Abstract
Aims
Because benefits of cardiac resynchronization therapy (CRT) appear to be less favourable in non-left bundle branch block (LBBB) patients, this prospective longitudinal study tested the hypothesis that QRS morphology and echocardiographic mechanical dyssynchrony were associated with long-term outcome after CRT.
Methods and results
Two-hundred and seventy-eight consecutive New York Heart Association class III and IV CRT patients with QRS ≥120 ms and ejection fraction ≤35% were studied. The pre-specified primary endpoint was death, heart transplant, or left ventricular assist device over 4 years. Dyssynchrony assessed before CRT included interventricular mechanical delay (IVMD) and speckle-tracking radial strain using pre-specified cut-offs for each. Of 254 with baseline quantitative echocardiographic data available, 128 had LBBB, 81 had intraventricular conduction delay (IVCD), and 45 had right bundle branch block (RBBB). Radial dyssynchrony was observed in 85% of the patients with LBBB, 59% with IVCD*, and 40% with RBBB* (*P < 0.01 vs. LBBB). Of 248 (98%) with follow-up, LBBB patients had a significantly more favourable long-term survival than non-LBBB patients. However, non-LBBB patients with dyssynchrony had a more favourable event-free survival than those without dyssynchrony: radial dyssynchrony hazard ratio 2.6, 95% confidence interval (CI) 1.47–4.53 (P = 0.0008) and IVMD hazard ratio 4.9, 95% CI 2.60–9.16 (P = 0.0007). Right bundle branch block patients who lacked dyssynchrony had the least favourable outcome.
Conclusion
Non-LBBB patients with dyssynchrony had a more favourable long-term survival than non-LBBB patients who lacked dyssynchrony. Mechanical dyssynchrony and QRS morphology are associated with outcome following CRT.
Keywords: Cardiac resynchronization therapy, Heart failure, Conduction disturbance
Introduction
Cardiac resynchronization therapy (CRT) has been established as a useful therapy for heart failure patients with prolonged QRS duration and low ejection fraction (EF).1–4 However, clinical response rate to CRT remains at approximately two-thirds using routine guidelines and precise reasons for non-response are unclear. Although QRS widening ≥120 ms is currently one of the selection criteria for CRT, the specific QRS morphologies of left bundle branch block (LBBB), intraventricular conduction delay (IVCD), and right bundle branch block (RBBB) have been associated with different outcomes following CRT.1,3,5,6 In particular, non-LBBB patients have benefited less from CRT than those with LBBB. Recent observations from the MADIT-CRT trial and others have resulted in an expanded indication for CRT in less symptomatic heart failure patients, but in those only with LBBB morphology.7 Mechanical dyssynchrony is widely believed to be the derangement treated by CRT with QRS width as its surrogate. However, the degree of mechanical dyssynchrony may not be reflected by QRS width or morphology, and there is evidence to support that response to CRT is related, at least in part, to the degree of mechanical dyssynchrony.8–13 Accordingly, the objective of this study was to test the hypotheses that patient outcome following CRT is related to the degree of baseline mechanical dyssynchrony when considering QRS morphology.
Methods
We prospectively studied 278 consecutive patients referred for CRT with left ventricular (LV) EF ≤35%, QRS ≥120 ms, and symptomatic New York Heart Association (NYHA) class III or IV heart failure, despite the optimal pharmacological therapy. Patients with failed CRT implantation or who had right ventricular (RV) paced rhythm before CRT were not included. The protocol was approved by the Institutional Review Board on Biomedical Research, and patients were given informed consent consistent with this protocol. The mean age was 65 ± 12 years, EF was 24 ± 6%, and QRS duration was 159 ± 27 ms. Eighty-one patients (29%) were female. One-hundred and fifty-eight patients (57%) had ischaemic cardiomyopathy, defined by coronary angiography. Cardiac resynchronization therapy was initiated with implantation of routine CRT pacing defibrillators in all with LV lead placement via the coronary sinus targeting the posterior or lateral wall using angiographic and fluoroscopic guidance.
Electrocardiograms
Baseline 12-lead electrocardiograms (ECG) were recorded at a paper speed of 25 mm/s. Left bundle branch block was defined as: (i) broad, notched R-wave in lateral precordial leads (V5 and V6) and usually leads І and aVL, (ii) smaller or absent initial r waves in right precordial leads (V1 and V2) followed by deep S-waves, and (iii) absent q waves in left-sided leads. Right bundle branch block was defined as: (i) broad, notched R waves (rsr′, rsR′, or rSR′ patterns) in right precordial leads (V1 and V2) and (ii) wide and deep S waves in the left precordial leads (V5 and V6).14 Intraventricular conduction delay was defined as a conduction disturbance which does not meet the criteria of LBBB and RBBB.15 Experienced observers who were blind to all other patient data interpreted QRS morphology.
Echocardiography
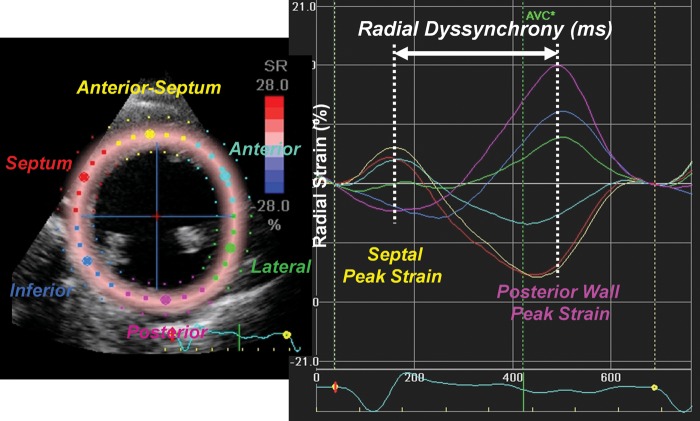
Echocardiograms were performed with a GE Vivid 7 system (GE-Vigmed, Horten, Norway), or Aplio 80 or Aplio Artida systems (Toshiba Medical Systems Tokyo, Japan) before and ∼6 months after the CRT implantation. Routine digital grey-scale two-dimensional and tissue Doppler cineloops were obtained from standard apical and mid-LV short-axis views.8,16,17 Quantitative analyses were performed using off-line software (EchoPac, version BT09, GE-Vingmed or Toshiba Medical Systems). Speckle-tracking radial dyssynchrony was evaluated using mid-short-axis images as previously described in detail.16 Briefly, a circular region of interest was traced on the endocardial and epicardial borders and manually adjusted for time–strain curves. Significant radial dyssynchrony was defined as the time difference between the peak strain in anterior-septum and posterior wall segments predefined as ≥130 ms (Figure 1).16 Left ventricular and RV pre-ejection delay (PED) was determined as the time from the QRS onset to the onset of pulsed Doppler flow in the LV and RV outflow tracts. Interventricular mechanical delay (IVMD) was calculated as the difference in LV and RV PED with a predefined cut-off of ≥40 ms.18,19 Tissue Doppler longitudinal dyssynchrony was defined as the maximum opposing wall time difference in peak systolic velocities with the predefined cut-off of ≥65 ms.11,17,20 The Yu index was calculated as the standard deviation (SD) of the 12 segments' time-to-peak velocities with the predefined cut-off of ≥32 ms.21 Left ventricular EF was assessed by biplane Simpson's rule.
Figure 1.
Representative speckle-tracking radial strain curves in a heart failure patient with left bundle branch block. Segmental strain curves in mid-left ventricular segments are shown in yellow (anterior septum), light blue (anterior), green (lateral), purple (posterior), dark blue (inferior), and red (septum). Time difference in peak strain in the anterior septum to the peak strain in the posterior wall was used to measure radial dyssynchrony.
Analysis of effects of cardiac resynchronization therapy on outcome
The pre-specified long-term outcome variable was the combined endpoint of death, cardiac transplant, or ventricular assist device (LVAD) implantation. This combined endpoint was pre-determined because only patients with end-stage heart failure with a limited anticipated survival would undergo transplant or LVAD implantation in our institution. Follow-up was obtained from a combination of the patient's medical records, vital status registry, and when needed telephone contact with the patient's physicians. Analysis of effects of CRT on LV function and reverse remodeling was assessed at ∼6 months in the subgroup of patients who had quantitative echocardiographic data available for EF and end-systolic volume (ESV).13,22,23
Statistical analysis
Statistical analyses were conducted using SPSS (version 17.0, SPSS Inc., Chicago, IL, USA). Group data were presented as means ± SD. Data from all continuous variables were determined to have a normal distribution and were compared using the two-sided Student's t-test for paired and unpaired data, where appropriate. One-way ANOVA followed by the Tukey post hoc tests was used to compare parameters, including LV volume and EF among patients with LBBB, IVCD, and RBBB. Proportional differences were evaluated using Fisher's exact test and χ2 was used for non-continuous variables. The Kaplan–Meier survival curves were plotted to measure outcome and the log-rank test used to compare survival between patients with or without dyssynchrony. A Cox proportional hazard model was used to assess for any potential influence of covariates. To test the appropriateness of the proportional hazards assumption in the Cox model, we determined that differences in QRS width and incidence of ischaemic aetiology at baseline were potential confounding covariates. Adjustments were made with the influence of these explanatory variables assumed to be constant over time. We verified the proportional hazards assumption using graphical methods and the log-rank test and observed the proportional hazards assumption to be appropriate. Data were reported as mean ± SD with a P-value of <0.05 considered as significant.
Results
Clinical characteristics
Of 278 consecutive patients referred for CRT, 24 (9%) had baseline echocardiographic images unsuitable for quantitative analysis and were eliminated from all further study. Accordingly, the study group consisted of 254 patients with the following QRS morphologies: LBBB (n = 128), IVCD (n = 81), and RBBB (n = 45). There were no differences in age or gender among the groups. Ischaemic disease was more prevalent in patients with IVCD or RBBB compared with LBBB patients. Patients with IVCD also had significantly narrower QRS duration than those with either LBBB or RBBB (Table 1).
Table 1.
Baseline patient characteristics
| LBBB (n = 128) | Non-LBBB (n = 126) | IVCD (n = 81) | RBBB (n = 45) | P-value | |
|---|---|---|---|---|---|
| Age (years) | 66 ± 12 | 65 ± 11 | 65 ± 11 | 63 ± 12 | 0.374 |
| Male gender (n) | 88 (69%) | 92 (73%) | 55 (68%) | 37 (82%) | 0.180 |
| NYHA class III/IV (n) | 113/15 | 114/12 | 73/8 | 41/4 | 0.839 |
| Ischaemic cardiomyopathy (n) | 63 (49%) | 83 (66%)* | 52 (64%)* | 31 (69%)* | 0.024 |
| QRS duration (ms) | 164 ± 26 | 153 ± 26* | 148 ± 25* | 163 ± 26† | <0.0001 |
| QRS >150 ms (n) | 90 (70%) | 58 (46%)* | 27 (36%)* | 29 (64%)† | <0.0001 |
| Atrial fibrillation (n) | 13 (10%) | 17 (13%) | 12 (15%) | 5 (11%) | 0.589 |
| Mitral regurgitation (1 to 4+) | 1.5 ± 1.2 | 1.7 ± 1.1 | 1.7 ± 1.1 | 1.6 ± 1.2 | 0.711 |
LBBB, left bundle branch block; IVCD, non-specific intraventricular conduction disturbance; RBBB, right bundle branch block; NS, not significant. P-value indicates comparison across LBBB, IVCD, and RBBB. Mitral regurgitation: 1+, mild; 2+, mild to moderate; 3+, moderate to severe; 4+, severe.
*P < 0.05 vs. LBBB.
†P < 0.05 vs. IVCD.
Baseline dyssynchrony analysis
Routine pulsed-Doppler, speckle-tracking radial strain, and tissue Doppler dyssynchrony results are shown in Table 2. Left bundle branch block patients overall had the most prevalent and significant degree of dyssynchrony by speckle-tracking radial strain compared with non-LBBB patients, and RBBB patients overall had the least. Left bundle branch block patients had time-to-peak radial strain occurred earlier in the anteroseptum and later in the posterior wall than non-LBBB patients (Figure 2). Left bundle branch block patients also had greater dyssynchrony by routine pulsed-Doppler methods than non-LBBB patients. Specifically, the LV PED and IVMD were greatest in LBBB patients and RV PED was greatest in RBBB patients, while the IVMD was usually minimal in patients with RBBB. However, there were no differences in the degree or prevalence of tissue Doppler longitudinal dyssynchrony between QRS morphology groups by opposing wall delay (at the pre-specified cut-off of 65 ms) or the Yu index among groups. Post hoc analysis of an opposing wall delay of ≥80 ms became statistically significant (P < 0.05). Of IVCD patients, the QRS width was 150 ± 26 (range 120–216 ms) in the 47 with radial dyssynchrony ≥130 ms and 142 ± 20 (range 120–200 ms) in the 33 patients without radial dyssynchrony (P = 0.117). Of 25 IVCD patients with IVMD ≥40 ms, QRS width was 162 ± 30 (range 120–216 ms) vs. 141 ± 19 (range 120–214 ms) in the 51 with no significant interventricular dyssynchrony (P = 0.0007). Of RBBB patients, the QRS width was 169 ± 32 (range 122–228 ms) in the 17 with radial dyssynchrony ≥130 ms vs. 160 ± 20 (range 120–200 ms) in the 26 without radial dyssynchrony (P = 0.240). Of the 6 RBBB patients with IVMD ≥40 ms, QRS width was 183 ± 31 (range 138–228 ms) vs. 159 ± 21 (range 120–212 ms) in the 30 with no significant IVMD (P = 0.027), supporting that significant IVMD occurred more often in non-LBBB patients with wider QRS.
Table 2.
Echocardiographic dyssynchrony
| LBBB (n = 128) | Non-LBBB (n = 126) | IVCD (n = 81) | RBBB (n = 45) | P-value | |
|---|---|---|---|---|---|
| Speckle-tracking radial strain | |||||
| Time-to-peak strain | |||||
| Anterior septum (ms) | 232 ± 115 | 310 ± 123* | 302 ± 128* | 325 ± 112* | <0.0001 |
| Posterior wall (ms) | 484 ± 84 | 449 ± 80* | 468 ± 70 | 414 ± 87*,† | <0.0001 |
| Septal-posterior Wall (ms) | 257 ± 112 | 160 ± 111* | 176 ± 117* | 130 ± 93* | <0.001 |
| Septal-posterior Wall ≥130 ms (n) | 105/124 (85%) | 64/123 (52%)* | 47/80 (59%)* | 17/43 (40%)* | <0.0001 |
| Pulsed Doppler | |||||
| LV PED (ms) | 156 ± 32 | 134 ± 33* | 137 ± 33* | 131 ± 33* | <0.0001 |
| LV PED ≥140 ms (n) | 84/115 (73%) | 49/119 (41%)* | 33/76 (43%)* | 16/43 (37%)* | <0.0001 |
| RV PED (ms) | 112 ± 22 | 118 ± 26 | 105 ± 22 | 135 ± 22*,† | <0.0001 |
| IVMD (ms) | 46 ± 22 | 21 ± 35* | 32 ± 24* | −3 ± 42*,† | <0.0001 |
| IVMD ≥40 ms (n) | 73/109 (70%) | 31/112 (28%)* | 25/76 (33%)* | 6/36 (17%)* | <0.0001 |
| Tissue Doppler velocity | |||||
| Opposing wall delay (ms) | 95 ± 36 | 86 ± 40 | 90 ± 39 | 77 ± 42 | 0.065 |
| Opposing wall ≥65 ms (n) | 95/123 (77%) | 71/102 (70%) | 55/74 (74%) | 16/28 (57%)* | 0.092 |
| Opposing wall ≥80 ms (n) | 87/123 (71%) | 59/102 (58%)* | 46/74 (62%) | 13/28 (46%)* | 0.043 |
| Yu index (ms) | 41 ± 17 | 38 ± 17 | 39 ± 17 | 34 ± 17 | 0.120 |
| Yu index ≥32 ms (n) | 88/123 (72%) | 61/102 (60%) | 44/74 (59%) | 17/28 (61%) | 0.178 |
LBBB, left bundle branch block; IVCD, non-specific intraventricular conduction disturbance; RBBB, right bundle branch block; LV PED, left ventricular pre-ejection delay; RV PED, right ventricular pre-ejection delay; IVMD, interventricular mechanical delay; NS, not significant. P-value indicates comparison across LBBB, IVCD, and RBBB.
*P< 0.05 vs. LBBB.
†P < 0.05 vs. IVCD.
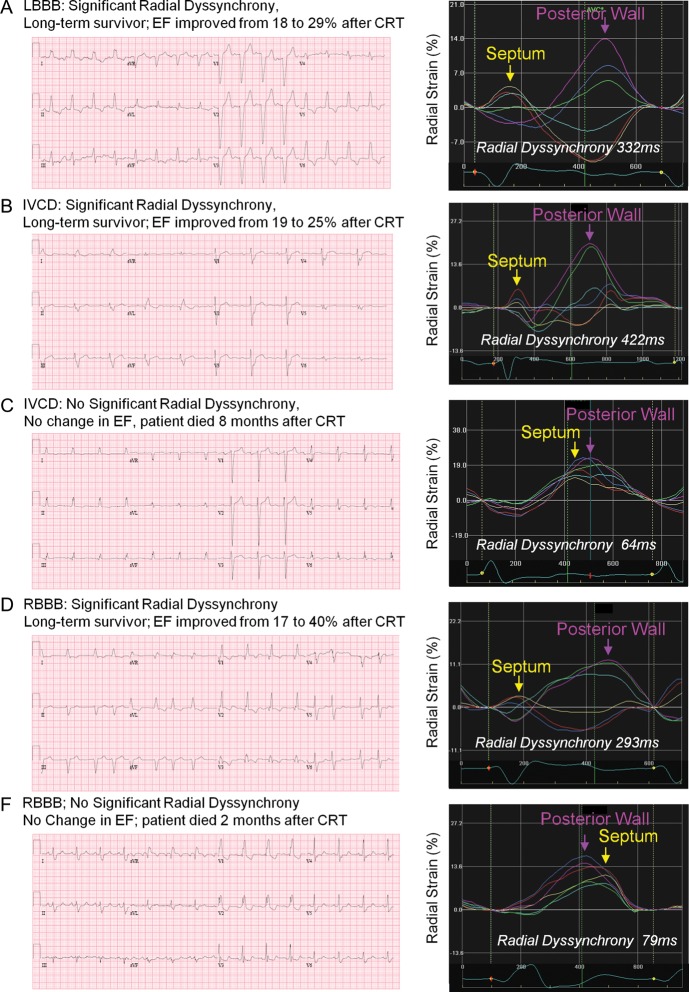
Figure 2.
(A–E) Five patient examples of different QRS morphology and radial strain dyssynchrony, with ejection fraction (EF) response and long-term outcome following cardiac resynchronization therapy (CRT). LBBB, left bundle branch block; IVCD, interventricular conduction delay; RBBB, right bundle branch block.
Since ischaemic cardiomyopathy was represented in patients with non-LBBB more often than those with LBBB, the details related to dyssynchrony were as follows: in the 47 IVCD patients with radial dyssynchrony ≥130 ms, ischaemic aetiology was observed in 27 (57%) vs. 25 in 33 patients (76%) without radial dyssynchrony (P = 0.146). Of the 25 IVCD patients with IVMD ≥40 ms, ischaemic aetiology was observed in 12 (48%) vs. 38 in 51 patients (75%) without significant IVMD (P = 0.042). Of the 17 RBBB patients with radial dyssynchrony ≥130 ms, ischaemic aetiology was observed in 12 (71%) vs. 17 in 26 patients (65%) without significant radial dyssynchrony (P = 0.982). Of the 6 RBBB patients with IVMD ≥40 ms, ischaemic aetiology was observed in 3 (50%) vs. 20 in 50 patients (67%) without significant IVMD (P = 0.756).
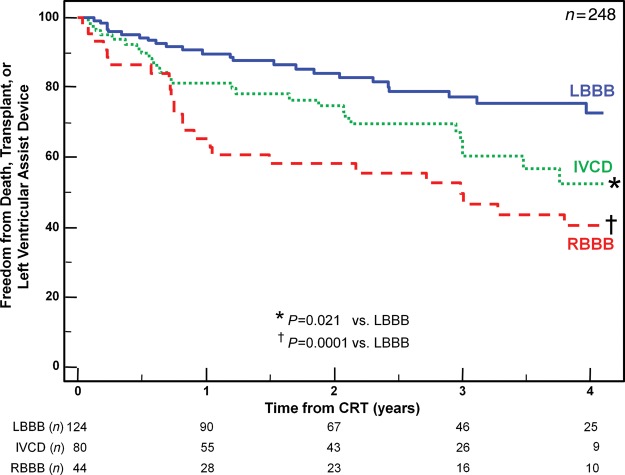
Long-term clinical outcomes
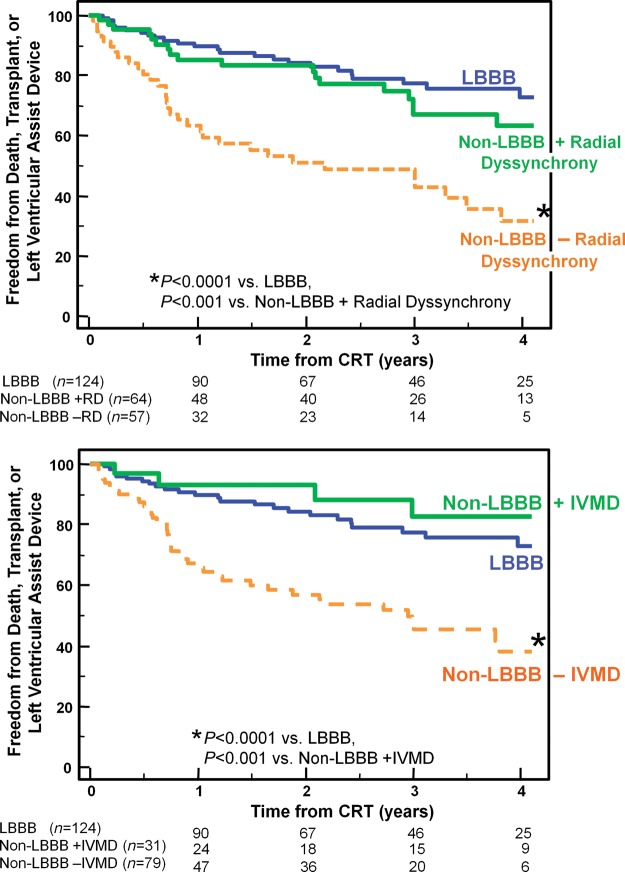
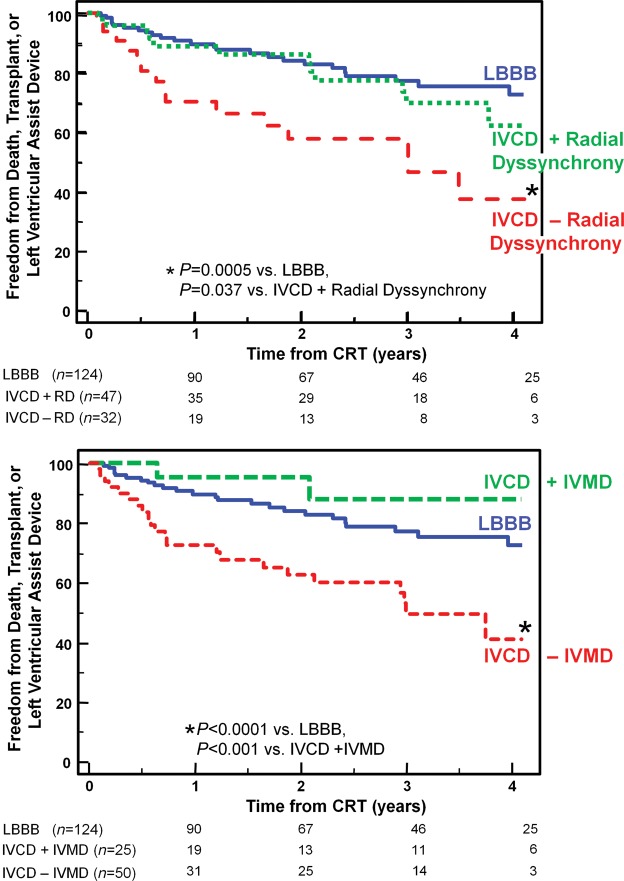
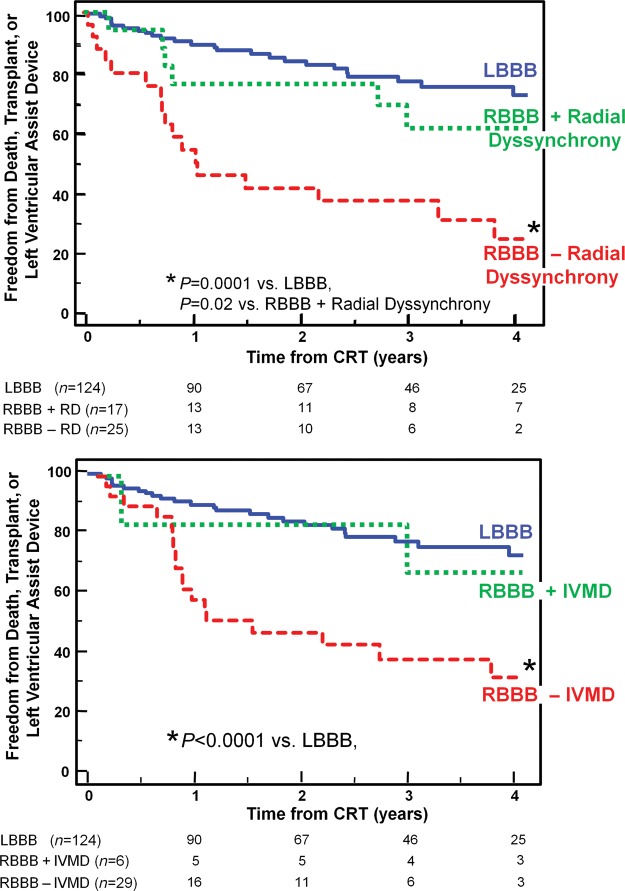
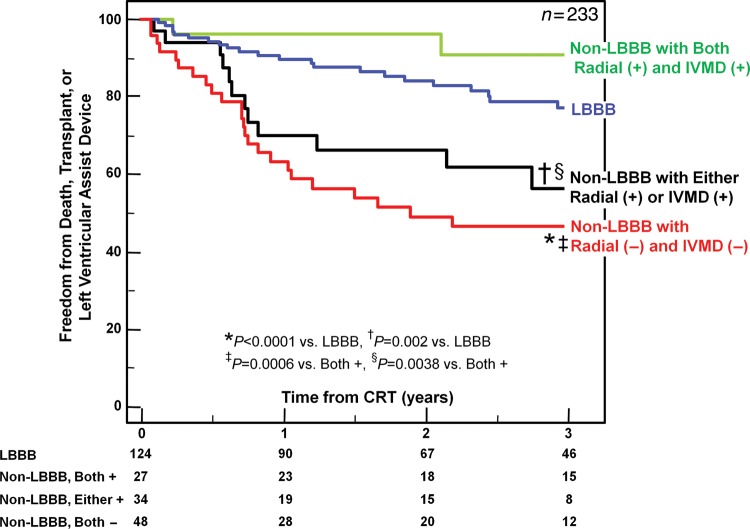
Over a 4-year period, 75 outcome events occurred after CRT including 56 deaths, 12 transplants, and 7 LVAD implantations. Six patients (2%) who were lost to follow-up could not be included in long-term analysis. Overall, patients with LBBB had the greatest freedom from death, transplant, or LVAD, compared with patients with IVCD with an intermediate outcome, and RBBB patients with the least favourable outcome (Figure 3). Non-LBBB patients with baseline dyssynchrony by radial strain or IVMD had significantly more favourable long-term outcome, which was similar to LBBB patients when compared with non-LBBB patients who lacked dyssynchrony (Figure 4). Specifically, in non-LBBB patients when associating dyssynchrony with survival, the hazard ratio of radial dyssynchrony was 2.58, with a 95% confidence interval (CI) of 1.47–4.53, P = 0.0008, and the hazard ratio of IVMD was 4.88, with a 95% CI of 2.60–9.16, P = 0.0007. When considered individually, both IVCD and RBBB patients who lacked radial dyssynchrony or IVMD had a significantly lower event-free survival following CRT than LBBB patients (Figures 5 and 6). Specifically when associating dyssynchrony with survival in IVCD patients, the hazard ratio of radial dyssynchrony was 2.25, with a 95% CI of 1.02–4.95, P = 0.03, and the hazard ratio of IVMD was 6.33, with a 95% CI of 2.76–14.52, P = 0.004. Finally when associating dyssynchrony with survival in RBBB patients, the hazard ratio of radial dyssynchrony was 2.75, with a 95% CI of 1.21–6.23, P = 0.02, although the hazard ratio of IVMD was not statistically significant. We then adjusted for the covariates of ischaemic disease and baseline QRS duration using a Cox proportional hazard model, and the presence of radial strain dyssynchrony remained independently associated with event-free survival in combined non-LBBB patients (adjusted hazard radio: 2.50, P = 0.002) as well as IVCD (adjusted hazard radio: 2.29, P = 0.047) and RBBB patients (adjusted hazard radio: 2.78, P = 0.033). Interventricular mechanical delay remained independently associated with event-free survival in grouped non-LBBB patients (adjusted hazard radio: 5.34, P = 0.002) and IVCD patients (adjusted hazard radio: 6.91, P = 0.013) individually. We then assessed the potential influence of a combination of interventricular and intraventricular dyssynchrony by examining IVMD and radial strain together (Figure 7). Patients with both IVMD and radial dyssynchrony appeared to have favourable outcome similar to LBBB after CRT, whereas patients who lacked significant IVMD and radial dyssynchrony had the least favourable outcome (Figure 7).
Figure 3.
The Kaplan–Meier curves demonstrating the probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT) comparing patients with left bundle branch block (LBBB), interventricular conduction delay (IVCD), and right bundle branch block (RBBB).
Figure 4.
The Kaplan–Meier curves demonstrating the probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT) comparing patients with left bundle branch block (LBBB), to those with non-LBBB with or without significant radial dyssynchrony (top panel) or with or without interventricular mechanical delay (IVMD) (bottom panel). Non-LBBB patients with dyssynchrony had similar outcome as those with LBBB, whereas Non-LBBB patients without dyssynchrony had a less favourable outcome.
Figure 5.
The Kaplan–Meier curves demonstrating the probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT) comparing patients with left bundle branch block (LBBB), to those with interventricular conduction delay (IVCD) with or without significant radial dyssynchrony (top panel) or with or without interventricular mechanical delay (IVMD) (bottom panel). Interventricular conduction delay patients with dyssynchrony had similar outcome as those with LBBB, whereas interventricular conduction delay patients without dyssynchrony had a less favourable outcome.
Figure 6.
The Kaplan–Meier curves demonstrating the probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT) comparing patients with left bundle branch block (LBBB), to those with right bundle branch block (RBBB) with or without significant radial dyssynchrony (top panel) or with or without interventricular mechanical delay (IVMD) (bottom panel). Right bundle branch block patients with dyssynchrony had similar outcome as those with left bundle branch block, whereas right bundle branch block patients without dyssynchrony had a less favourable outcome.
Figure 7.
The Kaplan–Meier curves demonstrating the probability of freedom from death, transplant, or ventricular assist device after cardiac resynchronization therapy (CRT) comparing patients with left bundle branch block (LBBB), to those with non-LBBB with or without significant dyssynchrony by a combined approach of interventricular mechanical delay (IVMD) and radial dyssynchrony by speckle-tracking strain. More favourable outcome was observed in non-LBBB with both markers of dyssynchrony, and least favourable outcome in patients who lacked dyssynchrony by either method. +, positive; −, negative.
Subgroup results of ventricular volumes and ejection fractions
Of 248 possible patients for near-term echocardiographic follow-up, 24 reached a primary endpoint <7 months after CRT as follows: 8/124 (6%) in LBBB (5 deaths, 2 transplants, and 1 LVAD), 9/80 (11%) in IVCD (6 deaths, 1 transplant, and 2 LVAD), and 7/44 (16%) in RBBB (6 deaths and 1 transplant) (not significant across groups, P = 0.170). The sample for LV functional analysis consisted of 204 out of 224 remaining patients (91%) who returned for quantitative echocardiography 7 ± 5 months after CRT. Baseline LV volumes and EFs were similar when classified by QRS morphology (Table 3). After CRT, LBBB patients were observed to have a significantly greater absolute increase in EF and relative decrease in ESV than IVCD or RBBB patients, P < 0.0001. Left bundle branch block patients had greater improvements in EF (23 ± 6–34 ± 12%*, P < 0.001). However, non-LBBB patients with dyssynchrony improved their EFs (23 ± 6–31 ± 10%*, P < 0.001) compared with those without dyssynchrony (25 ± 6–27 ± 8%, not significant P = 0.151). Specifically, IVCD and RBBB patients with dyssynchrony had improvements in EF and ESV following CRT, whereas those who lacked dyssynchrony had no significant changes in EF or ESV (Figure 8). Right bundle branch block patients who lacked dyssynchrony had the least improvement in ESV or EF following CRT.
Table 3.
Left ventricular volumes and ejection fractions
| LBBB | Non-LBBB | IVCD | RBBB | P-value | |
|---|---|---|---|---|---|
| Echo follow-up (n) | 104/116 (90%) | 100/108 (93%) | 66/71 (93%) | 34/37 (92%) | 0.638 |
| Follow-up (months) | 7 ± 5 | 7 ± 4 | 7 ± 4 | 8 ± 5 | 0.735 |
| LV end-diastolic volume | |||||
| Baseline (mL) | 194 ± 81 | 192 ± 67 | 195 ± 69 | 185 ± 61 | 0.764 |
| Follow-up (mL) | 169 ± 80 | 179 ± 62 | 182 ± 68 | 173 ± 48 | 0.527 |
| LV end-systolic volume | |||||
| Baseline (mL) | 152 ± 73 | 147 ± 57 | 151 ± 61 | 139 ± 49 | 0.535 |
| Follow-up (mL) | 118 ± 74 | 131 ± 57 | 134 ± 62 | 123 ± 42 | 0.295 |
| Relative decrease (%) | 24 ± 21 | 8 ± 24* | 10 ± 24* | 5 ± 22* | <0.0001 |
| Relative decrease ≥10% (n) | 66/104 (63%) | 38/100 (38%) | 27/66 (41%)* | 11/34 (32%)* | 0.001 |
| LV ejection fraction | |||||
| Baseline (%) | 23 ± 6 | 24 ± 6 | 24 ± 6 | 25 ± 4 | 0.070 |
| Follow-up (%) | 34 ± 12 | 28 ± 9* | 28 ± 10* | 29 ± 8 | 0.003 |
| Absolute EF increase (%) | 10 ± 9 | 5 ± 8* | 5 ± 8* | 4 ± 8* | <0.0001 |
| Absolute increase ≥5% (n) | 73/104 (70%) | 36/100 (36%) | 25/66 (38%)* | 11/34 (32%)* | <0.0001 |
LBBB, left bundle branch block; IVCD, non-specific intraventricular conduction disturbance; RBBB, right bundle branch block; LV, left ventricular; NS, not significant. P-value indicates comparison across LBBB, IVCD, and RBBB.
*P < 0.05 vs. LBBB.
Figure 8.
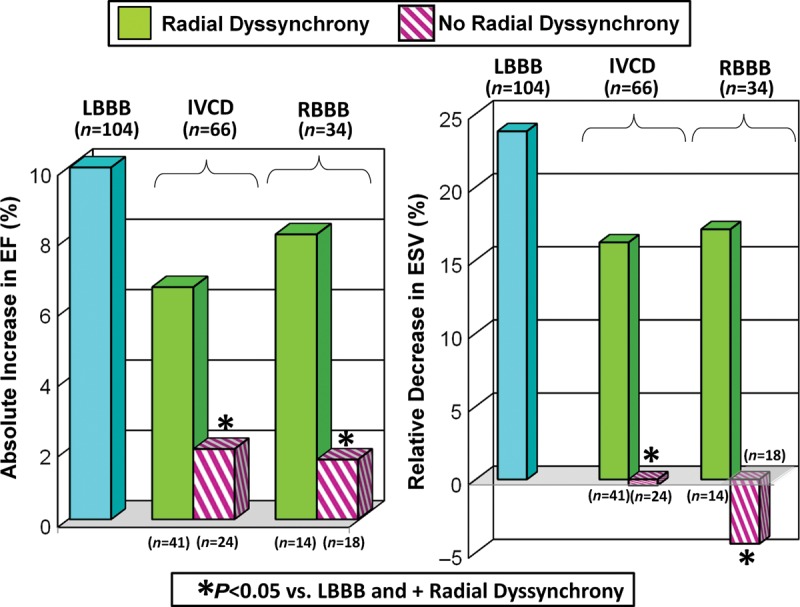
Bar graphs of subgroup analysis of absolute changes in ejection fraction (EF, %) and relative changes in end-systolic volume (ESV, %) after cardiac resynchronization therapy. Patients with left bundle branch block (LBBB) are shown in comparison to patients with interventricular conduction delay (IVCD) or right bundle branch block (RBBB) with or without significant radial dyssynchrony by speckle-tracking strain.
Discussion
This study of a large series of consecutive patients referred for CRT with routine indications demonstrated significant differences in long-term outcome when QRS morphology and mechanical dyssynchrony were considered. Overall, patients with LBBB had the most favourable outcome, patients with RBBB had the least favourable outcome, and patients with IVCD and an intermediate outcome.6 Subgroup analysis of LV functional response was supportive in terms of EF increases and ESV decreases followed a similar association with QRS morphology as did long-term survival free from transplant or LVAD. Importantly, patients with non-LBBB QRS morphology who demonstrated evidence of mechanical dyssynchrony by speckle-tracking radial strain or IVMD appeared to have a significantly greater benefit from CRT. Conversely, patients with QRS morphologies of IVCD or RBBB who lacked mechanical dyssynchrony had a comparatively less favourable outcome following CRT.
Recent attention has focused on QRS morphology and outcome following CRT after the MADIT-CRT randomized trial.7 Zareba et al.6 reported on 1817 patients with NYHA class I or II heart failure, EF ≤30%, and QRS ≥120 ms of whom 70% were LBBB and 30% were non-LBBB morphologies. The patients with LBBB derived substantial clinical benefits from CRT with reduction in heart failure hospitalizations, whereas patients with non-LBBB QRS morphology did not appear to benefit overall. The LBBB patients were more uniform in their favourable response to CRT, whereas subgroup analysis of the non-LBBB patients suggested a much wider variation in response as shown in their relative risk and hazard ratio analysis.6 Although we included only NYHA class III or IV patients, our data add a potential explanation for the variable response in non-LBBB patients in its association with degree of dyssynchrony. Data from the RAFT trial suggested similar results with more favourable benefit in the LBBB patients vs. the non-LBBB patients.24 Since LBBB patients had the greatest incidence and degree of dyssynchrony overall, our data suggest that dyssynchrony by imaging may be of less predictive value in these patients. However, patients with non-LBBB, including those with RBBB, appear to show the greatest association of mechanical dyssynchrony with LV functional improvements and overall survival. Previous observations in the COMPANION trial3 were that CRT benefited patients with LBBB the most by reducing death or hospitalization from any cause compared with pharmacology therapy. In the analysis of pooled data of RBBB patients in the MIRACLE and CONTAK CD trials, there were no demonstrable benefits in any variables studied at follow-up, except NYHA functional class.1,25 Other large retrospective CRT studies of patients with different QRS morphologies also reported that improvements in symptoms, LV function, and survival were comparatively lower in patients with non-LBBB.5,26,27
Ventricular contraction abnormalities in LBBB patients are typically characterized as early septal contraction and lateral stretch, followed by septum stretch and lateral contraction later in systole.27–32 We observed similar dyssynchrony patterns using radial strain where LBBB had an earlier anterior-septum peak radial strain and later posterior wall peak radial strain than those of the non-LBBB patients. Interventricular mechanical delay was also greater in LBBB patients compared with IVCD or RBBB patients associated with larger LV PED. Non-LBBB patients in contrast showed later time-to-peak radial strain in the anterior-septal region and earlier time-to-peak strain in the posterior region, which resulted in lesser degrees of radial dyssynchrony, overall. However, non-LBBB patients had a wide range of dyssynchrony patterns associated with variable CRT response. Ventricular activation patterns in IVCD patients are more complex and heterogeneous than those of the LBBB patients.33–35 Less data are available on dyssynchrony in RBBB. An experimental study of right bundle ablation showed less intraventricular dyssynchrony and less haemodynamic response to CRT than LBBB.28 We observed variable mechanical activation patterns in patients with RBBB using radial strain analysis. Although RBBB patients had similar QRS duration compared with those of the LBBB patients (Table 1), the majority of the RBBB patients lacked significant dyssynchrony. On the other hand, a subgroup showed left-sided activation delay similar to those of the LBBB patients associated with a favourable response to CRT.33,35
Several earlier echocardiographic studies reported on the potential utility of dyssynchrony as a means to predict response to CRT.8,11,17,21 The PROSPECT multicentre study with a 6-month follow-up suggested that dyssynchrony indices had an insufficient predictive value to alter the current selection criteria for CRT.36 More recent data have demonstrated echocardiographic measures of dyssynchrony to be associated with patient outcome including heart failure hospitalization or long-term survival following CRT. In particular, speckle-tracking echocardiography, which was not studied in PROSPECT, is a promising newer method. The STAR study of 132 CRT patients showed that dyssynchrony by speckle-tracking radial or transverse strain was associated with EF response and long-term event-free survival.12 Another study of 229 CRT patients with routine indications demonstrated that dyssynchrony measured by IVMD, tissue Doppler opposing wall delay, or Yu Index and speckle-tracking radial strain were all significantly associated with the long-term survival.10 Delgado et al.9 recently reported a study of 397 CRT patients with ischaemic heart disease that there was a significant association of radial strain dyssynchrony with survival and heart failure hospitalization over 3 years. Furthermore, the VALIANT study suggested that dyssynchrony may play a role in post-myocardial infarction prognosis of death or heart failure using dyssynchrony by velocity vector speckle tracking in 381 myocardial infarction patients with LV dysfunction, heart failure, or both.37 Most recently, dyssynchrony by SD of speckle-tracking transverse strain from apical views was associated with clinical outcomes in the MADIT-CRT trial.38,39 These previous studies along with our present study combine to support the association of echocardiographic dyssynchrony with patient outcome following CRT.
Study limitations
This study was not part of a randomized trial, and the relationship of echocardiographic dyssynchrony to survival in those who do not undergo CRT remains unknown. Although this study reports important associations of echocardiographic dyssynchrony with patient outcome in QRS morphology subgroups, a direct recommendation for patient selection for CRT would require further study by a randomized controlled trial. Another limitation was that dyssynchrony analysis could not be performed on ∼10% of the patients and speckle tracking is operator-dependent and requires user experience.16,20,40 However, IVMD requires no specialized equipment or training and was shown to have the greatest feasibility and reproducibility in the PROSPECT study.36 Dyssynchrony by the tissue Doppler longitudinal velocities was not associated with long-term outcome in QRS morphology groups. The reason for this observation is unclear, but longitudinal velocity appears to measure different mechanical phenomena than radial strain or IVMD and tissue Doppler velocities could not differentiate between active and passive motion like speckle-tracking strain imaging. Previous comparisons of tissue Doppler by QRS morphology have not been made. A limitation was that not all patients had quantitative follow-up echo data available for near-term LV functional analysis, but this was performed on a subgroup of 91%. Another limitation was that other CRT response markers such as heart failure hospitalizations, 6 min walk distance, or peak myocardial oxygen consumption were not part of the present study. Also, specific LV lead positioning data were not part of this study. It may be considered a limitation that ischaemic aetiology may influence response to CRT from scar burden or lead positioning and have confounding effects.41–43 We demonstrated that when adjusting specifically for ischaemic aetiology, IVMD and speckle-tracking radial strain remained independently associated with survival. Also, LBBB-like QRS morphology that is categorized as IVCD may be difficult to define. Future ECG methods of quantifying ventricular activation might be considered instead of QRS pattern recognition.44 This study concludes that mechanical dyssynchrony and QRS morphology are associated with outcome following CRT and future study is warranted.
Funding
This study was supported in part by NIH award K24 HL04503-01 and AHA grant-in-aid (0855526D).
Conflict of interest: J.G. received research grant support from Biotronik, GE, Medtronic, St. Jude Medical, and Toshiba Medical. All other co-authors: none declared.
Acknowledgements
The authors are grateful to Stephanie Haberman for her skillful assistance with the figures and we thank the entire University of Pittsburgh Medical Center Electrophysiology Laboratory faculty and staff for their continued support and cooperation.
References
- 1.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. doi:10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 2.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. J Am Med Assoc. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. doi:10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 3.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. doi:10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 4.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. doi:10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 5.Adelstein EC, Saba S. Usefulness of baseline electrocardiographic QRS complex pattern to predict response to cardiac resynchronization. Am J Cardiol. 2009;103:238–242. doi: 10.1016/j.amjcard.2008.08.069. doi:10.1016/j.amjcard.2008.08.069. [DOI] [PubMed] [Google Scholar]
- 6.Zareba W, Klein H, Cygankiewicz I, Hall WJ, McNitt S, Brown M, Cannom D, Daubert JP, Eldar M, Gold MR, Goldberger JJ, Goldenberg I, Lichstein E, Pitschner H, Rashtian M, Solomon S, Viskin S, Wang P, Moss AJ. Effectiveness of cardiac resynchronization therapy by QRS morphology in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. doi:10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 7.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. doi:10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 8.Bax JJ, Bleeker GB, Marwick TH, Molhoek SG, Boersma E, Steendijk P, van der Wall EE, Schalij MJ. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. doi:10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Delgado V, van Bommel RJ, Bertini M, Borleffs CJ, Marsan NA, Arnold CT, Nucifora G, van de Veire NR, Ypenburg C, Boersma E, Holman ER, Schalij MJ, Bax JJ. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78. doi: 10.1161/CIRCULATIONAHA.110.945345. doi:10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 10.Gorcsan J, 3rd, Oyenuga O, Habib PJ, Tanaka H, Adelstein EC, Hara H, McNamara DM, Saba S. Relationship of echocardiographic dyssynchrony to long-term survival after cardiac resynchronization therapy. Circulation. 2010;122:1910–1918. doi: 10.1161/CIRCULATIONAHA.110.954768. doi:10.1161/CIRCULATIONAHA.110.954768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sogaard P, Egeblad H, Kim WY, Jensen HK, Pedersen AK, Kristensen BO, Mortensen PT. Tissue Doppler imaging predicts improved systolic performance and reversed left ventricular remodeling during long-term cardiac resynchronization therapy. J Am Coll Cardiol. 2002;40:723–730. doi: 10.1016/s0735-1097(02)02010-7. doi:10.1016/S0735-1097(02)02010-7. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Nesser HJ, Buck T, Oyenuga O, Janosi RA, Winter S, Saba S, Gorcsan J., 3rd Dyssynchrony by speckle-tracking echocardiography and response to cardiac resynchronization therapy: results of the Speckle Tracking and Resynchronization (STAR) study. Eur Heart J. 2010;31:1690–1700. doi: 10.1093/eurheartj/ehq213. doi:10.1093/eurheartj/ehq213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yu CM, Bleeker GB, Fung JW, Schalij MJ, Zhang Q, van der Wall EE, Chan YS, Kong SL, Bax JJ. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. doi:10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 14.Mirvis DM, Goldberger AL. Braunwald's Heart Disease, CH12, Intraventricular Conduction Delays. 8th ed. Philadelphia, PA: Saunders, Elsevier; 2007. pp. 167–172. [Google Scholar]
- 15.Willems JL, Robles de Medina EO, Bernard R, Coumel P, Fisch C, Krikler D, Mazur NA, Meijler FL, Mogensen L, Moret P. Criteria for intraventricular conduction disturbances and pre-excitation.: World Health Organizational/International Society and Federation for Cardiology Task Force Ad Hoc. J Am Coll Cardiol. 1985;5:1261–1275. doi: 10.1016/s0735-1097(85)80335-1. doi:10.1016/S0735-1097(85)80335-1. [DOI] [PubMed] [Google Scholar]
- 16.Suffoletto MS, Dohi K, Cannesson M, Saba S, Gorcsan J., 3rd Novel speckle-tracking radial strain from routine black-and-white echocardiographic images to quantify dyssynchrony and predict response to cardiac resynchronization therapy. Circulation. 2006;113:960–968. doi: 10.1161/CIRCULATIONAHA.105.571455. doi:10.1161/CIRCULATIONAHA.105.571455. [DOI] [PubMed] [Google Scholar]
- 17.Gorcsan J, 3rd, Kanzaki H, Bazaz R, Dohi K, Schwartzman D. Usefulness of echocardiographic tissue synchronization imaging to predict acute response to cardiac resynchronization therapy. Am J Cardiol. 2004;93:1178–1181. doi: 10.1016/j.amjcard.2004.01.054. doi:10.1016/j.amjcard.2004.01.054. [DOI] [PubMed] [Google Scholar]
- 18.Richardson M, Freemantle N, Calvert MJ, Cleland JG, Tavazzi L. Predictors and treatment response with cardiac resynchronization therapy in patients with heart failure characterized by dyssynchrony: a pre-defined analysis from the CARE-HF trial. Eur Heart J. 2007;28:1827–1834. doi: 10.1093/eurheartj/ehm192. doi:10.1093/eurheartj/ehm192. [DOI] [PubMed] [Google Scholar]
- 19.Ghio S, Constantin C, Klersy C, Serio A, Fontana A, Campana C, Tavazzi L. Interventricular and intraventricular dyssynchrony are common in heart failure patients, regardless of QRS duration. Eur Heart J. 2004;25:571–578. doi: 10.1016/j.ehj.2003.09.030. doi:10.1016/j.ehj.2003.09.030. [DOI] [PubMed] [Google Scholar]
- 20.Gorcsan J, 3rd, Tanabe M, Bleeker GB, Suffoletto MS, Thomas NC, Saba S, Tops LF, Schalij MJ, Bax JJ. Combined longitudinal and radial dyssynchrony predicts ventricular response after resynchronization therapy. J Am Coll Cardiol. 2007;50:1476–1483. doi: 10.1016/j.jacc.2007.06.043. doi:10.1016/j.jacc.2007.06.043. [DOI] [PubMed] [Google Scholar]
- 21.Yu CM, Chau E, Sanderson JE, Fan K, Tang MO, Fung WH, Lin H, Kong SL, Lam YM, Hill MR, Lau CP. Tissue Doppler echocardiographic evidence of reverse remodeling and improved synchronicity by simultaneously delaying regional contraction after biventricular pacing therapy in heart failure. Circulation. 2002;105:438–445. doi: 10.1161/hc0402.102623. doi:10.1161/hc0402.102623. [DOI] [PubMed] [Google Scholar]
- 22.Bleeker GB, Mollema SA, Holman ER, Van de Veire N, Ypenburg C, Boersma E, van der Wall EE, Schalij MJ, Bax JJ. Left ventricular resynchronization is mandatory for response to cardiac resynchronization therapy: analysis in patients with echocardiographic evidence of left ventricular dyssynchrony at baseline. Circulation. 2007;116:1440–1448. doi: 10.1161/CIRCULATIONAHA.106.677005. doi:10.1161/CIRCULATIONAHA.106.677005. [DOI] [PubMed] [Google Scholar]
- 23.Di Biase L, Gasparini M, Lunati M, Santini M, Landolina M, Boriani G, Curnis A, Bocchiardo M, Vincenti A, Denaro A, Valsecchi S, Natale A, Padeletti L. Antiarrhythmic effect of reverse ventricular remodeling induced by cardiac resynchronization therapy: the InSync ICD (Implantable Cardioverter-Defibrillator) Italian Registry. J Am Coll Cardiol. 2008;52:1442–1449. doi: 10.1016/j.jacc.2008.07.043. doi:10.1016/j.jacc.2008.07.043. [DOI] [PubMed] [Google Scholar]
- 24.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. doi:10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 25.Egoavil CA, Ho RT, Greenspon AJ, Pavri BB. Cardiac resynchronization therapy in patients with right bundle branch block: analysis of pooled data from the MIRACLE and Contak CD trials. Heart Rhythm. 2005;2:611–615. doi: 10.1016/j.hrthm.2005.03.012. doi:10.1016/j.hrthm.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 26.Wokhlu A, Rea RF, Asirvatham SJ, Webster T, Brooke K, Hodge DO, Wiste HJ, Dong Y, Hayes DL, Cha YM. Upgrade and de novo cardiac resynchronization therapy: impact of paced or intrinsic QRS morphology on outcomes and survival. Heart Rhythm. 2009;6:1439–1447. doi: 10.1016/j.hrthm.2009.07.009. doi:10.1016/j.hrthm.2009.07.009. [DOI] [PubMed] [Google Scholar]
- 27.Nelson GS, Curry CW, Wyman BT, Kramer A, Declerck J, Talbot M, Douglas MR, Berger RD, McVeigh ER, Kass DA. Predictors of systolic augmentation from left ventricular preexcitation in patients with dilated cardiomyopathy and intraventricular conduction delay. Circulation. 2000;101:2703–2709. doi: 10.1161/01.cir.101.23.2703. [DOI] [PubMed] [Google Scholar]
- 28.Byrne MJ, Helm RH, Daya S, Osman NF, Halperin HR, Berger RD, Kass DA, Lardo AC. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol. 2007;50:1484–1490. doi: 10.1016/j.jacc.2007.07.011. doi:10.1016/j.jacc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 29.Breithardt OA, Stellbrink C, Herbots L, Claus P, Sinha AM, Bijnens B, Hanrath P, Sutherland GR. Cardiac resynchronization therapy can reverse abnormal myocardial strain distribution in patients with heart failure and left bundle branch block. J Am Coll Cardiol. 2003;42:486–494. doi: 10.1016/s0735-1097(03)00709-5. doi:10.1016/S0735-1097(03)00709-5. [DOI] [PubMed] [Google Scholar]
- 30.Prinzen FW, Hunter WC, Wyman BT, McVeigh ER. Mapping of regional myocardial strain and work during ventricular pacing: experimental study using magnetic resonance imaging tagging. J Am Coll Cardiol. 1999;33:1735–1742. doi: 10.1016/s0735-1097(99)00068-6. doi:10.1016/S0735-1097(99)00068-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Grines CL, Bashore TM, Boudoulas H, Olson S, Shafer P, Wooley CF. Functional abnormalities in isolated left bundle branch block. The effect of interventricular asynchrony. Circulation. 1989;79:845–853. doi: 10.1161/01.cir.79.4.845. doi:10.1161/01.CIR.79.4.845. [DOI] [PubMed] [Google Scholar]
- 32.Sade LE, Kanzaki H, Severyn D, Dohi K, Gorcsan J., 3rd Quantification of radial mechanical dyssynchrony in patients with left bundle branch block and idiopathic dilated cardiomyopathy without conduction delay by tissue displacement imaging. Am J Cardiol. 2004;94:514–518. doi: 10.1016/j.amjcard.2004.04.071. doi:10.1016/j.amjcard.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 33.Peichl P, Kautzner J, Cihak R, Bytesnik J. The spectrum of inter- and intraventricular conduction abnormalities in patients eligible for cardiac resynchronization therapy. Pacing Clin Electrophysiol. 2004;27:1105–1112. doi: 10.1111/j.1540-8159.2004.00592.x. doi:10.1111/j.1540-8159.2004.00592.x. [DOI] [PubMed] [Google Scholar]
- 34.Auricchio A, Fantoni C, Regoli F, Carbucicchio C, Goette A, Geller C, Kloss M, Klein H. Characterization of left ventricular activation in patients with heart failure and left bundle-branch block. Circulation. 2004;109:1133–1139. doi: 10.1161/01.CIR.0000118502.91105.F6. doi:10.1161/01.CIR.0000118502.91105.F6. [DOI] [PubMed] [Google Scholar]
- 35.Fantoni C, Kawabata M, Massaro R, Regoli F, Raffa S, Arora V, Salerno-Uriarte JA, Klein HU, Auricchio A. Right and left ventricular activation sequence in patients with heart failure and right bundle branch block: a detailed analysis using three-dimensional non-fluoroscopic electroanatomic mapping system. J Cardiovasc Electrophysiol. 2005;16:112–119. doi: 10.1046/j.1540-8167.2005.40777.x. discussion 120–111 doi:10.1046/j.1540-8167.2005.40777.x. [DOI] [PubMed] [Google Scholar]
- 36.Chung ES, Leon AR, Tavazzi L, Sun JP, Nihoyannopoulos P, Merlino J, Abraham WT, Ghio S, Leclercq C, Bax JJ, Yu CM, Gorcsan J, 3rd, St John Sutton M, De Sutter J, Murillo J. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. doi:10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 37.Shin SH, Hung CL, Uno H, Hassanein AH, Verma A, Bourgoun M, Kober L, Ghali JK, Velazquez EJ, Califf RM, Pfeffer MA, Solomon SD. Mechanical dyssynchrony after myocardial infarction in patients with left ventricular dysfunction, heart failure, or both. Circulation. 2010;121:1096–1103. doi: 10.1161/CIRCULATIONAHA.109.863795. doi:10.1161/CIRCULATIONAHA.109.863795. [DOI] [PubMed] [Google Scholar]
- 38.Knappe D, Pouleur AC, Shah AM, Cheng S, Uno H, Hall WJ, Bourgoun M, Foster E, Zareba W, Goldenberg I, McNitt S, Pfeffer MA, Moss AJ, Solomon SD. Dyssynchrony, contractile function, and response to cardiac resynchronization therapy. Circ Heart Fail. 2011;4:433–440. doi: 10.1161/CIRCHEARTFAILURE.111.962902. [DOI] [PubMed] [Google Scholar]
- 39.Pouleur AC, Knappe D, Shah AM, Uno H, Bourgoun M, Foster E, McNitt S, Hall WJ, Zareba W, Goldenberg I, Moss AJ, Pfeffer MA, Solomon SD. Relationship between improvement in left ventricular dyssynchrony and contractile function and clinical outcome with cardiac resynchronization therapy: the MADIT-CRT trial. Eur Heart J. 2011;32:1720–1729. doi: 10.1093/eurheartj/ehr185. [DOI] [PubMed] [Google Scholar]
- 40.Delgado V, Ypenburg C, van Bommel RJ, Tops LF, Mollema SA, Marsan NA, Bleeker GB, Schalij MJ, Bax JJ. Assessment of left ventricular dyssynchrony by speckle tracking strain imaging comparison between longitudinal, circumferential, and radial strain in cardiac resynchronization therapy. J Am Coll Cardiol. 2008;51:1944–1952. doi: 10.1016/j.jacc.2008.02.040. doi:10.1016/j.jacc.2008.02.040. [DOI] [PubMed] [Google Scholar]
- 41.Adelstein EC, Saba S. Scar burden by myocardial perfusion imaging predicts echocardiographic response to cardiac resynchronization therapy in ischemic cardiomyopathy. Am Heart J. 2007;153:105–112. doi: 10.1016/j.ahj.2006.10.015. doi:10.1016/j.ahj.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Bleeker GB, Kaandorp TA, Lamb HJ, Boersma E, Steendijk P, de Roos A, van der Wall EE, Schalij MJ, Bax JJ. Effect of posterolateral scar tissue on clinical and echocardiographic improvement after cardiac resynchronization therapy. Circulation. 2006;113:969–976. doi: 10.1161/CIRCULATIONAHA.105.543678. doi:10.1161/CIRCULATIONAHA.105.543678. [DOI] [PubMed] [Google Scholar]
- 43.Adelstein EC, Tanaka H, Soman P, Miske G, Haberman SC, Saba SF, Gorcsan J., 3rd Impact of scar burden by single-photon emission computed tomography myocardial perfusion imaging on patient outcomes following cardiac resynchronization therapy. Eur Heart J. 2011;32:93–103. doi: 10.1093/eurheartj/ehq389. doi:10.1093/eurheartj/ehq389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010;121:626–634. doi: 10.1161/CIRCULATIONAHA.109.894774. doi:10.1161/CIRCULATIONAHA.109.894774. [DOI] [PubMed] [Google Scholar]