Abstract
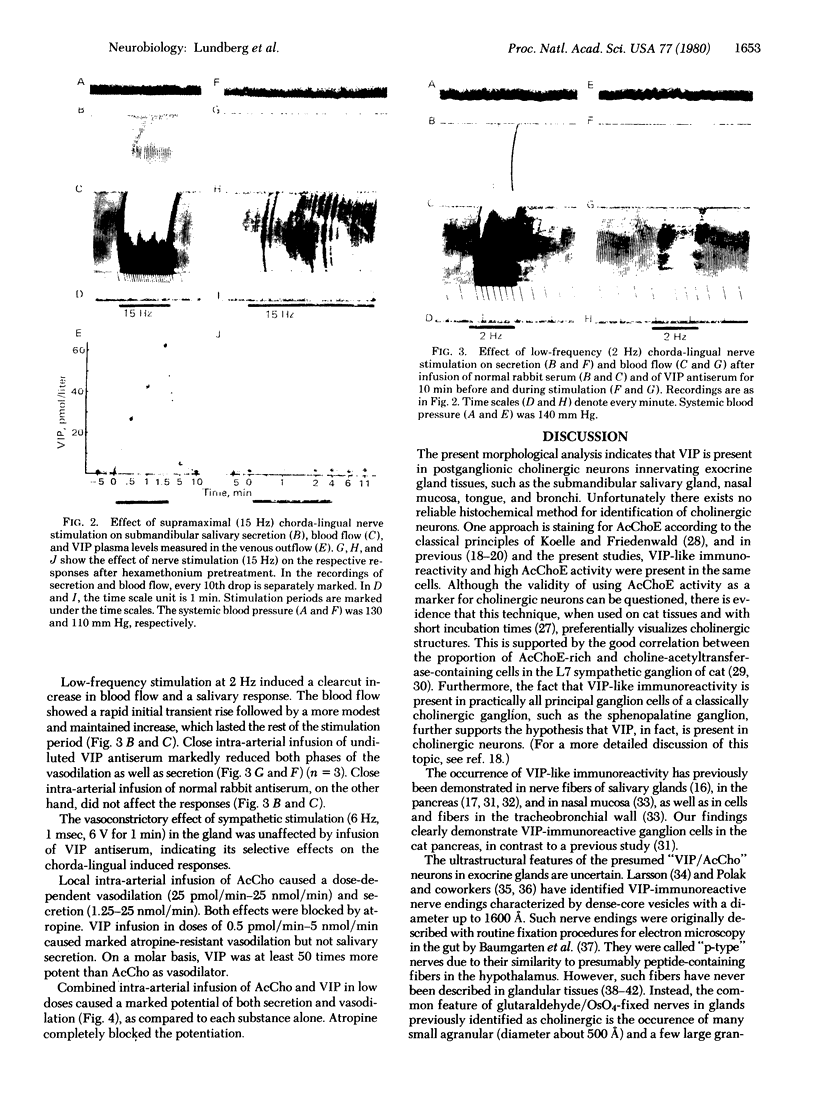
By a combination of the indirect immunofluorescence technique with acetylcholinesterase (acetylcholine acetylhydrolase, EC 3.1.1.7) staining, it was shown that vasoactive intestinal polypeptide (VIP) is present in cholinergic (acetylcholinesterase-rich) neurons involved in control of secretion and vasodilation in exocrine glands of cat. The submandibular salivary gland was used as a functional model. Preganglionic nerve stimulation induced an atropine-resistant, hexamethonium-sensitive vasodilation and release of VIP into the venous outflow from the gland and an atropine- and hexamethonium-sensitive secretion. Infusion of VIP antiserum reduced both the vasodilation and secretion. Infusion of VIP caused vasodilation only, whereas acetylcholine caused both vasodilation and secretion. Simultaneous infusion of VIP and acetylcholine in low doses resulted in a marked potentiation of both vasodilation and secretion. The present morphological and functional data support the following hypothesis for regulation of vasodilation and secretion in exocrine glands. Preganglionic cholinergic nerves activate, via nicotinic receptors, postganglionic neurons, causing concomitant release from the same nerve endings of two coexisting putative transmitters, acetylcholine and VIP. Acetylcholine produces mainly secretion by a muscarinic action and VIP causes mainly vasodilation, but the two substances seem to cooperate directly or indirectly in both types of response. Thus, the coexistence of two putative neurotransmitters, VIP and acetylcholine, in one neuron may explain the dual effector response (i.e., the cholinergic secretion and the atropine-resistant vasodilation) caused by nerve stimulation in exocrine glands.
Keywords: acetylcholinesterase, atropine-resistant vasodilation
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anggård A., Densert O. Adrenergic innervation of the nasal mucosa in cat. A histological and physiological study. Acta Otolaryngol. 1974 Sep-Oct;78(3-4):232–241. doi: 10.3109/00016487409126349. [DOI] [PubMed] [Google Scholar]
- BROOKS V. B. An intracellular study of the action of repetitive nerve volleys and of botulinum toxin on miniature end-plate potentials. J Physiol. 1956 Nov 28;134(2):264–277. doi: 10.1113/jphysiol.1956.sp005642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURGEN A. S. V., DICKENS F., ZATMAN L. J. The action of botulinum toxin on the neuro-muscular junction. J Physiol. 1949 Aug;109(1-2):10–24. doi: 10.1113/jphysiol.1949.sp004364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgarten H. G., Holstein A. F., Owman C. Auerbach's plexus of mammals and man: electron microscopic identification of three different types of neuronal processes in myenteric ganglia of the large intestine from rhesus monkeys, guinea-pigs and man. Z Zellforsch Mikrosk Anat. 1970;106(3):376–397. doi: 10.1007/BF00335780. [DOI] [PubMed] [Google Scholar]
- Beilenson S., Schachter M., Smaje L. H. Secretion of kallikrein and its role in vasodilatation in the submaxillary gland. J Physiol. 1968 Dec;199(2):303–317. doi: 10.1113/jphysiol.1968.sp008655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant M. G., Polak M. M., Modlin I., Bloom S. R., Albuquerque R. H., Pearse A. G. Possible dual role for vasoactive intestinal peptide as gastrointestinal hormone and neurotransmitter substance. Lancet. 1976 May 8;1(7967):991–993. doi: 10.1016/s0140-6736(76)91863-8. [DOI] [PubMed] [Google Scholar]
- Buckley G., Consolo S., Giacobini E., Sjöqvist F. Cholinacetylase in innervated and denervated sympathetic ganglia and ganglion cells of the cat. Acta Physiol Scand. 1967 Dec;71(4):348–356. doi: 10.1111/j.1748-1716.1967.tb03742.x. [DOI] [PubMed] [Google Scholar]
- COONS A. H. Fluorescent antibody methods. Gen Cytochem Methods. 1958;1:399–422. [PubMed] [Google Scholar]
- Darke A. C., Smaje L. H. Dependence of functional vasodilatation in the cat submaxillary gland upon stimulation frequency. J Physiol. 1972 Oct;226(1):191–203. doi: 10.1113/jphysiol.1972.sp009980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERICI I., UVNAS B. Efferent and antidromic vasodilator impulses to the tongue in the chorda-lingual nerve of the cat. Acta Physiol Scand. 1952;25(1):10–14. doi: 10.1111/j.1748-1716.1952.tb00854.x. [DOI] [PubMed] [Google Scholar]
- Edwards A. V., Birchman P. M., Mitchell S. J., Bloom S. R. Changes in the concentration of vasoactive intestinal peptide in intestinal lymph in response to vagal stimulation in the calf. Experientia. 1978 Sep 15;34(9):1186–1187. doi: 10.1007/BF01922948. [DOI] [PubMed] [Google Scholar]
- FOX R. H., HILTON S. M. Bradykinin formation in human skin as a factor in heat vasodilatation. J Physiol. 1958 Jul 14;142(2):219–232. doi: 10.1113/jphysiol.1958.sp006011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Haglund U., Jodal M., Lundgren O., Olbe L., de Muckadell O. B. Nervous release of vasoactive intestinal polypeptide in the gastrointestinal tract of cats: possible physiological implications. J Physiol. 1978 Nov;284:291–305. doi: 10.1113/jphysiol.1978.sp012541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fahrenkrug J., Schaffalitzky de Muckadell O. B. Distribution of vasoactive intestinal polypeptide (VIP) in the porcine central nervous system. J Neurochem. 1978 Dec;31(6):1445–1451. doi: 10.1111/j.1471-4159.1978.tb06571.x. [DOI] [PubMed] [Google Scholar]
- Fahrenkrug J., Schaffalitzky de Muckadell O. V. Radioimmunoassay of vasoactive intestinal polypeptide (VIP) in plasma. J Lab Clin Med. 1977 Jun;89(6):1379–1388. [PubMed] [Google Scholar]
- Ferreira S. H., Smaje L. H. Bradykinin and functional vasodilatation in the salivary gland. Br J Pharmacol. 1976 Oct;58(2):201–209. doi: 10.1111/j.1476-5381.1976.tb10397.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett J. R. The innervation of salivary glands. IV. The effects of certain experimental procedures on the ultrastructure of nerves in glands of the cat. J R Microsc Soc. 1966 Oct;86(1):15–31. [PubMed] [Google Scholar]
- Gautvik K. Parasympathetic neuro-effector transmission and functional vasodilatation in the submandibular salivary gland of cats. Acta Physiol Scand. 1970 Jun;79(2):204–215. doi: 10.1111/j.1748-1716.1970.tb04720.x. [DOI] [PubMed] [Google Scholar]
- Geneser-Jensen F. A., Blackstad T. W. Distribution of acetyl cholinesterase in the hippocampal region of the guinea pig. I. Entorhinal area, parasubiculum, and presubiculum. Z Zellforsch Mikrosk Anat. 1971;114(4):460–481. doi: 10.1007/BF00325634. [DOI] [PubMed] [Google Scholar]
- Giachetti A., Said S. I., Reynolds R. C., Koniges F. C. Vasoactive intestinal polypeptide in brain: localization in and release from isolated nerve terminals. Proc Natl Acad Sci U S A. 1977 Aug;74(8):3424–3428. doi: 10.1073/pnas.74.8.3424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacobini E., Palmborg B., Sjöqvist F. Cholinesterase activity in innervated and denervated sympathetic ganglion cells of the cat. Acta Physiol Scand. 1967 Apr;69(4):355–361. doi: 10.1111/j.1748-1716.1967.tb03532.x. [DOI] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The cause of the vasodilatation accompanying activity in the submandibular salivary gland. J Physiol. 1955 May 27;128(2):235–248. doi: 10.1113/jphysiol.1955.sp005302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The mechanism of the functional hyperaemia in the submandibular salivary gland. J Physiol. 1955 Aug 29;129(2):253–271. doi: 10.1113/jphysiol.1955.sp005351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HILTON S. M., LEWIS G. P. The relationship between glandular activity, bradykinin formation and functional vasodilatation in the submandibular salivary gland. J Physiol. 1956 Nov 28;134(2):471–483. doi: 10.1113/jphysiol.1956.sp005658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidd A., Garrett J. R. New observations on the innervation of striated ducts in submandibular glands of cats, including possible peptidergic nerves. Cell Tissue Res. 1979 Aug;200(2):205–214. doi: 10.1007/BF00236413. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Holst J. J., Schaffalitzky de Muckadell O. B. Innervation of the pancreas by vasoactive intestinal polypeptide (VIP) immunoreactive nerves. Life Sci. 1978 Mar;22(9):773–780. doi: 10.1016/0024-3205(78)90246-1. [DOI] [PubMed] [Google Scholar]
- Larsson L. I., Fahrenkrug J., Schaffalitzky De Muckadell O., Sundler F., Håkanson R., Rehfeld J. R. Localization of vasoactive intestinal polypeptide (VIP) to central and peripheral neurons. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3197–3200. doi: 10.1073/pnas.73.9.3197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg J. M., Hökfelt T., Schultzberg M., Uvnäs-Wallensten K., Köhler C., Said S. I. Occurrence of vasoactive intestinal polypeptide (VIP)-like immunoreactivity in certain cholinergic neurons of the cat: evidence from combined immunohistochemistry and acetylcholinesterase staining. Neuroscience. 1979;4(11):1539–1559. doi: 10.1016/0306-4522(79)90018-6. [DOI] [PubMed] [Google Scholar]
- Mutt V., Said S. I. Structure of the porcine vasoactive intestinal octacosapeptide. The amino-acid sequence. Use of kallikrein in its determination. Eur J Biochem. 1974 Mar 1;42(2):581–589. doi: 10.1111/j.1432-1033.1974.tb03373.x. [DOI] [PubMed] [Google Scholar]
- Norberg K. A., Hökfelt T., Eneroth C. M. The autonomic innervation of human submandibular and parotid glands. J Neurovisc Relat. 1969;31(3):280–290. doi: 10.1007/BF02323407. [DOI] [PubMed] [Google Scholar]
- Nustad K., Orstavik T. B., Gautvik K. M., Pierce J. V. Glandular kallikreins. Gen Pharmacol. 1978;9(1):1–9. doi: 10.1016/0306-3623(78)90049-6. [DOI] [PubMed] [Google Scholar]
- Pickel V. M., Reis D. J., Leeman S. E. Ultrastructural localization of substance P in neurons of rat spinal cord. Brain Res. 1977 Feb 25;122(3):534–540. doi: 10.1016/0006-8993(77)90463-2. [DOI] [PubMed] [Google Scholar]
- SCOTT B. L., PEASE D. C. Electron microscopy of the salivary and lacrimal glands of the rat. Am J Anat. 1959 Jan;104:115–161. doi: 10.1002/aja.1001040106. [DOI] [PubMed] [Google Scholar]
- Said S. I., Rosenberg R. N. Vasoactive intestinal polypeptide: abundant immunoreactivity in neural cell lines and normal nervous tissue. Science. 1976 May 28;192(4242):907–908. doi: 10.1126/science.1273576. [DOI] [PubMed] [Google Scholar]
- Schaffalitzky de Muckadell O. B., Fahrenkrug J., Watt-Boolsen S., Worning H. Pancreatic response and plasma secretin concentration during infusion of low dose secretin in man. Scand J Gastroenterol. 1978;13(3):305–311. doi: 10.3109/00365527809179825. [DOI] [PubMed] [Google Scholar]
- Schaffalitzky de Muckadell O. B., Fahrenkrug J., Watt-Boolsen S., Worning H. Pancreatic response and plasma secretin concentration during infusion of low dose secretin in man. Scand J Gastroenterol. 1978;13(3):305–311. doi: 10.3109/00365527809179825. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Taira N. Assessment of the effects of vasoactive intestinal peptide (VIP) on blood flow through and salivation of the dog salivary gland in comparison with those of secretin, glucagon and acetylcholine. Br J Pharmacol. 1979 Apr;65(4):683–687. doi: 10.1111/j.1476-5381.1979.tb07882.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu T., Taira N. Vasodilatation by prostaglandin F2alpha in the canine tongue through a parasympathetic mechanism. Br J Pharmacol. 1978 Jul;63(3):567–574. doi: 10.1111/j.1476-5381.1978.tb07813.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundler F., Alumets J., Håkanson R., Fahrenkrug J., Schaffalitzky de Muckadell O. Peptidergic (VIP) nerves in the pancreas. Histochemistry. 1978 Mar 2;55(2):173–176. doi: 10.1007/BF00493519. [DOI] [PubMed] [Google Scholar]
- Uddman R., Alumets J., Densert O., Håkanson R., Sundler F. Occurrence and distribution of VIP nerves in the nasal mucosa and tracheobronchial wall. Acta Otolaryngol. 1978 Nov-Dec;86(5-6):443–448. doi: 10.3109/00016487809107524. [DOI] [PubMed] [Google Scholar]