Abstract
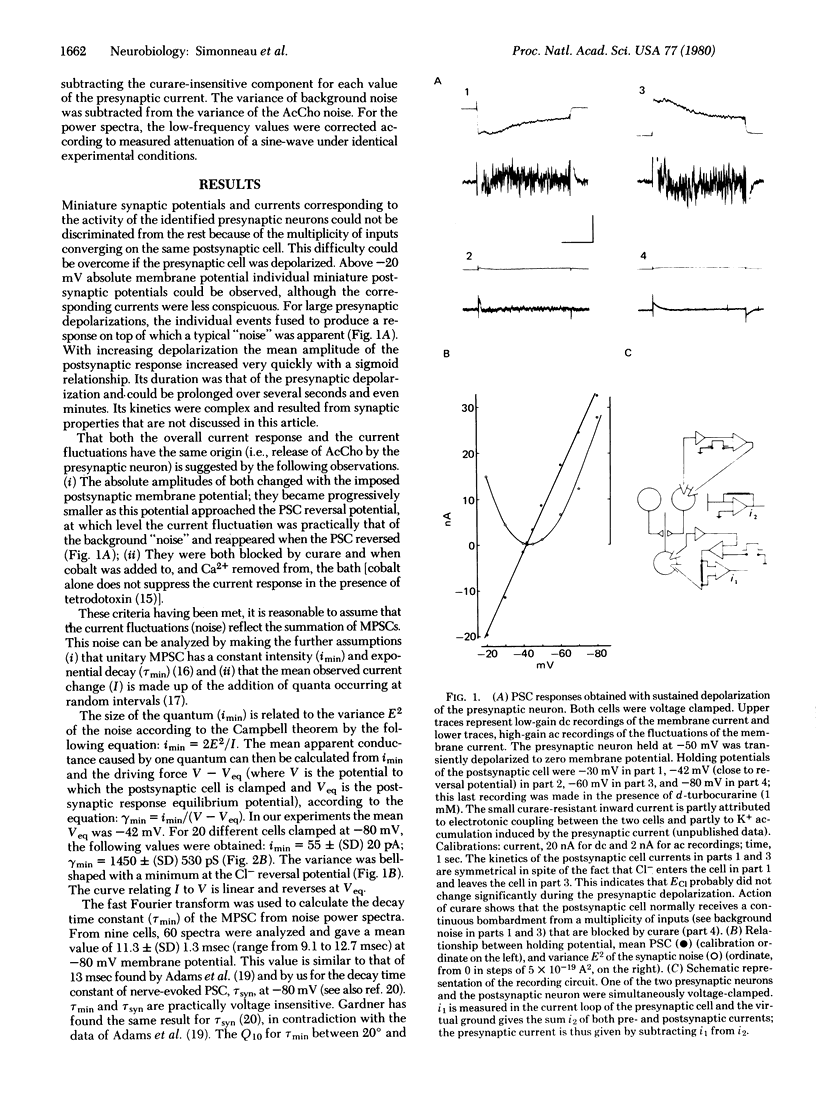
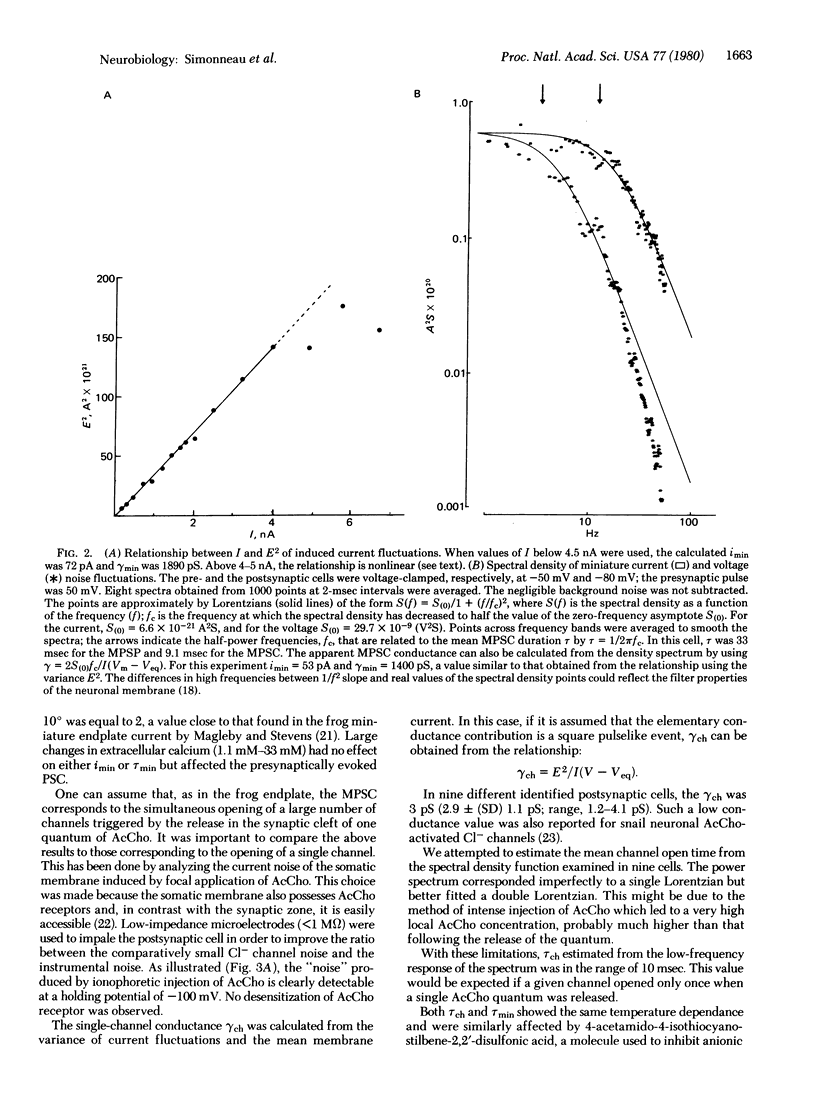
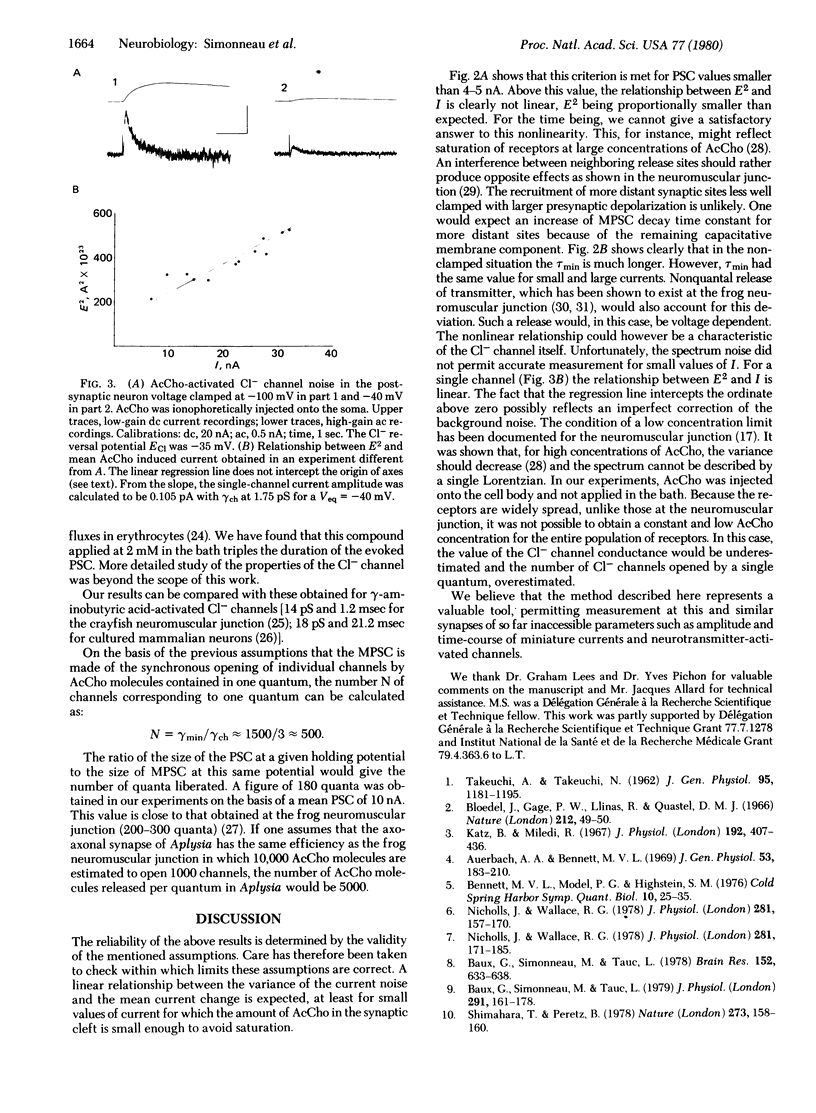
Quantal events have been analyzed at a neuro-neuronal synapse of Aplysia where the nature of the transmitter is established and both presynaptic and postsynaptic neurons are identified and can be voltage-clamped. Prolonged depolarizations were applied to the presynaptic neuron, which gave rise in the postsynaptic cell to a current response characterized by current fluctuations or noise. Acetylcholine was also applied ionophoretically on the same postsynaptic cell. Amplitude and time course of miniature currents and acetylcholine-activated chloride channels of the same cell were examined by using a current fluctuation analysis. It was estimated that one presynaptic spike releases about 180 quanta, each opening 500 chloride channels.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. J., Gage P. W., Hamill O. P. Voltage sensitivity of inhibitory postsynaptic current in Aplysia buccal ganglia. Brain Res. 1976 Oct 22;115(3):506–511. doi: 10.1016/0006-8993(76)90368-1. [DOI] [PubMed] [Google Scholar]
- Anderson C. R., Cull-Candy S. G., Miledi R. Glutamate current noise: post-synaptic channel kinetics investigated under voltage clamp. J Physiol. 1978 Sep;282:219–242. doi: 10.1113/jphysiol.1978.sp012459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Auerbach A. A., Bennett M. V. Chemically mediated transmission at a giant fiber synapse in the central nervous system of a vertebrate. J Gen Physiol. 1969 Feb;53(2):183–210. doi: 10.1085/jgp.53.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L. Blocking action of Ruthenium Red on cholinergic and non-cholinergic synapses: possible involvment of sialic acid-containing substrates in neurotransmission. Brain Res. 1978 Sep 8;152(3):633–638. doi: 10.1016/0006-8993(78)91122-8. [DOI] [PubMed] [Google Scholar]
- Baux G., Simonneau M., Tauc L. Transmitter release: ruthenium red used to demonstrate a possible role of sialic acid containing substrates. J Physiol. 1979 Jun;291:161–178. doi: 10.1113/jphysiol.1979.sp012805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett M. V., Model P. G., Highstein S. M. Stimulation-induced depletion of vesicles, fatigue of transmission and recovery processes at a vertebrate central synapse. Cold Spring Harb Symp Quant Biol. 1976;40:25–35. doi: 10.1101/sqb.1976.040.01.005. [DOI] [PubMed] [Google Scholar]
- Bloedel J., Gage P. W., Llinás R., Quastel D. M. Transmitter release at the squid giant synapse in the presence of tetrodotoxin. Nature. 1966 Oct 1;212(5057):49–50. doi: 10.1038/212049a0. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Akaike N., Lee K. S. The calcium conductance of neurons. Ann N Y Acad Sci. 1978 Apr 28;307:330–344. doi: 10.1111/j.1749-6632.1978.tb41960.x. [DOI] [PubMed] [Google Scholar]
- Castellucci V. F., Kandel E. R. A quantal analysis of the synaptic depression underlying habituation of the gill-withdrawal reflex in Aplysia. Proc Natl Acad Sci U S A. 1974 Dec;71(12):5004–5008. doi: 10.1073/pnas.71.12.5004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gage P. W. Generation of end-plate potentials. Physiol Rev. 1976 Jan;56(1):177–247. doi: 10.1152/physrev.1976.56.1.177. [DOI] [PubMed] [Google Scholar]
- Gardner D. Bilateral symmetry and interneuronal organization in the buccal ganglia of Aplysia. Science. 1971 Aug 6;173(3996):550–553. doi: 10.1126/science.173.3996.550. [DOI] [PubMed] [Google Scholar]
- Geduldig D., Junge D. Sodium and calcium components of action potentials in the Aplysia giant neurone. J Physiol. 1968 Dec;199(2):347–365. doi: 10.1113/jphysiol.1968.sp008657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Yoshikami D. Post-synaptic potentiation: interaction between quanta of acetylcholine at the skeletal neuromuscular synapse. J Physiol. 1975 Oct;251(2):427–463. doi: 10.1113/jphysiol.1975.sp011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. A study of synaptic transmission in the absence of nerve impulses. J Physiol. 1967 Sep;192(2):407–436. doi: 10.1113/jphysiol.1967.sp008307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. The statistical nature of the acetycholine potential and its molecular components. J Physiol. 1972 Aug;224(3):665–699. doi: 10.1113/jphysiol.1972.sp009918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B., Miledi R. Transmitter leakage from motor nerve endings. Proc R Soc Lond B Biol Sci. 1977 Feb 11;196(1122):59–72. doi: 10.1098/rspb.1977.0029. [DOI] [PubMed] [Google Scholar]
- Kuffler S. W., Yoshikami D. The number of transmitter molecules in a quantum: an estimate from iontophoretic application of acetylcholine at the neuromuscular synapse. J Physiol. 1975 Oct;251(2):465–482. doi: 10.1113/jphysiol.1975.sp011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magleby K. L., Stevens C. F. The effect of voltage on the time course of end-plate currents. J Physiol. 1972 May;223(1):151–171. doi: 10.1113/jphysiol.1972.sp009839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBurney R. N., Barker J. L. GABA-induced conductance fluctuations in cultured spinal neurones. Nature. 1978 Aug 10;274(5671):596–597. doi: 10.1038/274596a0. [DOI] [PubMed] [Google Scholar]
- Neher E., Sakmann B. Noise analysis of drug induced voltage clamp currents in denervated frog muscle fibres. J Physiol. 1976 Jul;258(3):705–729. doi: 10.1113/jphysiol.1976.sp011442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Modulation of transmission at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:157–170. doi: 10.1113/jphysiol.1978.sp012414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholls J., Wallace B. G. Quantal analysis of transmitter release at an inhibitory synapse in the central nervous system of the leech. J Physiol. 1978 Aug;281:171–185. doi: 10.1113/jphysiol.1978.sp012415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein A., Cabantchik Z. I., Knauf P. Mechanism of anion transport in red blood cells: role of membrane proteins. Fed Proc. 1976 Jan;35(1):3–10. [PubMed] [Google Scholar]
- Shimahara T., Peretz B. Soma potential of an interneurone controls transmitter release in a monosynaptic pathway in Aplysia. Nature. 1978 May 11;273(5658):158–160. doi: 10.1038/273158a0. [DOI] [PubMed] [Google Scholar]
- TAKEUCHI A., TAKEUCHI N. Electrical changes in pre- and postsynaptic axons of the giant synapse of Loligo. J Gen Physiol. 1962 Jul;45:1181–1193. doi: 10.1085/jgp.45.6.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TAUC L., GERSCHENFELD H. M. Cholinergic transmission mechanisms for both excitation and inhibition in molluscan central synapses. Nature. 1961 Oct 28;192:366–367. doi: 10.1038/192366a0. [DOI] [PubMed] [Google Scholar]
- Tauc L., Hinzen D. H. Neuraminidase: its effect on synaptic transmission. Brain Res. 1974 Nov 15;80(2):340–344. doi: 10.1016/0006-8993(74)90697-0. [DOI] [PubMed] [Google Scholar]
- Tauc L., Hoffmann A., Tsuji S., Hinzen D. H., Faille L. Transmission abolished on a cholinergic synapse after injection of acetylcholinesterase into the presynaptic neurone. Nature. 1974 Aug 9;250(5466):496–498. doi: 10.1038/250496a0. [DOI] [PubMed] [Google Scholar]
- Vyskocil F., Illés P. Non-quantal release of transmitter at mouse neuromuscular junction and its dependence on the activity of Na+-K+ ATP-ase. Pflugers Arch. 1977 Sep 16;370(3):295–297. doi: 10.1007/BF00585542. [DOI] [PubMed] [Google Scholar]



