Abstract
Objective
The aim of this study was to evaluate the effect of hemiplegia on diaphragmatic movements using motion-mode ultrasonography.
Methods
23 hemiplegic patients who were diagnosed with a single-hemisphere lesion (mean age 60.5 years; 13 males and 10 females) and a control group of 20 patients (13 males and 7 females) were all evaluated by ultrasonography. Ultrasonography recordings were made of the amplitude of diaphragmatic movement during spontaneous and deep breathing. The patients underwent lung function tests.
Results
When the hemiplegic and control groups were compared, the forced vital capacity, forced expired volume in 1 s, maximum inspiratory pressure and maximum expiratory pressure values were significantly lower in the groups with right and left hemiplegia (p<0.05). When a comparison was made between the right hemiplegic group and the control group and between the left hemiplegic group and the control group in terms of diaphragmatic excursions, for both groups, no significant difference was determined between the movements of the right hemidiaphragm during spontaneous and deep breathing and those of the left hemidiaphragm in spontaneous respiration. In contrast, for both hemiplegic groups, a significant decrease was noted in the movements of the left hemidiaphragm in deep respiration.
Conclusion
The diaphragm is both contralaterally innervated and ipsilaterally innervated, and innervation exhibits marked variations from person to person. This provides an explanation for varying diaphragmatic movements in hemiplegic cases during deep respiration.
The diaphragm is the primary respiratory muscle that accounts for about 75% of airflow into the lungs [1]. Breathing is activated either volitionally via the corticospinal pathway or automatically via the bulbospinal pathway [2]. It has been demonstrated by transcranial magnetic stimulation that an oligosynaptic pathway exists running from the cortex to the diaphragm [3].
It has been suggested that hemiplegia caused by a lesion superior to the brain stem will impair diaphragmatic motion [4]. Retrospective viewing of chest radiographs in patients with hemiplegia has shown that the hemidiaphragm on the paralysed side was often higher than the hemidiaphragm on the normal side [5,6]. Fluoroscopic measurements of diaphragmatic excursion have also shown a smaller excursion of the hemidiaphragm on the paralysed side during a deep volitional breath in some patients with hemiplegia [6].
Although fluoroscopy is an effective tool for assessing diaphragmatic movement, it has disadvantages because it requires patient transportation and uses ionising radiation. By contrast, sonography is ubiquitous in medical facilities, requires no radiation and may be used at the patient's bedside; therefore, some authors have stated that it would be ideal if sonography could replace fluoroscopy for the evaluation of motion of the diaphragm [7,8].
This study was performed to evaluate the effect of hemiplegia on diaphragmatic movement in hemiplegic patients using motion-mode (M-mode) ultrasonography.
Materials and methods
The local ethics committee approved the study protocol, and written informed consent was obtained from all patients and control subjects.
Patient selection
Volunteer patients who were diagnosed with a stroke and who had had an ischaemic cerebrovascular attack associated with a single hemisphere were selected for the study in accordance with criteria set by the World Health Organization.
A history of a neuropathy (autonomous neuropathy etc.), a systemic illness or medication usage leading to neuropathy, and the presence of a brain stem lesion, loss of consciousness or lack of co-operative behaviour, dementia, sensory or global aphasia, smoking, extreme obesity and chest deformity were determined to be exclusion criteria.
Selection of the control group
Volunteers who were receiving treatment in the Physical Medicine and Rehabilitation Department, Gazi University School of Medicine, for other reasons as inpatients and those who did not fulfil the exclusion criteria were included as the control group.
Ultrasonography
All sonographic examinations were performed by the same experienced radiologist (NV) using a GE Logiq 7 ultrasound machine (GE Healthcare, Milwaukee, WI) with a multifrequency convex probe. The radiologist was blinded to the presence or side of hemiplegia. Ultrasonography examinations were carried out 2–3 h after a meal. After the patients were allowed to rest for 5–10 min in the supine position, first, conventional B-mode sonography was performed with a sector transducer to evaluate the upper quadrants of the abdomen and the lower chest to exclude adjacent pathology. Those who had adjacent pathology were excluded from the study. Both hemidiaphragms were examined in the supine position in the longitudinal semicoronal plane through a subcostal or intercostal approach. The liver was used as a window on the right, whereas the spleen was used for the left hemidiaphragm. Diaphragmatic movement was evaluated based on the posterior and lateral regions, where the movements have the greatest amplitude.
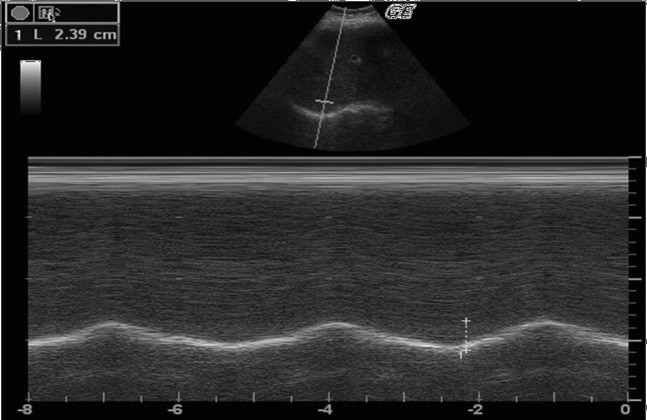
The movements of the right and left hemidiaphragms during spontaneous and deep inspiration in the same position were recorded by M-mode ultrasonography during a few respiratory cycles (Figure 1). The distance between the echogenic lines was measured in frozen images during a minimum of three different cycles. The averages of the findings were calculated in centimetres.
Figure 1.
M-mode ultrasonography during a few respiratory cycles.
Spirometric studies
Spirometric parameters were measured with a Vmax 20 spirometer (Sensormedics, Anaheim, CA) on the same day as ultrasonography was performed. Values for forced vital capacity (FVC), forced expiratory volume in 1 s (FEV1), the FEV1/FVC ratio, maximum inspiratory pressure (PImax) and maximum expiratory pressure (PEmax) were recorded.
Statistics
The findings were assessed using SPSS for Windows v.13 (IBM Corporation, Armonk, NY). The difference between the hemiplegic cases and the control group, the diaphragmatic movements in all groups during spontaneous and deep breathing and the findings for lung function tests were analysed with the Wilcoxon test and the Mann–Whitney U-test; p<0.05 was considered to be statistically significant.
Results
The study included a total of 23 hemiplegic patients [mean age 60.5±10.7, range 34–82 years; 13 males (56.5%) and 10 females (43.5%)], diagnosed with a stroke. All the patients had a lesion in only one hemisphere and had no exclusion criteria for participation in this study. 15 (65.2%) patients had right-sided hemiplegia and 8 (34.8%) had left-sided hemiplegia.
The control group included a total of 20 volunteers, 13 of whom were males (65%) and 7 of whom were females (35%).
The mean age of the patients with right hemiplegia was 58.8±11.6 years (range 34–82 years) and that of the patients with left hemiplegia was 63.6±8.3 years (range 54–81 years), and the control group had a mean age of 61.2±12.1 years (range 34–81 years); the differences between the groups were not statistically significant.
The 86 hemidiaphragms in the 43 cases were evaluated in the patient and control groups during spontaneous and deep breathing. In all the cases, the liver was used as the acoustic window on the right. Except for only one patient who had undergone splenectomy, the spleen was used as a window on the left. The kidney of the splenectomised patient was used as the acoustic window through a subcostal approach. There were no patients whose hemidiaphragms could not be assessed.
The mean measured movement of the right hemidiaphragm in the right hemiplegic cases was 1.8±0.7 cm during spontaneous breathing and 5.1±2.2 cm during deep respiration, and the mean measured movement of the left hemidiaphragm was 2.2±0.7 cm during spontaneous breathing and 4.9±1.9 cm during deep respiration. The mean measured movement of the right hemidiaphragm in the left hemiplegic cases was 1.9±0.5 cm during spontaneous breathing and 4.9±1.4 cm during deep respiration, and that of the left hemidiaphragm was 1.9±0.9 cm during spontaneous breathing and 3.5±1.1 cm during deep respiration. The mean measured movement of the right hemidiaphragm in the control group was found to be 1.6±0.8 cm during spontaneous breathing and 6.1±2.5 cm during deep respiration, and that of the left hemidiaphragm was 2.1±0.6 cm during spontaneous breathing and 6.1±1.6 cm during deep respiration.
When the control group and the left hemiplegic group and the control group and the right hemiplegic group were compared in terms of diaphragmatic excursions, for both groups, no significant difference was observed between the movements of the right hemidiaphragm in spontaneous and deep respiration and those of the left hemidiaphragm in spontaneous respiration. In contrast, for both groups, there was a significant decrease in the activity of the left hemidiaphragm in deep respiration (p<0.05) (Table 1).
Table 1. Findings of diaphragmatic movement measurements for all groups.
| Right hemiplegia (n=15) |
Left hemiplegia (n=8) |
Control (n=20) |
||||
| Amplitudes | Mean, cm | (Range, cm) | Mean, cm | (Range, cm) | Mean, cm | (Range, cm) |
| RSB | 1.8 | (0–2.9) | 1.9 | (1.3–2.8) | 1.6 | (0.6–3.7) |
| RDR | 5.1 | (0–8.3) | 4.9 | (2.8–7.3) | 6.1 | (1.5–10.9) |
| LSB | 2.2 | (1.2–4.0) | 1.9 | (0.9–3.6) | 2.1 | (1.2–3.1) |
| LDR | 4.9a | (2.5–9.5) | 3.5b | (1.9–5.0) | 6.1 | (3.0–9.6) |
LSB, the amplitude of left diaphragmatic movement during spontaneous breathing
RDR, the amplitude of right diaphragmatic movement during deep respiration
RSB, the amplitude of right diaphragmatic movement during spontaneous breathing
LDR, the amplitude of left diaphragmatic movement during deep respiration.
ap<0.05, significantly different from right hemiplegia and controls.
bp<0.05, significantly different from left hemiplegia and controls.
When hemidiaphragmatic excursions during spontaneous and deep respiration on both the hemiplegic and non-hemiplegic sides of the hemiplegic patients were further compared, no statistically significant difference was observed.
All the cases underwent a lung function test. However, it was not possible to carry out the test in three cases because of poor co-operation (one patient in the group with right and left hemiplegia and two patients in the control group). In the remainder of the cases, three patients from the right hemiplegic group and one case from the control group did not co-operate during measurement of PImax and PEmax, which made it impossible to obtain the PImax and PEmax values in these four cases.
When the hemiplegic and control groups were compared, the values for FVC, FEV1, FEV1/FVC ratio, PImax and PEmax were found to be significantly lower in the hemiplegic group (Table 2).
Table 2. Findings of lung function tests for all groups.
| Lung function tests | Right hemiplegia | Left hemiplegia | Control |
| FVC (litres) | 64.3±27.2 (n=14)a | 68.3±12.8 (n=7)b | 88.4±20.7 (n=18) |
| FEV1 (litres) | 72.5±32.4 (n=14)a | 79.1±18.8 (n=7)b | 100.8±18.9 (n=18) |
| FEV1/FVC (%) | 82.0±14.8 (n=14)a | 85.1±10.9 (n=7)b | 83.1±10.3 (n=18) |
| PImax (cmH2O) | 31.8±10.1 (n=11)a | 32.9±10.5 (n=7)b | 62.4±26.1 (n=17) |
| PEmax (cmH2O) | 59.7±19.7 (n=11)a | 61.4±26.1 (n=7)b | 92.1±36.7 (n=17) |
FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; PEmax, maximum expiratory pressure; PImax, maximum inspiratory pressure.
ap<0.05, significantly different from right hemiplegia and controls.
bp<0.05, significantly different from left hemiplegia and controls.
Discussion
The diaphragm is the fibromuscular layer separating the thorax and abdominal cavities. It makes a great contribution to the expansion of the lungs during inspiration [7]. Respiration takes place volitionally or automatically. Volitional respiration is carried out by the cortex. It is thought that both hemidiaphragms are controlled by the contralateral primary motor cortex and, thus, in the presence of paralysis, the diaphragm is also affected on the same side as the paralysis [2,4,9].
Paralysis of the diaphragm has multiple aetiologies. Diaphragmatic movement can be affected by either central nervous system diseases or neck and chest pathologies along the phrenic nerve tract or neuromuscular junction transmission abnormalities, diaphragmatic muscle diseases and thorax and abdomen pathologies [7]. In order to exclude other variables that affect diaphragmatic movement, we only included patients diagnosed with a stroke in which only one hemisphere was involved and excluded those with other known pathologies or with pathologies that were identified on examination.
In this study, we did not find any significant differences between the hemiplegic and control groups in terms of diaphragmatic movement in spontaneous respiration such as those detected by Cohen et al [4]. However, we demonstrated that, for both hemiplegic groups, there was a decrease in the movement of the left hemidiaphragm during deep breathing. Houston et al [10] reported reduced movements in both hemidiaphragms during an acute ischaemic infarction. However, in the group with right hemiplegia, a decrease on the left rather than on the right is not an anticipated finding. By contrast, Cohen et al [4] demonstrated that the diaphragm in all hemiplegic cases may not be affected and argued that this can be attributed to a number of reasons. One is the probability that the hemisphere may be affected, whereas the phrenic nucleus may not. Another is that the ipsilateral projection of the corticospinal fibres of the diaphragm is more evident in some patients than in others [4,11]. In our opinion, the decrease we observed in this group of right hemiplegic patients on the right may be due to ipsilateral innervation.
Fluoroscopy is a technique that is used quite frequently in evaluating diaphragmatic movement. However, it has drawbacks. For instance, it requires patient transportation and uses ionising radiation. Also, on an anteroposterior view, it shows only the least active anterior region of the diaphragm, and paralysis of the diaphragm may be overlooked when it is bilateral [12-15].
Ultrasonography is described as a simple and harmless method for evaluating the diaphragm [8,16,17]. It allows diaphragmatic excursions to be recorded. The M-mode is convenient for measurements, as the beginning and end of each breath can be seen easily [4]. Some authors described the diaphragm as three echogenic lines and others as two echogenic lines [18,19]. In our study, the diaphragm was seen as one echogenic line, similar to Gerscovich et al [7], and we did not encounter any technical difficulties obtaining ultrasonographic images of the diaphragm [20].
Another finding of decreased diaphragmatic movement is a restrictive defect in spirometric measurements defined by a matched decrease in both FEV1 and FVC values, and a normal or high FEV1/FVC ratio, which was also shown in our study. We also found that hemiplegic patients had lower PImax and PEmax values, which directly reflected decreased respiratory muscle strength. The correlation found between ultrasonographic findings and spirometric findings also supports the effective role of M-mode ultrasonography in evaluating the diaphragm and determining its movements [21].
In conclusion, diaphragmatic movement in hemiplegic patients may be affected not only on the hemiplegic side but also on the non-hemiplegic side. In this respect, there may be variations from person to person.
References
- 1.Shields TW. Diaphragmatic function, diaphragmatic paralysis and eventration of the diaphragm. Shields TW, General thoracic surgery. Baltimore, MD: Williams and Wilkins; 1994. pp. 607–11 [Google Scholar]
- 2.Aminoff MJ, Sears TA. Spinal integration of segmental, cortical and breathing inputs to thoracic respiratory motoneurones. J Physiol 1971;215:557–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol 1987;384:109–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen E, Mier A, Heywood P, Murphy K, Boultbee J, Guz A. Diaphragmatic movement in hemiplegic patients measured by ultrasonography. Thorax 1994;49:890–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith M. The effect of hemiplegia on the diaphragm. Am Rev Respir Dis 1964;89:450–2 [DOI] [PubMed] [Google Scholar]
- 6.Korczyn A, Hermann G, Don R. Diaphragmatic involvement in hemiplegia and hemiparesis. J Neurol Neurosurg Psychiatry 1969;32:588–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gerscovich EO, Cronan M, McGahan JP, Jain K, Jones D, McDonald C. Ultrasonographic evaluation of diaphragmatic motion. J Ultrasound Med 2001;20:597–604 [DOI] [PubMed] [Google Scholar]
- 8.Kantarci F, Mihmanli I, Demirel MK, Harmanci K, Akman C, Aydoğan F, et al. Normal diaphragmatic motion and the effects of body composition: determination with M-mode sonography. J Ultrasound Med 2004;23:255–60 [DOI] [PubMed] [Google Scholar]
- 9.Similowski T, Catala M, Orcel B, Willer J-C, Derenne J-P. Unilaterality of the motor cortical representation of the human diaphragm. J Physiol 1991;438:37P [Google Scholar]
- 10.Houston JG, Angus RM, Cowan MD. Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax 1994;49:500–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maskill D, Murphy K, Mier A, Owen M, Guz A. Motor cortical representation of the diaphragm in man. J Physiol 1991;443:105–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Epelman M, Navarro OM, Daneman A, Miller SF. M-mode sonography of diaphragmatic motion: description of technique and experience in 278 pediatric patients. Pediatr Radiol 2005;35:661–7 [DOI] [PubMed] [Google Scholar]
- 13.Verschakelen JA, Deschepper K, Jiang TX, Demedts M. Diaphragmatic displacement measured by fluoroscopy and derived by Respitrace. J Appl Physiol 1989;67:694–8 [DOI] [PubMed] [Google Scholar]
- 14.Laing IA, Teele RL, Stark AR. Diaphragmatic movement in newborn infants. J Pediatr 1988;112:638–43 [DOI] [PubMed] [Google Scholar]
- 15.Urvoas E, Pariente D, Fausser C, Lipsich J, Taleb R, Devictor D. Diaphragmatic paralysis in children: diagnosis with TM-mode US. Pediatr Radiol 1994;24:564–8 [DOI] [PubMed] [Google Scholar]
- 16.Riccabona M, Sorantin E, Ring E. Application of M-mode sonography to functional evaluation in pediatric patients. Eur Radiol 1998;8:1457–61 [DOI] [PubMed] [Google Scholar]
- 17.Ayoub J, Metge L, Dauzat M, Lemerre C, Pourcelot L, Préfaut C, et al. Diaphragm kinetics coupled with spirometry. M-mode ultrasonographic and fluoroscopic study: preliminary result. J Radiol 1997;78:563–8 [PubMed] [Google Scholar]
- 18.Lewandowski BJ, Winsberg F. Echographic appearance of the right hemidiaphragm. J Ultrasound Med 1983;2:243–9 [DOI] [PubMed] [Google Scholar]
- 19.Mead J. Functional significance of the area of apposition of diaphragm to rib cage. Am Rev Respir Dis 1979;119:31–2 [DOI] [PubMed] [Google Scholar]
- 20.Zifko U, Hartmann M, Girsch W, Zoder G, Rokitansky A, Grisold W, et al. Diaphragmatic paresis in newborns due to phrenic nerve injury. Neuropediatrics 1995;26:281–4 [DOI] [PubMed] [Google Scholar]
- 21.Culver BH. Pulmonary function and exercise testing. Albert RK, Spiro SG, Jett JR, Clinical respiratory medicine, 2nd edn Philadelphia, PA: Mosby; 2004. pp. 117–28 [Google Scholar]