Abstract
Background
In order to assess the depressant effects of alcohol on the level of consciousness of patients admitted with head injuries, this study examined the changes that occur in the Glasgow Coma Scale (GCS) of traumatic brain injury patients over time.
Methods
The records of 269 head trauma patients consecutively admitted to the neurosurgery ICU were examined retrospectively. 81 patients were excluded due to incomplete data. The remaining 188 patients were divided further into an intoxicated group (BAC ≥ 0.08%, n= 100 [53%]) and a non-intoxicated group (BAC < 0.08%, n= 88 [47%]). The GCS in the prehospital setting, in the emergency room, and the highest GCS achieved during the first 24 hours post-injury were compared.
Results
The change between ER GCS and the best day 1 GCS in the intoxicated group was greater than the non-intoxicated group and deemed clinically and statistically significant; median change (3 vs. 0) p value< 0.001. To assess if these results were directly related to the BAC%, piecewise regression using a general linear model was utilized to assess the intercept and slope of alcohol on the changes of GCS with cutting point at BAC%=0.08. The analysis showed that in the non-intoxicated range, the effect of alcohol was not significantly related to the changes of GCS. But in the intoxicated range BAC% was significantly positively related to the changes of GCS.
Conclusion
This study concludes that the GCS increases significantly over time in alcohol intoxicated patients with TBI.
Keywords: Alcohol, Intoxication, Traumatic brain injury, Glasgow Coma Scale
INTRODUCTION
Alcohol intoxication is reported to be present at hospital admission in 35% to 50% of traumatic brain injury (TBI) patients1. The effect of alcohol intoxication on the level of consciousness has been recognized for at least a century2. As a potent central nervous system (CNS) depressant, alcohol may reduce consciousness level and result in stupor, coma, and even death due to respiratory depression3. The effects of alcohol vary between individuals and they are dose dependent. Some motor and cognitive impairment occur at a blood alcohol concentration (BAC) of 0.08% (the legal limit for driving in most of the United States), significant motor and cognitive impairments occur at 0.15%, amnesia may occur at levels greater than 0.2%3–6, and at level 0.3% and higher; disorientation and loss of consciousness is a common occurrence.
The Glasgow Coma Scale (GCS), first introduced by Teasdale and Jennett in 1974, has been the standard for objectively assessing individuals with TBI7. The GCS has 2 main advantages in that it provides a reproducible, objective evaluation of neurological status, and it is a relatively simple way to monitor a patient's neurological condition over time8. It is widely used for clinical decision-making and triage in pre-hospital settings and to guide diagnosis and management of TBI patients in emergency departments and trauma centers9. A GCS score of 13 to 15 is considered a mild head injury, scores between 9 and 12 are considered moderate, whereas patients with a GCS score of 8 or less are considered to have severe TBI10. When examining the change in GCS over time, an increase or decrease in the score by 2 or more points that cannot be accounted for by alterations in sedation is often considered clinically significant.
Predicting the change in GCS of intoxicated TBI patients had always been difficult. Because; while the rate of elimination of alcohol is roughly 0.015 to 0.02 per hour, the effects of any given BAC on the consciousness level of humans and GCS varies by several factors such as: Age, sex, gender, ethnicity, body weight, fat and water content, and frequency of consumption/tolerance and, even in the same person the effects vary from one occasion to another depending on the amount and rate of consumption, and associated food and drug intake. In TBI patients the assessment of neurological functions at times following the emergency department (ED) assessment is further confounded by anesthesia if surgery is required for treatment of injuries, and by sedation needed for ICU procedures and for Intracranial pressure (ICP) control.
The purpose of this study is to examine the changes that occur in the GCS of TBI patients over time. The hypothesis is that the GCS of intoxicated patients would gradually increase over time as the body metabolizes the alcohol. We used (best Day 1 GCS) which is the highest GCS recorded in the first 24 hours after the patient’s admission to the Neurosurgical Intensive Care Unit (NICU) and compared it to the ED-GCS. It would be ideal to have an assessment of GCS at a fixed time when the alcohol is completely metabolized, in addition to the initial GCS assessment in the field after the injury, and the in the ED, however, due to the retrospective nature of the study, the best Day 1 GCS was used. The best Day 1 GCS has been observed in previous studies to be the most reliable neurological examination for indicating injury severity in TBI patients, after mass lesions have been evacuated and patients are free from initial intoxication11–12.
PATIENTS AND METHODS
TBI patients included in this study were cared for in the Neurosurgical Intensive Care Unit at Ben Taub General Hospital (BTGH). BTGH is a Level I trauma center, one of three in the Houston - Galveston area. The data for the study were extracted from a prospectively collected TBI database. The patients included in the database were screened for and/or enrolled in research studies approved by the Investigational Review Board (IRB) of Baylor College of Medicine. The patients consented for their de-identified data to be maintained in the database and used in future studies. The data examined for this study included: demographic data, pre-hospital records, including the pre-hospital GCS (PH-GCS) as reported by the EMS crew at the scene, emergency department records, including: vital signs, lab work, and admission neurological examination, and the initial computed tomography (CT) scan of the head.
Testing for BAC is part of the management protocol for severe head trauma admissions to the neurosurgical ICU. Compliance with this requirement was not 100%, but the information was recorded in the database when it was available. The ED-GCS was often obtained prior to sedation for intubation, and in all cases was obtained as free from sedation as circumstances permitted. The initial CT scan was characterized by the Marshall CT categories13. The severity of the overall injuries was graded using the Abbreviated Injury Score (AIS). Finally, after admission to the ICU the nursing staff recorded GCS at least on hourly basis. The best Day 1 GCS was determined from these bedside ICU records. Often the best examination was obtained during a daily sedation holiday one or two hours before the time of morning rounds for the neurosurgeons.
Patients were selected retrospectively among the 269 consecutive patients admitted to the neurosurgery ICU with varying degrees of head trauma in the period from May 2006 to February 2008. A total of 81 patients were excluded due to incomplete data, including 75 with unknown BAC, 1 patient with unknown ED-GCS, and 5 without best Day 1 GCS, leaving 188 for the analysis.
While alcohol impairment occurs with any significant departure from zero BAC, in this study we considered BAC of 0.08% and above to be intoxication. Based on a report prepared for the U.S. Department of Transportation and National Highway Traffic Safety Administration, and published in April 2000, after reviewing one hundred and twelve articles from 1981 to 1997; more than 94% of the studies reviewed found that by BAC of 0.08%, skills impairment were exhibited 14.
By this criterion, 100 (53%) of the 188 patients were intoxicated on admission, and 88 (47%) were not intoxicated. The 2 groups were compared and all the continuous data were found to be approximately normally distributed. For the relation of continuous variables, such as BAC% ED-GCS, age, and AIS, to the changes in GCS, scatter plots were first used to find the patterns of the relationship, then the significance of the relations were tested using Pearson’s correlation coefficient. Statistical results showed all of these four variables were significantly related to the changes in GCS. For the categorical variables, t-test or ANOVA model was used. Since very few patients had hypotension, no test was done for that, there were no significant relationships for: mechanism, intubation, hypoxia, and drugs with the changes in GCS.
The scatter plot of BAC% to the changes in GCS showed two different patterns with a cut point of BAC% =0.08, a piecewise regression using a general linear model (GLM) was used to assess the relationship of BAC% to the changes in GCS, ED-GCS, age, and AIS were controlled, but after other more important variables were controlled, the effect of age was not significant any more, therefore it was dropped from the model. The assumptions of the GLM were tested, none was violated. The distribution of the changes of GCS were also examined within each group (BAC% ≥0.08 and BAC% <0.08, the median for the changes of GCS within each group was tested using Wilcoxon’s Signed Rank test to see if it was equal to zero between group difference on the median was also tested using Wilcoxon Signed Rank test. Group differences on the other variables in Table 1 were also tested the same way.
Table 1.
Characteristics of Intoxicated and Non-Intoxicated Patients
| Intoxicated (n = 100) median (25%, 75%) |
Non-intoxicated (n = 88) median (25%, 75%) |
p Value | |
|---|---|---|---|
| BAC% | 0.23 (0.15, 0.27) | 0 (0, 0.002) | <0.001 |
| PH GCS | 5 (3, 8) | 8 (5, 11) | <0.001 |
| ED GCS | 6 (3, 8) | 7 (4, 9) | 0.130 |
| Best Day 1 GCS | 10 (7, 14) | 7 (6, 10) | <0.001 |
| Change in GCS (PH→ED) | 0 (0, 2) | 0 (−2, 1) | 0.004 |
| Change in GCS (ER→Day 1) | 3 (0, 6) | 0 (−1, 2) | <0.001 |
| Age (years) | 31 (25,41) | 34 (22,48) | 0.738 |
| AIS | 26 (25, 27) | 26 (25, 30) | 0.051 |
| Mechanism | |||
| Low velocity | 33% | 43% | 0.198 |
| High velocity | 67% | 57% | |
| Gender (%) | |||
| Male | 88% | 77% | 0.079 |
| Female | 12% | 23% | |
| Prehospital hypoxia (%) | 12% | 11% | 0.530 |
| Systolic BP>90 (%) | 91% | 95% | 0.162 |
| Urine Drug screening (%) | |||
| Positive or suspected | 38% | 50% | |
| Negative | 42% | 30% | 0.085 |
| Intubation (%) | |||
| PH | 21% | 14% | 0.258 |
| ED or in ICU | 79% | 86% | |
| ED CT (%) | |||
| Diffuse injury | 51.0% | 58.0% | |
| Mass lesion | 32% | 35.2% | |
| Normal | 15% | 5.7% | 0.203 |
| Unknown | 2% | 1.1% | |
| Survival (%) | 86% | 75% | 0.084 |
Abbreviations:
BAC% = blood alcohol concentration
PH = prehospital
ED = emergency department
GCS = Glasgow Coma Score
AIS = Abbreviated Injury Score
BP = blood pressure
ICU = intensive care unit
CT = computed tomography
A p value <0.05 was considered statistically significant. Since a decrease in GCS by 2 points had been established as a measure of neuro-worsening in prospective pharmacological trials15, a change of at least 2 points in GCS between the ED and Day 1 was considered clinically significant.
RESULTS
A comparison of the demographic features, injury mechanism and severity, co-morbidities, and survival for the intoxicated and non-intoxicated patients is listed in Table 1. There were no significant differences in age, ED-GCS, AIS, presence of pre-hospital hypoxia, or hypotension in the two groups of patients. Although the ED-GCS was not significantly different by level of intoxication, the PH-GCS was significantly lower in the intoxicated group, the median was 5 compared to 8 in the non-intoxicated group (p <0.001). A greater fraction of the intoxicated patients had a PH-GCS of 3, 39% compared to 17% in the non-intoxicated group (p =0.002). The median change in GCS from PH to ED was 0 in all patients (p =0.004). However, when testing the change in individual groups, the non-intoxicated group worsened (mean =0.646, variance =6.552, and p =0.039) and the intoxicated group improved (mean =0.275, variance =10.379, and p =0.09).
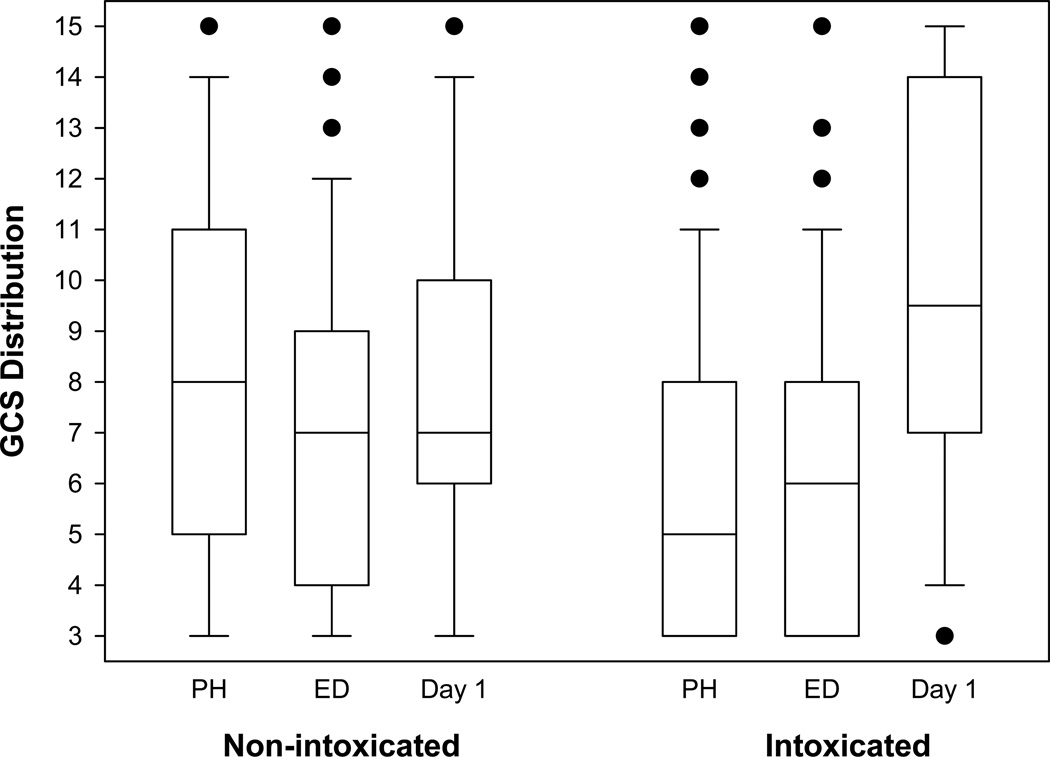
The change in GCS between the ED and Day 1 in the intoxicated group was significantly greater than the non-intoxicated group (median change =3 vs. 0, p value< 0.001) (Figure 1). Although this change in GCS was small and did not indicate return to a normal neurological examination for most patients, the median value moved from the severe TBI range (GCS 3–8) to the moderate TBI range (GCS 9–12). This would be a clinically important difference in GCS because the treatment and the prognosis would be changed. A patient with a severe TBI would probably require monitoring of ICP, while a patient with a moderate TBI would usually be followed with serial neurological exams.
Figure 1.
Box-plots of GCS from pre-hospital (PH), emergency department (ED), and day 1 after injury. The horizontal line in the box is the median of the distribution. The ends of the boxes are the 25th and 75th percentiles. The error bars give the 10th and 90th percentiles. The closed circles are outlying values.
To assess if these results were directly related to the BAC%, piecewise regression using a general linear model was utilized to assess the intercept and slope of alcohol on the changes of GCS with cutting point at BAC%=0.08. The ED-GCS and AIS were also controlled in the model, age by itself was significantly related to the changes of GCS, but when other more important variables were controlled, its effect was not significant any more, therefore was dropped.
The piecewise regression analysis showed that in the non-intoxicated range (BAC% <0.08), the effect of alcohol was not significantly related to the changes of GCS (t183 =1.48, p =0.1409). But in the intoxicated range (BAC% > 0.08) BAC% was significantly positively related to the changes of GCS, indicating that the higher the BAC% the better the improvement. GCS have increased by 1.3 units with every 0.1 increase of BAC%. The analysis showed that ED-GCS was also significantly related to the changes of GCS (t183 =-8.85, p <0.0001), indicating that the lower the ED-GCS (more sever) the greater the improvement. GCS increased by 0.06 of a unit with every one unit of ED-GCS less. Similar pattern for AIS (t183 =-5.45, p <0.0001), showing that the higher AIS (worse) for the body injury, the greater for the improvement, the magnitude was that GCS increased 0.01 unit with every one unit AIS less.
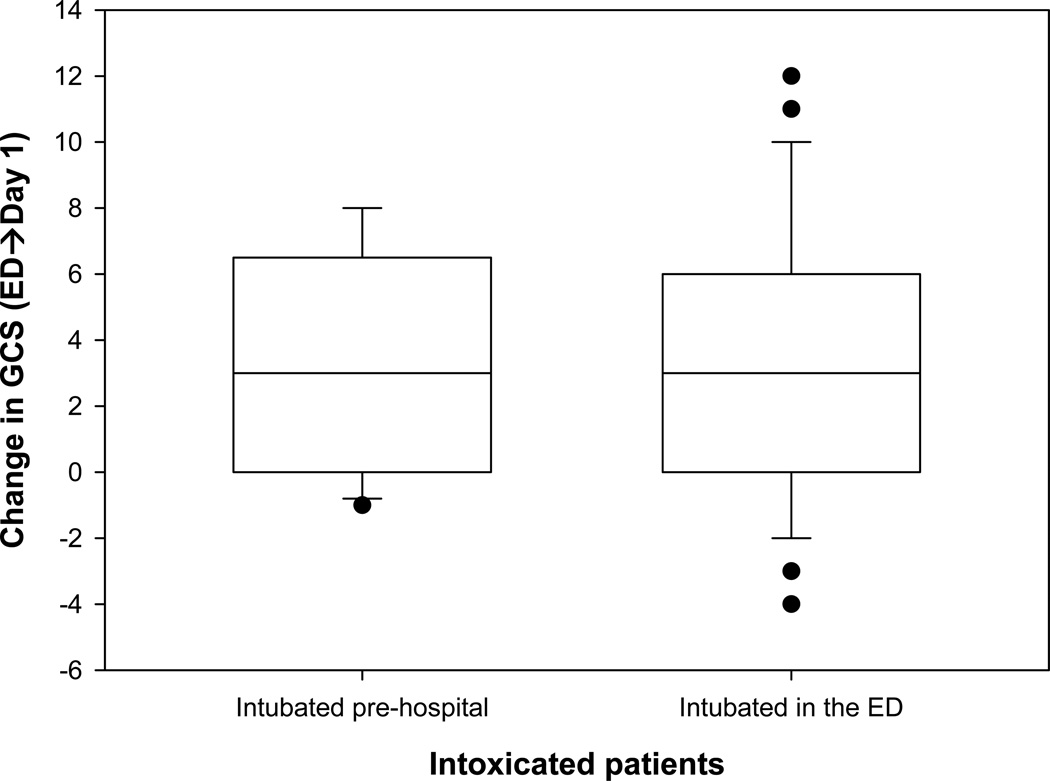
The following observations had no statistical significance and were found not to be related to the change in GCS but we felt they were worth noting; The distribution of the different types of brain injury observed on the initial CT scan of the head was not significantly different (p =0.15), however, there were almost three times as many patients in the intoxicated group with normal CT scans of the head compared to the non-intoxicated group, 15% compared to 5.7%. The patients with BAC% ≥ 0.08 were less likely to have other drug intoxication (than alcohol), 38% compared to 50% of those with BAC% < 0.08 (p =0.1). There also tended to be more males in the intoxicated group (88% vs. 77%; p =0.079). Survival rate among the intoxicated tended to be higher (86%) than the non-intoxicated (75%; p =0.084). 21% of the patients in the intoxicated group were intubated in the field or the scene before arriving to the ED versus 14% in the non-intoxicated group(p =0.258). The median change in GCS was 3 in the intoxicated group, regardless of the place of intubation (Figure 2).
Figure 2.
Change in GCS in Intoxicated patients by place of intubation.
DISCUSSION
Assessment of injury severity is important in the clinical management of patients who suffer traumatic brain injuries, as well as for the design of clinical trials for brain injured patients. The GCS7 is the most widely used method of quantifying the level of consciousness by trauma teams16. The classification of a severe TBI was originally defined for traumatically injured patients as coma (GCS< 7) for at least 6 hours, either immediately after injury or after a lucid interval17. This 6 hour duration of coma was chosen to exclude patients who might temporarily be in coma because of factors other than the head injury; most notably hypoxia, hypotension, and drug or alcohol intoxication18. GCS correlates with survival and functional outcome; however, addition of information about age, pupillary reactivity, computerized tomography scan findings, and the presence of secondary insults such as hypoxia and hypotension and information about other systemic injuries significantly improve prediction reliability of the initial GCS. In addition, the GCS can be unreliable if the patient has been sedated or paralyzed in the field to facilitate intubation and the verbal score cannot be assessed in intubated patients.
Published data on the effects of alcohol on GCS scores of TBI patients are conflicting19. In evaluating patients of all grades of TBI severity, alcohol consistently had a depressive effect on the level of consciousness1, 5, 20–23. Some studies even suggest that the increase of GCS in TBI patients subsequent to alcohol intake is due to a neuro-protective effect. Animal studies have reported this for alcohol either alone, or in combination with caffeine24–31. Other clinical studies, have reported a positive association between high BAC% on admission and lower in-hospital mortality rates in trauma patients32–34. This is thought to be mainly a result of inhibition of N-methyl D-aspartate (NMDA) mediated excitotoxicity35–38.
Furthermore, pre- or early post-injury administration of ethanol has demonstrated to reduce the amount of intracellular calcium accumulation, hyper-glycolysis, lesion size, and improve functional recovery36, 39–43. However, at higher doses ethanol had many adverse effects on the CNS injury by depressing hemodynamic and respiratory centers and the resulting cerebral ischemia20, 36, 44, decreasing regional cerebral blood flow (rCBF)45, impairment of blood brain barrier (BBB), and formation of brain edema32, 35–36, 46–49, impairment of platelet function and post-traumatic coagulopathy49–53, and formation of free radicals20, 54.
On the other hand, some publications55–57 have reported no relationship between the BAC% and GCS in patients with TBI. Two large recently published studies compared the Glasgow Coma Scale recorded in the emergency department (ED-GCS) of intoxicated and non-intoxicated TBI patients and found no statistically significant difference between the two groups19, 9. Although these studies involved large numbers of patients from retrospective analysis of a trauma center registry19, and from the National Trauma Data Bank of the American College of Surgery9, one limitation in both studies was that the GCS was a single observation and was not observed over time, and in one study it was not clear if the ED-GCS was unaffected by sedation.
The main findings of this study are that the GCS increases over time in alcohol intoxicated patients with TBI. The CNS depressant effects of alcohol cause the initial neurological assessment to overestimate the severity of the brain injury. The true injury severity then is revealed as the alcohol is metabolized. The findings in our study that suggest this interpretation are: The amount of improvement in the GCS between the emergency department exam and the best exam on day 1 was directly related to the BAC%. In addition, the number of cases with a normal CT scan was higher in the intoxicated group, even though the pre-hospital GCS was lower in the intoxicated group.
Also the change in GCS was not affected in both groups by either other drug intoxication or intubation. The very rapid improvement in neurological status, as was seen in our study in the intoxicated group, which co-insides with the likely metabolism of alcohol, favors the explanation that alcohol confounds the initial assessment of injury severity rather than it could be the results of neuro-protection with alcohol.
Some have also reported findings similar to ours. Jagger, et al, examined the change in the GCS of 257 brain-injured adults admitted to the University of Virginia Hospital, between arrival in the emergency department and 6 to 10 hours later, and found improvement in the level of consciousness between the first and second measurements, that was significantly related to the BAC% on admission, Patients with the highest blood alcohol concentrations showed the greatest improvement20.
CONCLUSION
This study concludes that alcohol intoxication is an important confounder for the early assessment of neurological examination in trauma patients. GCS increases significantly over time in alcohol intoxicated patients with TBI. Nevertheless, significant brain injuries requiring emergency treatment frequently occur in conjunction with alcohol intoxication. If there is any evidence of head trauma it is necessary to assess and treat such injuries without delay.
REFERENCES
- 1.Sloan EP, et al. Toxicology screening in urban trauma patients: drug prevalence and its relationship to trauma severity and management. J Trauma. 1989;29(12):1647–1653. [PubMed] [Google Scholar]
- 2.W M. The diagnosis of alcoholic coma. Glasgow Med J. 1879;1:1–14. [PMC free article] [PubMed] [Google Scholar]
- 3.Johnston JJ, MS Alcohol related falls: an interesting pattern of injuries. Emerg Med J. 2004;21:185–188. doi: 10.1136/emj.2003.006130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.KA D. Management of the multiply injured or intoxicated patient. In: PR C, editor. Head Injury. New York: McGraw-Hill; 2000. [Google Scholar]
- 5.Galbraith S, et al. The relationship between alcohol and head injury and its effect on the conscious level. Br J Surg. 1976;63(2):128–130. [PubMed] [Google Scholar]
- 6.Holt S, et al. Alcohol and the emergency service patient. Br Med J. 1980;281(6241):638–640. doi: 10.1136/bmj.281.6241.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Teasdale G, JB Assessment of coma and impaired consciousness. A practical scale. Lancet. 1974 Jul 13;7872:81–84. doi: 10.1016/s0140-6736(74)91639-0. [DOI] [PubMed] [Google Scholar]
- 8.Salcman M, SR, Ducker TB. Calculated recovery rates in severe head trauma. Neurosurgery. 1981;8(3):301–308. doi: 10.1227/00006123-198103000-00001. [DOI] [PubMed] [Google Scholar]
- 9.Stuke L, et al. Effect of alcohol on Glasgow Coma Scale in head-injured patients. Ann Surg. 2007;245(4):651–655. doi: 10.1097/01.sla.0000250413.41265.d3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.A V. Injury to the cranium. In: Mattox KLFD, Moore EE, editors. Trauma. New York: McGraw-Hill; 2000. pp. 377–399. [Google Scholar]
- 11.Teasdale G, Jennett B. Assessment and prognosis of coma after head injury. Acta Neurochir (Wien) 1976;34(1–4):45–55. doi: 10.1007/BF01405862. [DOI] [PubMed] [Google Scholar]
- 12.Pastorek NJ, Hannay HJ, Contant CS. Prediction of global outcome with acute neuropsychological testing following closed-head injury. J Int Neuropsychol Soc. 2004;10(6):807–817. doi: 10.1017/s1355617704106012. [DOI] [PubMed] [Google Scholar]
- 13.Marshall LF. A new classification of head injury based on computerized tomography. Journal of neurosurgery. 1991;75(suppl.):S14–S20. [Google Scholar]
- 14.Moskowitz H, DF A Review of the Literature on the Effects of Low Doses of Alcohol on Driving-Related Skills. U.S. Department of Transportation, National Highway Traffic Safety Administration. 2000 (Report HS-809-028) [Google Scholar]
- 15.Morris GF, et al. Neurological deterioration as a potential alternative endpoint in human clinical trials of experimental pharmacological agents for treatment of severe traumatic brain injuries. Executive Committee of the International Selfotel Trial. Neurosurgery. 1998;43(6):1369–1372. discussion 1372-4. [PubMed] [Google Scholar]
- 16.Sternbach GL. The Glasgow coma scale. J Emerg Med. 2000;19(1):67–71. doi: 10.1016/s0736-4679(00)00182-7. [DOI] [PubMed] [Google Scholar]
- 17.Jennett B, Teasdale G. Aspects of coma after severe head injury. Lancet. 1977;1(8017):878–881. doi: 10.1016/s0140-6736(77)91201-6. [DOI] [PubMed] [Google Scholar]
- 18.Golan JD, et al. Traumatic brain injury in intoxicated patients. J Trauma. 2007;63(2):365–369. doi: 10.1097/TA.0b013e31811ec178. [DOI] [PubMed] [Google Scholar]
- 19.Sperry JL, et al. Waiting for the patient to "sober up": Effect of alcohol intoxication on glasgow coma scale score of brain injured patients. J Trauma. 2006;61(6):1305–1311. doi: 10.1097/01.ta.0000240113.13552.96. [DOI] [PubMed] [Google Scholar]
- 20.Jagger J, et al. Effect of alcohol intoxication on the diagnosis and apparent severity of brain injury. Neurosurgery. 1984;15(3):303–306. doi: 10.1227/00006123-198409000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Brickley MR, Shepherd JP. The relationship between alcohol intoxication, injury severity and Glasgow Coma Score in assault patients. Injury. 1995;26(5):311–314. doi: 10.1016/0020-1383(95)00034-7. [DOI] [PubMed] [Google Scholar]
- 22.Chatham-Showalter PE, et al. Alcohol level at head injury and subsequent psychotropic treatment during trauma critical care. Psychosomatics. 1996;37(3):285–288. doi: 10.1016/S0033-3182(96)71567-0. [DOI] [PubMed] [Google Scholar]
- 23.Kelly MP, et al. Substance abuse, traumatic brain injury and neuropsychological outcome. Brain Inj. 1997;11(6):391–402. doi: 10.1080/026990597123386. [DOI] [PubMed] [Google Scholar]
- 24.Kelly DF, et al. Paradoxical effects of acute ethanolism in experimental brain injury. J Neurosurg. 1997;86(5):876–882. doi: 10.3171/jns.1997.86.5.0876. [DOI] [PubMed] [Google Scholar]
- 25.Tureci E, et al. Acute ethanol intoxication in a model of traumatic brain injury: the protective role of moderate doses demonstrated by immunoreactivity of synaptophysin in hippocampal neurons. Neurol Res. 2004;26(1):108–112. doi: 10.1179/016164104773026633. [DOI] [PubMed] [Google Scholar]
- 26.Janis LS, et al. Acute ethanol administration reduces the cognitive deficits associated with traumatic brain injury in rats. J Neurotrauma. 1998;15(2):105–115. doi: 10.1089/neu.1998.15.105. [DOI] [PubMed] [Google Scholar]
- 27.Dash PK, et al. Post-trauma administration of caffeine plus ethanol reduces contusion volume and improves working memory in rats. J Neurotrauma. 2004;21(11):1573–1583. doi: 10.1089/neu.2004.21.1573. [DOI] [PubMed] [Google Scholar]
- 28.Hayes RL, Jenkins LW, Lyeth BG. Neurotransmitter-mediated mechanisms of traumatic brain injury: acetylcholine and excitatory amino acids. J Neurotrauma. 1992;9(Suppl 1):S173–S187. [PubMed] [Google Scholar]
- 29.Crews FT, et al. Ethanol, stroke, brain damage, and excitotoxicity. Pharmacol Biochem Behav. 1998;59(4):981–991. doi: 10.1016/s0091-3057(97)00538-8. [DOI] [PubMed] [Google Scholar]
- 30.Strong R, Grotta JC, Aronowski J. Combination of low dose ethanol and caffeine protects brain from damage produced by focal ischemia in rats. Neuropharmacology. 2000;39(3):515–522. doi: 10.1016/s0028-3908(99)00156-2. [DOI] [PubMed] [Google Scholar]
- 31.Piriyawat P, et al. Pilot dose-escalation study of caffeine plus ethanol (caffeinol) in acute ischemic stroke. Stroke. 2003;34(5):1242–1245. doi: 10.1161/01.STR.0000067706.23777.04. [DOI] [PubMed] [Google Scholar]
- 32.Ward RE, et al. Effects of ethanol ingestion on the severity and outcome of trauma. Am J Surg. 1982;144(1):153–157. doi: 10.1016/0002-9610(82)90617-1. [DOI] [PubMed] [Google Scholar]
- 33.Blondell RD, et al. A comparison of alcohol-positive and alcohol-negative trauma patients. J Stud Alcohol. 2002;63(3):380–383. doi: 10.15288/jsa.2002.63.380. [DOI] [PubMed] [Google Scholar]
- 34.Tien HC, et al. Association between alcohol and mortality in patients with severe traumatic head injury. Arch Surg. 2006;141(12):1185–1191. doi: 10.1001/archsurg.141.12.1185. discussion 1192. [DOI] [PubMed] [Google Scholar]
- 35.Yamakami I, et al. Effects of acute ethanol intoxication on experimental brain injury in the rat: neurobehavioral and phosphorus-31 nuclear magnetic resonance spectroscopy studies. J Neurosurg. 1995;82(5):813–821. doi: 10.3171/jns.1995.82.5.0813. [DOI] [PubMed] [Google Scholar]
- 36.Kelly DF. Alcohol and head injury: an issue revisited. J Neurotrauma. 1995;12(5):883–890. doi: 10.1089/neu.1995.12.883. [DOI] [PubMed] [Google Scholar]
- 37.Shapira Y, et al. The influence of acute and chronic alcohol treatment on brain edema, cerebral infarct volume and neurological outcome following experimental head trauma in rats. J Neurosurg Anesthesiol. 1997;9(2):118–127. doi: 10.1097/00008506-199704000-00003. [DOI] [PubMed] [Google Scholar]
- 38.Chandler LJ, Sumners C, Crews FT. Ethanol inhibits NMDA receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin Exp Res. 1993;17(1):54–60. doi: 10.1111/j.1530-0277.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- 39.Fineman I, et al. Concussive brain injury is associated with a prolonged accumulation of calcium: a 45Ca autoradiographic study. Brain Res. 1993;624(1–2):94–102. doi: 10.1016/0006-8993(93)90064-t. [DOI] [PubMed] [Google Scholar]
- 40.Shapira Y, et al. Therapeutic time window and dose response of the beneficial effects of ketamine in experimental head injury. Stroke. 1994;25(8):1637–1643. doi: 10.1161/01.str.25.8.1637. [DOI] [PubMed] [Google Scholar]
- 41.Inglis FM, et al. Ischaemic brain damage associated with tissue hypermetabolism in acute subdural haematoma: reduction by a glutamate antagonist. Acta Neurochir Suppl (Wien) 1990;51:277–279. doi: 10.1007/978-3-7091-9115-6_94. [DOI] [PubMed] [Google Scholar]
- 42.Kawamata T, et al. Administration of excitatory amino acid antagonists via microdialysis attenuates the increase in glucose utilization seen following concussive brain injury. J Cereb Blood Flow Metab. 1992;12(1):12–24. doi: 10.1038/jcbfm.1992.3. [DOI] [PubMed] [Google Scholar]
- 43.Shapira Y, et al. Protective effect of MK801 in experimental brain injury. J Neurotrauma. 1990;7(3):131–139. doi: 10.1089/neu.1990.7.131. [DOI] [PubMed] [Google Scholar]
- 44.Modell JG, Mountz JM. Drinking and flying--the problem of alcohol use by pilots. N Engl J Med. 1990;323(7):455–461. doi: 10.1056/NEJM199008163230706. [DOI] [PubMed] [Google Scholar]
- 45.Altura BM, Altura BT, Gebrewold A. Alcohol-induced spasms of cerebral blood vessels: relation to cerebrovascular accidents and sudden death. Science. 1983;220(4594):331–333. doi: 10.1126/science.6836278. [DOI] [PubMed] [Google Scholar]
- 46.Albin MS, Bunegin L. An experimental study of craniocerebral trauma during ethanol intoxication. Crit Care Med. 1986;14(10):841–846. doi: 10.1097/00003246-198610000-00001. [DOI] [PubMed] [Google Scholar]
- 47.Brodner RA, Van Gilder JC, Collins WF., Jr Experimental spinal cord trauma: potentiation by alcohol. J Trauma. 1981;21(2):124–129. doi: 10.1097/00005373-198102000-00005. [DOI] [PubMed] [Google Scholar]
- 48.Baughman VL, et al. Hypothermia versus ethanol: neurologic outcome after incomplete cerebral ischemia in midazolam-anesthetized rats. J Neurosurg Anesthesiol. 1990;2(4):290–295. [PubMed] [Google Scholar]
- 49.Persson L, Rosengren L. Increased blood-brain barrier permeability around cerebral stab wounds, aggravated by acute ethanol intoxication. Acta Neurol Scand. 1977;56(1):7–16. doi: 10.1111/j.1600-0404.1977.tb01404.x. [DOI] [PubMed] [Google Scholar]
- 50.Pikaar NA, et al. Effects of moderate alcohol consumption on platelet aggregation, fibrinolysis, and blood lipids. Metabolism. 1987;36(6):538–543. doi: 10.1016/0026-0495(87)90163-6. [DOI] [PubMed] [Google Scholar]
- 51.Deykin D, Janson P, McMahon L. Ethanol potentiation of aspirin-induced prolongation of the bleeding time. N Engl J Med. 1982;306(14):852–854. doi: 10.1056/NEJM198204083061406. [DOI] [PubMed] [Google Scholar]
- 52.Elmer O, Goransson G, Zoucas E. Impairment of primary hemostasis and platelet function after alcohol ingestion in man. Haemostasis. 1984;14(2):223–228. doi: 10.1159/000215060. [DOI] [PubMed] [Google Scholar]
- 53.Haut MJ, Cowan DH. The effect of ethanol on hemostatic properties of human blood platelets. Am J Med. 1974;56(1):22–33. doi: 10.1016/0002-9343(74)90747-5. [DOI] [PubMed] [Google Scholar]
- 54.Flamm ES, et al. Ethanol potentiation of central nervous system trauma. J Neurosurg. 1977;46(3):328–335. doi: 10.3171/jns.1977.46.3.0328. [DOI] [PubMed] [Google Scholar]
- 55.Nath FP, Beastal G, Teasdale GM. Alcohol and traumatic brain damage. Injury. 1986;17(3):150–153. doi: 10.1016/0020-1383(86)90320-7. [DOI] [PubMed] [Google Scholar]
- 56.Huth JF, et al. Effect of acute ethanolism on the hospital course and outcome of injured automobile drivers. J Trauma. 1983;23(6):494–498. doi: 10.1097/00005373-198306000-00009. [DOI] [PubMed] [Google Scholar]
- 57.Pories SE, et al. Intoxication and injury. J Trauma. 1992;32(1):60–64. doi: 10.1097/00005373-199201000-00013. [DOI] [PubMed] [Google Scholar]