed] [Google Scholar]
- 87.Wagenaar JFP, Goris MGA, Gasem MH, et al. Long pentraxin PTX3 is associated with mortality and disease severity in severe Leptospirosis. Journal of Infection. 2009;58(6):425–432. doi: 10.1016/j.jinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 88.Poltorak A, He X, Smirnova I, et al. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282(5396):2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 89.Pereira MM, Andrade J, Marchevsky RS, Ribeiro Dos Santos R. Morphological characterization of lung and kidney lesions in C3H/HeJ mice infected with Leptospira interrogans serovar icterohaemorrhagiae: defect of CD4+ and CD8+ T-cells are prognosticators of the disease progression. Experimental and Toxicologic Pathology. 1998;50(3):191–198. doi: 10.1016/S0940-2993(98)80083-3. [DOI] [PubMed] [Google Scholar]
- 90.Silva JBD, Ramos TMV, de Franco M, et al. Chemokines expression during Leptospira interrogans serovar copenhageni infection in resistant BALB/c and susceptible C3H/HeJ mice. Microbial Pathogenesis. 2009;47(2):87–93. doi: 10.1016/j.micpath.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 91.Nally JE, Fishbein MC, Blanco DR, Lovett MA. Lethal infection of C3H/HeJ and C3H/SCID mice with an isolate of Leptospira interrogans serovar copenhageni . Infection and Immunity. 2005;73(10):7014–7017. doi: 10.1128/IAI.73.10.7014-7017.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Viriyakosol S, Matthias MA, Swancutt MA, Kirkland TN, Vinetz JM. Toll-like receptor 4 protects against lethal Leptospira interrogans serovar icterohaemorrhagiae infection and contributes to in vivo control of leptospiral burden. Infection and Immunity. 2006;74(2):887–895. doi: 10.1128/IAI.74.2.887-895.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Murgia R, Garcia R, Cinco M. Leptospires are killed in vitro by both oxygen-dependent and -independent reactions. Infection and Immunity. 2002;70(12):7172–7175. doi: 10.1128/IAI.70.12.7172-7175.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Cinco M. New insights into the pathogenicity of leptospires: evasion of host defences. New Microbiologica. 2010;33(4):283–292. [PubMed] [Google Scholar]
- 95.Cinco M, Banfi E. Activation of complement by leptospires and its bactericidal activity. Zentralblatt fur Bakteriologie Mikrobiologie und Hygiene A. 1983;254(2):261–265. [PubMed] [Google Scholar]
- 96.Meri T, Murgia R, Stefanel P, Meri S, Cinco M. Regulation of complement activation at the C3-level by serum resistant leptospires. Microbial Pathogenesis. 2005;39(4):139–147. doi: 10.1016/j.micpath.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 97.Castiblanco-Valencia MM, Fraga TR, Silva LBD, et al. Leptospiral immunoglobulin-like proteins interact with human complement regulators factor H, FHL-1, FHR-1, and C4BP. Journal of Infectious Diseases. 2012;205(6):995–1004. doi: 10.1093/infdis/jir875. [DOI] [PubMed] [Google Scholar]
- 98.Choy HA. Multiple activities of ligb potentiate virulence of Leptospira interrogans: inhibition of alternative and classical pathways of complement. PLoS ONE. 2012;7(7) doi: 10.1371/journal.pone.0041566.e41566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Souza NM, Vieira ML, Alves IJ, de Morais ZM, Vasconcellos SA, Nascimento ALTO. Lsa30, a novel adhesin of Leptospira interrogans binds human plasminogen and the complement regulator C4bp. Microbial Pathogenesis. 2012;53(3-4):125–134. doi: 10.1016/j.micpath.2012.06.001. [DOI] [PubMed] [Google Scholar]
- 100.Domingos RF, Vieira ML, Romero EC, et al. Features of two proteins of Leptospira interrogans with potential role in host-pathogen interactions. BMC Microbiology. 2012;12, article 50 doi: 10.1186/1471-2180-12-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Barbosa AS, Monaris D, Silva LB, et al. Functional characterization of LcpA, a surface-exposed protein of Leptospira spp. that binds the human complement regulator C4BP. Infection and Immunity. 2010;78(7):3207–3216. doi: 10.1128/IAI.00279-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adler B, Faine S. Host immunological mechanisms in the resistance of mice to leptospiral infections. Infection and Immunity. 1977;17(1):67–72. doi: 10.1128/iai.17.1.67-72.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Haake DA, Mazel MK, Mccoy AM, et al. Leptospiral outer membrane proteins OmpL1 and LipL41 exhibit synergistic immunoprotection. Infection and Immunity. 1999;67(12):6572–6582. doi: 10.1128/iai.67.12.6572-6582.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Chassin C, Picardeau M, Goujon JM, et al. TLR4- and TLR2-mediated B cell responses control the clearance of the bacterial pathogen, Leptospira interrogans . Journal of Immunology. 2009;183(4):2669–2677. doi: 10.4049/jimmunol.0900506. [DOI] [PubMed] [Google Scholar]
- 105.Naiman BM, Alt D, Bolin CA, Zuerner R, Baldwin CL. Protective killed Leptospira borgpetersenii vaccine induces potent Th1 immunity comprising responses by CD4 and γδ T lymphocytes. Infection and Immunity. 2001;69(12):7550–7558. doi: 10.1128/IAI.69.12.7550-7558.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Tuero I, Vinetz JM, Kumpel GR. Lack of demonstrable memory T cell responses in humans who have spontaneously recovered from leptospirosis in the peruvian amazon. Journal of Infectious Diseases. 2010;201(3):420–427. doi: 10.1086/650300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Finco DR, Low DG. Endotoxin properties of Leptospira canicola. American Journal of Veterinary Research. 1967;28(127):1863–1872. [PubMed] [Google Scholar]
- 108.Abdulkader RCRM, Daher EF, Camargo ED, Spinosa C, Da Silva MV. Leptospirosis severity may be associated with the intensity of humoral immune response. Revista do Instituto de Medicina Tropical de Sao Paulo. 2002;44(2):79–83. doi: 10.1590/s0036-46652002000200005. [DOI] [PubMed] [Google Scholar]
- 109.Tu V, Adler B, Faine S. The role of macrophages in the protection of mice against leptospirosis: in vitro and in vivo studies. Pathology. 1982;14(4):463–468. doi: 10.3109/00313028209092128. [DOI] [PubMed] [Google Scholar]
- 110.Diament D, Brunialti MKC, Romero EC, Kallas EG, Salomao R. Peripheral blood mononuclear cell activation induced by Leptospira interrogans glycolipoprotein. Infection and Immunity. 2002;70(4):1677–1683. doi: 10.1128/IAI.70.4.1677-1683.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Nieuwenhuizen L, De Groot PG, Grutters JC, Biesma DH. A review of pulmonary coagulopathy in acute lung injury, acute respiratory distress syndrome and pneumonia. European Journal of Haematology. 2009;82(6):413–425. doi: 10.1111/j.1600-0609.2009.01238.x. [DOI] [PubMed] [Google Scholar]
- 112.Marchiori E, Lourenço S, Setúbal S, Zanetti G, Gasparetto TD, Hochhegger B. Clinical and imaging manifestations of hemorrhagic pulmonary leptospirosis: a state-of-the-art review. Lung. 2011;189(1):1–9. doi: 10.1007/s00408-010-9273-0. [DOI] [PubMed] [Google Scholar]
- 113.Chen HI, Kao SJ, Hsu YH. Pathophysiological mechanism of lung injury in patients with leptospirosis. Pathology. 2007;39(3):339–344. doi: 10.1080/00313020701329740. [DOI] [PubMed] [Google Scholar]
- 114.Arean VM. The pathologic anatomy and pathogenesis of fatal human leptospirosis (Weil’s disease) The American Journal of Pathology. 1962;40:393–423. [PMC free article] [PubMed] [Google Scholar]
- 115.Pereira MM, Pereira Da Silva JJ, Pinto MA, et al. Experimental leptospirosis in marmoset monkeys (Callithrix jacchus): a new model for studies of severe pulmonary leptospirosis. American Journal of Tropical Medicine and Hygiene. 2005;72(1):13–20. [PubMed] [Google Scholar]
- 116.Croda J, Neto AND, Brasil RA, Pagliari C, Nicodemo AC, Duarte MIS. Leptospirosis pulmonary haemorrhage syndrome is associated with linear deposition of immunoglobulin and complement on the alveolar surface. Clinical Microbiology and Infection. 2010;16(6):593–599. doi: 10.1111/j.1469-0691.2009.02916.x. [DOI] [PubMed] [Google Scholar]
- 117.Sznajder JI, Ridge KM, Harris ZL, et al. Alveolar type II cell Na,K-ATPase is upregulated during mechanical ventilation-induced pulmonary edema. Chest. 1994;105(3, supplement):116S–117S. doi: 10.1378/chest.105.3_supplement.116s. [DOI] [PubMed] [Google Scholar]
- 118.Sznajder JI. Alveolar edema must be cleared for the acute respiratory distress syndrome patient to survive. American Journal of Respiratory and Critical Care Medicine. 2001;163(6):1293–1294. doi: 10.1164/ajrccm.163.6.ed1801d. [DOI] [PubMed] [Google Scholar]
- 119.Matthay MA. Alveolar fluid clearance in patients with ARDS: does it make a difference? Chest. 2002;122(6, supplement):340S–343S. doi: 10.1378/chest.122.6_suppl.340s. [DOI] [PubMed] [Google Scholar]
- 120.Vadász I, Morty RE, Kohstall MG, et al. Oleic acid inhibits alveolar fluid reabsorption: a role in acute respiratory distress syndrome? American Journal of Respiratory and Critical Care Medicine. 2005;171(5):469–479. doi: 10.1164/rccm.200407-954OC. [DOI] [PubMed] [Google Scholar]
- 121.Yang C-W, Wu M-S, Pan M-J. Leptospirosis renal disease. Nephrology Dialysis Transplantation. 2001;16(supplement 5):73–77. doi: 10.1093/ndt/16.suppl_5.73. [DOI] [PubMed] [Google Scholar]
- 122.Younes-Ibrahim M, Buffin-Meyer B, Cheval L, et al. Na,K-ATPase: a molecular target for Leptospira interrogans endotoxin. Brazilian Journal of Medical and Biological Research. 1997;30(2):213–223. doi: 10.1590/s0100-879x1997000200009. [DOI] [PubMed] [Google Scholar]
- 123.Ko AI, Goarant C, Picardeau M. Leptospira: the dawn of the molecular genetics era for an emerging zoonotic pathogen. Nature Reviews Microbiology. 2009;7(10):736–747. doi: 10.1038/nrmicro2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seguro AC, Lomar AV, Rocha AS. Acute renal failure of leptospirosis: nonoliguric and hypokalemic forms. Nephron. 1990;55(2):146–151. doi: 10.1159/000185943. [DOI] [PubMed] [Google Scholar]
- 125.Andrade L, Rodrigues AC, Sanches TRC, Souza RB, Seguro AC. Leptospirosis leads to dysregulation of sodium transporters in the kidney and lung. American Journal of Physiology. 2007;292(2):F586–F592. doi: 10.1152/ajprenal.00102.2006. [DOI] [PubMed] [Google Scholar]
- 126.Pereira MM, Andrade J, Lacerda MD, Batoréu NM, Marchevsky RS, Ribeiro Dos Santos R. Demonstration of leptospiral antigens on tissues using monoclonal antibodies and avidin-biotin peroxidase staining. Experimental and Toxicologic Pathology. 1997;49(6):505–511. doi: 10.1016/s0940-2993(97)80155-8. [DOI] [PubMed] [Google Scholar]
- 127.Younes-Ibrahim M, Burth P, Castro Faria MV, et al. Inhibition of Na,K-ATPase by an endotoxin extracted from Leptospira interrogans: a possible mechanism for the physiopathology of leptospirosis. Comptes Rendus de l’Academie des Sciences III. 1995;318(5):619–625. [PubMed] [Google Scholar]
- 128.Burth P, Younes-Ibrahim M, Santos MCB, Castro-Faria Neto HC, De Castro Faria MV. Role of nonesterified unsaturated fatty acids in the pathophysiological processes of leptospiral infection. Journal of Infectious Diseases. 2005;191(1):51–57. doi: 10.1086/426455. [DOI] [PubMed] [Google Scholar]
- 129.Boden G, She P, Mozzoli M, et al. Free fatty acids produce insulin resistance and activate the proinflammatory nuclear factor-κb pathway in rat liver. Diabetes. 2005;54(12):3458–3465. doi: 10.2337/diabetes.54.12.3458. [DOI] [PubMed] [Google Scholar]
- 130.Wood LG, Scott HA, Garg ML, Gibson PG. Innate immune mechanisms linking non-esterified fatty acids and respiratory disease. Progress in Lipid Research. 2009;48(1):27–43. doi: 10.1016/j.plipres.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 131.Martins De Lima T, Gorjão R, Hatanaka E, et al. Mechanisms by which fatty acids regulate leucocyte function. Clinical Science. 2007;113(1-2):65–77. doi: 10.1042/CS20070006. [DOI] [PubMed] [Google Scholar]
- 132.Weatherill AR, Lee JY, Zhao L, Lemay DG, Youn HS, Hwang DH. Saturated and polyunsaturated fatty acids reciprocally modulate dendritic cell functions mediated through TLR4. Journal of Immunology. 2005;174(9):5390–5397. doi: 10.4049/jimmunol.174.9.5390. [DOI] [PubMed] [Google Scholar]
- 133.Lee JY, Ye J, Gao Z, et al. Reciprocal modulation of toll-like receptor-4 signaling pathways involving MyD88 and phosphatidylinositol 3-kinase/AKT by saturated and polyunsaturated fatty acids. Journal of Biological Chemistry. 2003;278(39):37041–37051. doi: 10.1074/jbc.M305213200. [DOI] [PubMed] [Google Scholar]
- 134.Chi SS, Tikhonova IG, Neumann S, et al. Identification of residues important for agonist recognition and activation in GPR40. Journal of Biological Chemistry. 2007;282(40):29248–29255. doi: 10.1074/jbc.M705077200. [DOI] [PubMed] [Google Scholar]
- 135.Costanzi S, Neumann S, Gershengorn MC. Seven transmembrane-spanning receptors for free fatty acids as therapeutic targets for diabetes mellitus: pharmacological, phylogenetic, and drug discovery aspects. Journal of Biological Chemistry. 2008;283(24):16269–16273. doi: 10.1074/jbc.R800014200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Toborek M, Lee YW, Garrido R, Kaiser S, Hennig B. Unsaturated fatty acids selectively induce an inflammatory environment in human endothelial cell. American Journal of Clinical Nutrition. 2002;75(1):119–125. doi: 10.1093/ajcn/75.1.119. [DOI] [PubMed] [Google Scholar]
- 137.Bagrov AY, Shapiro JI, Fedorova OV. Endogenous cardiotonic steroids: physiology, pharmacology, and novel therapeutic targets. Pharmacological Reviews. 2009;61(1):9–38. doi: 10.1124/pr.108.000711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Xie Z, Cai T. Na+-K+–ATPase-mediated signal transduction: from protein interaction to cellular function. Mol Interv. 2003;3(3):157–168. doi: 10.1124/mi.3.3.157. [DOI] [PubMed] [Google Scholar]
- 139.Tian J, Xie ZJ. The Na-K-ATPase and calcium-signaling microdomains. Physiology. 2008;23(4):205–211. doi: 10.1152/physiol.00008.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Aperia A. New roles for an old enzyme: Na,K-ATPase emerges as an interesting drug target. Journal of Internal Medicine. 2007;261(1):44–52. doi: 10.1111/j.1365-2796.2006.01745.x. [DOI] [PubMed] [Google Scholar]
- 141.Miyakawa-Naito A, Uhlén P, Lal M, et al. Cell signaling microdomain with Na,K-ATPase and inositol 1,4,5-trisphosphate receptor generates calcium oscillations. Journal of Biological Chemistry. 2003;278(50):50355–50361. doi: 10.1074/jbc.M305378200. [DOI] [PubMed] [Google Scholar]
- 142.Nesher M, Shpolansky U, Rosen H, Lichtstein D. The digitalis-like steroid hormones: new mechanisms of action and biological significance. Life Sciences. 2007;80(23):2093–2107. doi: 10.1016/j.lfs.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 143.Liang M, Tian J, Liu L, et al. Identification of a pool of non-pumping Na/K-ATPase. Journal of Biological Chemistry. 2007;282(14):10585–10593. doi: 10.1074/jbc.M609181200. [DOI] [PubMed] [Google Scholar]
- 144.Rodrigues-Mascarenhas S, De Oliveira ADS, Amoedo ND, Affonso-Mitidieri OR, Rumjanek FD, Rumjanek VM. Modulation of the immune system by ouabain. Annals of the New York Academy of Sciences. 2009;1153:153–163. doi: 10.1111/j.1749-6632.2008.03969.x. [DOI] [PubMed] [Google Scholar]
- 145.Foey AD, Crawford A, Hall ND. Modulation of cytokine production by human mononuclear cells following impairment of Na,K-ATPase activity. Biochimica et Biophysica Acta. 1997;1355(1):43–49. doi: 10.1016/s0167-4889(96)00116-4. [DOI] [PubMed] [Google Scholar]
- 146.Blasi E, Ardizzoni A, Colombari B, et al. NF-kB activation and p38 phosphorilation in microglial cells infected with Leptospira or exposed to partially purified leptospiral lipoproteins. Microbial Pathogenesis. 2007;42(2-3):80–87. doi: 10.1016/j.micpath.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 147.Cinco M, Vecile E, Murgia R, Dobrina P, Dobrina A. Leptospira interrogans and Leptospira peptidoglycans induce the release of tumor necrosis factor α from human monocytes. FEMS Microbiology Letters. 1996;138(2-3):211–214. doi: 10.1111/j.1574-6968.1996.tb08159.x. [DOI] [PubMed] [Google Scholar]
- 148.Burth P, Younes-Ibrahim M, Goncalez FHFS, Costa ER, Faria MVC. Purification and characterization of a Na+,K+ ATPase inhibitor found in an endotoxin of Leptospira interrogans . Infection and Immunity. 1997;65(4):1557–1560. doi: 10.1128/iai.65.4.1557-1560.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Tajiki MH, Salonião R. Association of plasma levels of tumor necrosis factor α with severity of disease and mortality among patients with leptospirosis. Clinical Infectious Diseases. 1996;23(5):1177–1178. doi: 10.1093/clinids/23.5.1177. [DOI] [PubMed] [Google Scholar]
- 150.Martinon F, Burns K, Tschopp J. The Inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β . Molecular Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 151.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006;124(4):783–801. doi: 10.1016/j.cell.2006.02.015. [DOI] [PubMed] [Google Scholar]
- 152.Lacroix-Lamandé S, D'Andon MF, Michel E, et al. Downregulation of the Na/K-ATPase pump by leptospiral glycolipoprotein activates the NLRP3 inflammasome. Journal of Immunology. 2012;188(6):2805–2814. doi: 10.4049/jimmunol.1101987. [DOI] [PubMed] [Google Scholar]


