Abstract
Context:
Previous 25-hydroxyvitamin D [25(OH)D] and mortality studies have included mostly individuals of European descent. Whether the relationship is similar in Blacks and to what extent differences in 25(OH)D explain racial disparities in mortality is unclear.
Objective:
The objective of the study was to examine the relationship between 25(OH)D, PTH, and mortality in Black and white community-dwelling older adults over 8.5 yr of follow-up.
Design and Setting:
Health ABC is a prospective cohort study conducted in Memphis, TN, and Pittsburgh, PA.
Participants:
Well-functioning Blacks and whites aged 71–80 yr with measured 25(OH)D and PTH (n = 2638; 49% male, 39% Black) were included in the study.
Main Outcome Measure:
Multivariate-adjusted proportional hazards models estimated the hazard ratios (HR) for all-cause, cardiovascular, cancer, and noncancer, noncardiovascular mortality (n = 691 deaths).
Results:
Mean 25(OH)D concentrations were higher in whites than in Blacks [mean (sd): 29.0 (9.9) and 20.8 (8.7) ng/ml, respectively; P < 0.001]. Serum 25(OH)D by race interactions were not significant, however. Lower 25(OH)D concentrations were associated with higher mortality in Blacks and whites combined [HR (95% confidence interval [CI] 2.27 (1.59–3.24), 1.48 (1.20–1.84), and 1.25 (1.02–1.52) for < 10, 10 to < 20, and 20 to < 30 vs. ≥30 ng/ml]. In the multivariate model without 25(OH)D, Blacks had 22% higher mortality than whites [HR (95% CI) 1.22 (1.01, 1.48)]; after including 25(OH)D in the model, the association was attenuated [1.09 (0.90–1.33)]. The mortality population attributable risks (95% CI) for 25(OH)D concentrations less than 20 ng/ml and less than 30 ng/ml in Blacks were 16.4% (3.1–26.6%) and 37.7% (11.6–55.1%) and in whites were 8.9% (3.9–12.7%) and 11.1% (−2.7 to 22.0%), respectively. PTH was also associated with mortality [HR (95% CI) 1.80 (1.33–2.43) for ≥70 vs. <23 pg/ml].
Conclusions:
Low 25(OH)D and high PTH concentrations were associated with increased mortality in Black and white community-dwelling older adults. Because 25(OH)D concentrations were much lower in Blacks, the potential impact of remediating low 25(OH)D concentrations was greater in Blacks than whites.
There has been a rapid growth in interest in the role of vitamin D in health and disease. Low 25-hydroxyvitamin D [25(OH)D], the accepted measure of vitamin D status, has been associated with an increased risk of outcomes ranging from infection to cardiovascular disease to some forms of cancer (1–3). There is circumstantial and direct evidence that supports the importance of vitamin D in a wide-variety of pathological processes (4, 5). A large number of tissues have vitamin D receptors and a number of tissues including colon, prostate, breast, β-islets, vascular smooth muscle cells, and macrophages are able to convert 25(OH)D to the most active metabolite, 1,25-dihydroxyvitamin D [1,25(OH)2D]. Furthermore, 25(OH)D can bind to the vitamin D receptor, although with much lower affinity than 1,25(OH)2D. The actions of 1,25(OH) 2D are antiproliferative, prodifferentiating, immunemodulating, and, for at least some pathogens, antiinfective. Given the variety of its biological actions, it is conceivable that low 25(OH)D concentrations could have an adverse effect on a variety of health outcomes.
Given the breadth of associations, it is not surprising that, with some exceptions, observational studies have found low 25(OH)D [<20 ng/ml (<50 nmol/liter)] to be associated with increased all-cause mortality (6, 7). Furthermore, post hoc analyses of vitamin D supplementation trials have indicated a potential mortality benefit (8). There are inconsistencies in the literature, however. First, there is some evidence that the association between 25(OH)D and mortality weakens considerably after adjustment for frailty-related measures and/or comorbidities, suggesting that low 25(OH)D may be a consequence rather than a cause of failing health (9, 10). Second, low 25(OH)D leads to elevations of PTH (secondary hyperparathyroidism), and it may be that 25(OH)D is not associated with mortality independently of elevated PTH (11). Lastly, although the distribution of 25(OH)D differs by race and/or ethnicity, most of the research on the health effects of low 25(OH)D has been conducted in populations of European origin.
The efficiency of the dermal conversion of 7-dehydroxycholesterol to previtamin D3, a metabolic precursor to 25(OH)D, is related to the amount of melanin in the skin, dressing habits, and the use of sunscreen. Observational studies show that Blacks have substantially lower 25(OH)D and higher PTH concentrations than whites (12–14). However, despite having lower 25(OH)D and higher PTH concentrations, Blacks, paradoxically, have lower rates of osteoporosis than whites, and the 25(OH)D-fracture association may differ substantially between Blacks and whites (13, 15). Data from the National Health and Nutrition Examination Surveys (NHANES) suggest that the association between 25(OH)D and chronic conditions such as hypertension, diabetes, and cardiovascular disease as well as mortality may differ in Blacks compared with whites (16, 17). However, if vitamin D-health relationships are similar between whites and Blacks, vitamin D may be an important factor in explaining racial health disparities (16, 18).
Data are presented from the Health, Aging, and Body Composition study (Health ABC), a prospective cohort study of well-functioning, community-dwelling older Black and white adults, to compare the 25(OH)D-mortality relationships between Black and white participants and to determine whether these relationships are independent of PTH after adjusting for functional and disease-related measures.
Subjects and Methods
Health ABC enrolled 3075 community-dwelling Black and white men and women aged 70–79 yr between April 1997 and June 1998. Participants were recruited from a random sample of white and all Black Medicare-eligible residents in the Pittsburgh, PA, and Memphis, TN, metropolitan areas. Participants were eligible if they reported no difficulty walking 0.25 mile, climbing 10 steps, and performing basic activities of daily living; were free of life-threatening illness; planned to remain in the geographic area for at least 3 yr; and were not enrolled in lifestyle intervention trials. All participants provided written informed consent and all protocols were approved by the institutional review boards at both study sites.
Participants attending the 12-month follow-up visit served as the baseline population for this analysis. Of the 2793 participants with 25(OH)D measured at the 12-month visit, 144 were excluded on the basis of missing covariate data. Participants with PTH greater than 250 pg/ml (n = 5) or 25(OH)D greater than 75.25 ng/ml (n = 6) were also excluded as outliers. The final analysis sample included 2638 participants.
Assessment of 25(OH)D, PTH, and calcium
Fasting blood samples were collected in the morning after a 12-h fast, centrifuged, and stored at −80 C. Serum 25(OH)D was measured using a two-step RIA (25-Hydroxyvitamin D 125I RIA kit; DiaSorin, Stillwater, MN) in a laboratory meeting the Vitamin D External Quality Assessment Scheme quality criteria. Intact PTH was measured in EDTA plasma with a two-site immunoradiometric assay kit (N-tact PTH SP; DiaSorin). Serum calcium was measured with direct quantitative colorimetric determination (Stanbio Total Calcium LiquiColor Procedure No. 0150; Stanbio Laboratory, Boerne, TX). The interassay coefficients of variation for 25(OH)D, PTH, and serum calcium were 6.8, 8.6, and 2.2%, respectively.
Mortality
Participants were contacted for in-person examinations or telephone interviews every 6 months. All participants identified proxies who were contacted if the participant could not be reached. Date and causes of death were obtained from the death certificate. Underlying causes of death were adjudicated by a panel of study physicians based on the review of medical records, death certificates, proxy information, and autopsy reports (when performed). Deaths included in this analysis are those that occurred between the 12-month follow-up visit and December 31, 2006, the censoring date for surviving participants, for a maximum follow-up time of 8.5 yr (median 7.8 yr). Vital status was known for 99.2% of participants.
Covariates
Demographic characteristics (age, gender, race, education, and field center), smoking, alcohol use, and physical activity were ascertained by interviewer-administered questionnaires at study baseline. Body mass index (BMI; kilograms per square meter) was calculated from measured weight and height. The season in which the blood sample was obtained was included to account for seasonal effects on 25(OH)D and PTH. Because impaired kidney function can lead to hyperphosphatemia and reduced calcium reabsorption, both of which increase PTH, glomerular filtration rate was estimated from serum creatinine using the Modification of Diet in Renal Disease formula. The Modified Mini-Mental State Examination was used as an indicator of general cognitive status. Depressive symptoms were measured using the 20-item Center for Epidemiologic Studies Depression Scale. The prevalence of chronic conditions (type 2 diabetes, hypertension, cardiovascular disease (CVD), cancer, and lung disease) was determined using algorithms based on self-report and medication use. Total serum cholesterol was measured in fasting serum on a commercially available analyzer (Vitros 950; Johnson & Johnson, Rochester, NY). IL-6 was measured in fasting serum in duplicate by ELISA (Quantikine HS; R & D Systems, Inc., Minneapolis, MN). Gait speed (meters per second) was assessed by instructing participants to walk at their usual pace over a 20-m course. Smoking, kidney function, Modified Mini-Mental State Examination (3MS), and Center for Epidemiologic Studies Depression Scale (CES-D) were measured at study baseline; all other covariates were measured at the 12-month follow-up.
Statistical analyses
Because there is no consensus definition of 25(OH)D sufficiency, we used commonly cited cut points: 10, 20, and 30 ng/ml (19). Elevated PTH was defined as 70 pg/ml or greater based on analyses from NHANES using the same assay (20). Three equal-width intervals in the normal PTH range were then created to give four categories (0 to <23, 23 to <46, 46 to <70, and ≥70 pg/ml). Differences in participant characteristics in Blacks and whites were compared using two-way ANOVA F tests for continuous variables and χ2 tests for categorical variables. Overall and race-stratified multivariable analyses of all-cause, CVD, cancer, and other noncancer, non-CVD mortality were performed using Cox proportional hazards models adjusting for potential confounding variables known to be associated with serum 25(OH)D and/or mortality. Models were first adjusted for age, gender, race, education, field center, and season. The fully adjusted model also included smoking status; cigarette pack-years; alcohol consumption; BMI; physical activity; usual 20-m walking speed; kidney function; cognitive function; depressive symptoms; IL-6; cholesterol; and a history of cancer, diabetes, hypertension, CVD, or lung disease. Tests for linear trends across categories of 25(OH)D or PTH were tested using the median value of each category as a continuous variable. The proportional hazards assumption was met for all models. Overall and race-specific population attributable risk was calculated for both the 20- and 30-ng/ml 25(OH)D cut points and the 70-pg/mL PTH cut point (21). Estimates of the predicted reduction in total mortality with specific increments in 25(OH)D were calculated from the adjusted model including continuous terms for 25(OH)D and 25(OH)D-squared to accommodate a nonlinear dose-response relationship. All statistical analyses were performed using SAS version 9.2 (SAS Institute Inc., Cary, NC).
Results
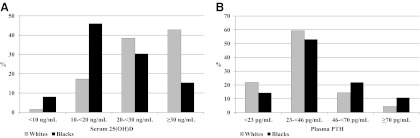
Of the 2793 participants with measured 25(OH)D, 155 (5.6%) were excluded due to missing or out-of-range data. Excluded participants were more likely to be Black, from Memphis, have less than high school education, and have higher all-cause mortality than the analysis sample (all P < 0.001). 25(OH)D concentrations were not significantly different, however (P = 0.26). The baseline participant characteristics are shown in Table 1. Blacks tended to have many characteristics associated with higher mortality risk including lower attained education; higher percentage of current smokers; slower walking speed; less physical activity; lower cognition scores; and a higher prevalence of diabetes, hypertension, and lung disease. The prevalence of 25(OH)D less than 10 ng/ml was 5 times higher in Blacks than in whites (8.1 vs. 1.6%, P < 0.001; Fig. 1). Only 15.4% of Blacks had 25(OH)D of 30 ng/ml or greater compared with 42.8% of whites (P < 0.001). For PTH, 10.8% of Blacks and 4.4% of whites had concentrations of 70 pg/ml or greater (P < 0.001; Fig. 1).
Table 1.
Participant characteristics: the Health ABC study, 1998–1999a
| Total sample (n = 2638) | White (n = 1615) | Black (n = 1023) | P valueb | |
|---|---|---|---|---|
| Age (yr) | 74.7 (2.9) | 74.8 (2.9) | 74.5 (2.9) | 0.049 |
| Female (%) | 51.2 | 47.5 | 57.1 | <0.001 |
| Field center (%) | ||||
| Memphis | 49.4 | 51.2 | 46.5 | 0.021 |
| Pittsburgh | 50.6 | 48.9 | 53.5 | |
| Less than high school education (%) | 23.1 | 11.6 | 41.4 | <0.001 |
| Current smoker (%) | 9.6 | 6.1 | 15.1 | <0.001 |
| Pack-years | 19.0 (28.0) | 20.8 (30.4) | 16.2 (23.4) | <0.001 |
| Alcohol consumption (%) | ||||
| None in past year | 62.1 | 54.1 | 74.6 | <0.001 |
| Seven or fewer drinks per week | 29.0 | 35.1 | 19.4 | |
| More than one per day | 9.0 | 10.8 | 6.1 | |
| Season (%) | ||||
| Winter (December-February) | 25. 8 | 27.8 | 22.6 | 0.009 |
| Spring (March-May) | 31.5 | 30.1 | 33.7 | |
| Summer (June-August) | 17.4 | 17.8 | 16.7 | |
| Fall (September-November) | 25.3 | 24.3 | 27.0 | |
| BMI (kg/m2) | 27.2 (4.8) | 26.4 (4.1) | 28.5 (5.5) | <0.001 |
| Usual 20-m walking speed (m/sec) | 1.14 (0.22) | 1.20 (0.20) | 1.05 (0.21) | <0.001 |
| Minutes walking per week (%) | ||||
| 0 min/wk | 40.0 | 33.9 | 49.7 | <0.001 |
| 1–149 min/wk | 31.5 | 33.1 | 28.8 | |
| 150+ min/wk | 28.5 | 33.0 | 21.5 | |
| Cognitive function (3MS score) | 90.2 (8.1) | 93.0 (5.5) | 85.9 (9.5) | <0.001 |
| Depressive symptoms (CES-D score ≥16; %) | 4.7 | 4.8 | 4.5 | 0.75 |
| Serum 25(OH)D (ng/ml)c | 25.8 (10.3) | 28.9 (10.0) | 20.8 (8.7) | <0.001 |
| Intact PTH (pg/ml) | 38.7 (22.9) | 35.3 (17.4) | 44.2 (28.7) | <0.001 |
| Serum calcium (mg/dl) | 8.9 (0.4) | 8.8 (0.4) | 8.9 (0.4) | <0.001 |
| Estimated glomerular filtration rate (ml/min per 1.73 m2) | 72.4 (16.0) | 70.0 (14.3) | 76.3 (17.8) | <0.001 |
| IL-6 (pg/ml) | 3.5 (3.6) | 3.3 (3.5) | 3.8 (3.8) | 0.002 |
| Cholesterol (mg/dl) | 205.8 (38.9) | 204.3 (37.9) | 208.4 (40.4) | 0.008 |
| Prevalent disease (%) | ||||
| Diabetes | 19.9 | 15.5 | 26.8 | <0.001 |
| Hypertension | 58.2 | 50.5 | 70.5 | <0.001 |
| CVD | 28.0 | 28.0 | 28.0 | 0.98 |
| Lung disease | 13.5 | 12.2 | 15.4 | 0.017 |
| Cancer | 19.0 | 20.1 | 17.3 | 0.08 |
| Cause of death (% of deaths)d | ||||
| CVD | 33.0 | 33.2 | 32.7 | 0.88 |
| Cancer | 31.6 | 31.4 | 31.8 | 0.91 |
| Noncancer/non-CVD | 35.5 | 35.4 | 35.5 | 0.97 |
Data are means (sd) or frequencies. Smoking, estimated glomerular filtration rate, cognitive function, and depressive symptoms were measured at study baseline (1997–1998); all other covariates were measured at the 12-month follow-up (1998–1999).
P value comparing Blacks and whites determined by two-way ANOVA F tests for continuous variables and χ2 tests for categorical variables.
To convert to nanomoles per liter, multiply by 2.496.
Percent of deaths with given cause over 8.5 yr of follow-up.
Fig. 1.
Distribution of 25(OH)D (A) and PTH (B) concentrations by race: the Health ABC Study, 1998–1999. Values differ significantly by race (P < 0.001).
Serum 25(OH)D and mortality
There were a total of 373 deaths in whites (23.1%) and 318 deaths in Blacks (31.1%). The distribution of the major causes of death was similar between Blacks and whites (Table 1). Race by 25(OH)D category interactions were not observed (P ≥ 0.20 for all); thus, models are presented in the combined sample as well as in whites and Blacks separately. In the combined sample, low 25(OH)D was associated with higher all-cause mortality (Table 2; P for trend < 0.001) as well as CVD, cancer, and noncancer, non-CVD mortality (Supplemental Table 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). When 25(OH)D and PTH were included together in the fully adjusted model, both independently predicted all-cause mortality. The 30 ng/ml or greater category was further subdivided in the combined sample to examine 25(OH)D concentrations of 40 ng/ml and above (9.1% of the total sample). All-cause mortality was not significantly different in those with 25(OH)D concentrations of 40 ng/ml or greater compared with those with concentrations of 30 to less than 40 ng/mL [hazard ratio (HR) [95% confidence interval (CI) 1.09 (0.79–1.51)].
Table 2.
Associations between serum 25(OH)D concentrations and all-cause mortality over 8.5 yr of follow-up: the Health ABC study
| Serum 25(OH)D |
P for trend | ||||
|---|---|---|---|---|---|
| <10 ng/ml | 10 to <20 ng/ml | 20 to <30 ng/ml | ≥30 ng/ml | ||
| Total sample (n = 2638) | |||||
| n | 108 | 750 | 931 | 849 | |
| Deaths (deaths per 100 P-Y) | 44 (6.19) | 241 (4.68) | 229 (3.45) | 177 (2.84) | |
| HR (95% CI), base modela | 2.39 (1.68–3.40) | 1.55 (1.25–1.91) | 1.19 (0.98–1.45) | 1.00 | <0.001 |
| HR (95% CI), full modelb | 2.27 (1.59–3.24) | 1.48 (1.20–1.84) | 1.25 (1.02–1.52) | 1.00 | <0.001 |
| HR (95% CI), full model with PTH | 1.91 (1.32–2.77) | 1.42 (1.14–1.77) | 1.24 (1.01–1.52) | 1.00 | <0.001 |
| Whites (n = 1615) | |||||
| n | 25 | 279 | 620 | 691 | |
| Deaths (deaths per 100 P-Y) | 10 (5.91) | 82 (4.26) | 138 (3.08) | 143 (2.81) | |
| HR (95% CI), base modela | 2.64 (1.38–5.06) | 1.54 (1.17–2.04) | 1.06 (0.84–1.34) | 1.00 | 0.001 |
| HR (95% CI), full modelb | 2.02 (1.02–3.99) | 1.54 (1.16–2.06) | 1.22 (0.96–1.55) | 1.00 | 0.001 |
| Blacks (n = 1023) | |||||
| n | 83 | 471 | 311 | 158 | |
| Deaths (deaths per 100 P-Y) | 34 (6.28) | 159 (4.94) | 91 (4.22) | 34 (2.94) | |
| HR (95% CI), base modela | 2.67 (1.64–4.34) | 1.74 (1.20–2.53) | 1.55 (1.04–2.30) | 1.00 | <0.001 |
| HR (95% CI), full modelb | 2.59 (1.57–4.26) | 1.76 (1.20–2.57) | 1.60 (1.07–2.39) | 1.00 | <0.001 |
P-Y, Person-years.
Hazard rate ratio and 95% confidence interval from model adjusted for age, gender, race (for total sample only), education (less than high school, high school or more), season, and field center.
Hazard rate ratio and 95% confidence interval from model adjusted for age, gender, race (for total sample only), education (less than high school, high school or more), season, field center, smoking status (current, former, never), pack-years, alcohol consumption (none in past year, seven or fewer drinks per week, more than one drink per day), BMI, time walking (0, 1–149, or 150+ min/wk), usual 20-m walking speed, estimated glomerular filtration rate, cognition (3MS score), depressive symptoms (CES-D score ≥16), IL-6 (picograms per milliliter), cholesterol (milligrams per deciliter), and prevalent diabetes, hypertension, CVD, cancer, or lung disease.
Low 25(OH)D was also associated with higher all-cause mortality in both Blacks and whites (Table 2). In the fully adjusted model for whites, compared with concentrations of 30 ng/ml or greater, 25(OH)D concentrations of less than 10 and 10 to less than 20 ng/ml were significantly associated with higher mortality. In Blacks, all categories of 25(OH)D less than 30 ng/ml were significantly associated with higher mortality. There was a significant linear trend between lower 25(OH)D and higher CVD and cancer mortality in whites and CVD and noncancer, non-CVD mortality in Blacks (Supplemental Table 1).
PTH and mortality
Race by PTH category interactions were not observed (P > 0.10 for all); thus, models are presented in the combined sample as well as in whites and Blacks separately. In the combined sample, elevated PTH (≥70 pg/ml) was associated with higher all-cause mortality (Table 3; P for trend < 0.001) as well as CVD and noncancer, non-CVD mortality (Supplemental Table 2). Higher PTH was also associated with increased all-cause mortality in both Blacks and whites (Table 3). In whites, PTH of 70 pg/ml or greater was significantly associated with an increased risk of mortality compared with concentrations of less than 23 pg/ml; however, the increased risk in Blacks did not reach statistical significance. There was a significant linear trend in the association between PTH and cancer mortality in whites and noncancer, non-CVD mortality in Blacks (Supplemental Table 2).
Table 3.
Associations between PTH concentrations and all-cause mortality over 8.5 yr of follow-up: the Health ABC study
| Plasma PTH |
P for trend | ||||
|---|---|---|---|---|---|
| <23 pg/ml | 23 to <46 pg/ml | 46 to <70 pg/ml | ≥70 pg/ml | ||
| Total sample (n = 2638) | |||||
| n | 499 | 1502 | 456 | 181 | |
| Deaths (deaths per 100 P-Y) | 117 (4.50) | 348 (5.01) | 141 (8.59) | 85 (18.18) | |
| HR (95% CI), base modela | 1.00 | 0.94 (0.76–1.16) | 1.26 (0.98–1.61) | 2.17 (1.63–2.89) | <0.001 |
| HR (95% CI), full modelb | 1.00 | 0.98 (0.79–1.21) | 1.21 (0.94–1.56) | 1.80 (1.33–2.43) | <0.001 |
| HR (95% CI), full model with 25(OH)D | 1.00 | 0.93 (0.75–1.15) | 1.09 (0.84–1.41) | 1.53 (1.12–2.10) | <0.001 |
| Whites (n = 1615) | |||||
| n | 353 | 959 | 232 | 71 | |
| Deaths (deaths per 100 P-Y) | 69 (2.66) | 211 (3.04) | 62 (3.78) | 31 (6.63) | |
| HR (95% CI), base modela | 1.00 | 1.07 (0.81–1.40) | 1.32 (0.94–1.86) | 2.33 (1.52–3.58) | <0.001 |
| HR (95% CI), full modelb | 1.00 | 1.14 (0.86–1.50) | 1.35 (0.95–1.93) | 1.86 (1.19–2.91) | 0.004 |
| Blacks (n = 1023) | |||||
| n | 146 | 543 | 224 | 110 | |
| Deaths (deaths per 100 P-Y) | 48 (4.86) | 137 (3.55) | 79 (5.15) | 54 (7.85) | |
| HR (95% CI), base modela | 1.00 | 0.71 (0.51–0.99) | 1.08 (0.75–1.55) | 1.75 (1.18–2.60) | <0.001 |
| HR (95% CI), full modelb | 1.00 | 0.71 (0.51–0.99) | 1.06 (0.73–1.55) | 1.50 (0.98–2.30) | <0.001 |
P-Y, Person-years.
Hazard rate ratio and 95% confidence interval from model adjusted for age, gender, race (for total sample only), education (less than high school, high school or more), season, and field center.
Hazard rate ratio and 95% confidence interval from model adjusted for age, gender, race (for total sample only), education (less than high school, high school or more), season, field center, smoking status (current, former, never), pack-years, alcohol consumption (none in past year, seven or fewer drinks per week, more than one drink per day), BMI, time walking (0, 1–149, or 150+ min/wk), usual 20-m walking speed, estimated glomerular filtration rate, cognition (3MS score), depressive symptoms (CES-D score ≥16), IL-6 (picograms per milliliter), cholesterol (milligrams per deciliter), and prevalent diabetes, hypertension, CVD, cancer, or lung disease.
Joint association of 25(OH)D and PTH on mortality
Table 4 shows the effects of high vs. low 25(OH)D and PTH on all-cause mortality. Participants with PTH of 70 pg/ml or greater were at the same elevated risk of all-cause mortality, regardless of 25(OH)D concentration. Low 25(OH)D (<20 ng/ml) was associated with mortality only in those with PTH of less than 70 pg/ml.
Table 4.
The joint association of categories of serum 25(OH)D and PTH for all-cause mortality [HR (95% CI)] over 8.5 yr of follow-up: the Health ABC Studya
| 25(OH)D category | PTH category |
|
|---|---|---|
| <70 pg/ml | ≥70 pg/ml | |
| Less than 20 ng/ml | 1.36 (1.13–1.63) | 1.96 (1.43–2.68) |
| 20 ng/ml or greater | 1.00 | 1.94 (1.35–2.79) |
Hazard rate ratios (95% CI) adjusted for age, gender, race, education (less than high school, high school or more), season, field center, smoking status (current, former, never), pack-years, alcohol consumption (none in past year, seven or fewer drinks per week, more than one drink per day), BMI, time walking (0, 1–149, or 150+ min/wk), usual 20-m walking speed, estimated glomerular filtration rate, cognition (3MS score), depressive symptoms (CES-D score ≥16), IL-6 (picograms per milliliter), cholesterol (milligrams per deciliter), and prevalent diabetes, hypertension, CVD, cancer, or lung disease.
Serum calcium and mortality
Serum calcium was not associated with all-cause mortality. Furthermore, adjusting for serum calcium did not affect the associations of either 25(OH)D or PTH on mortality.
Potential impact of 25(OH)D on racial disparities in mortality
Given the moderately strong relationship between 25(OH)D and all-cause mortality in both races and the strikingly lower 25(OH)D concentrations in Blacks compared with whites, 25(OH)D differences could potentially explain some of the Black-white mortality differences. In the fully adjusted model without 25(OH)D, Blacks had a 22% higher mortality rate than whites [HR (95% CI) 1.22 (1.01–1.48)]. When 25(OH)D was added to the model, the excess mortality attributed to race was attenuated and no longer statistically significant [HR (95% CI) comparing Blacks with whites: 1.09 (0.90–1.33)]. Table 5 presents the population attributable risk estimates of all-cause mortality for commonly used cut points of 25(OH)D and PTH. The population-attributable risk for mortality in Black participants is approximately double that of whites for 25(OH)D cut points of less than 20 and less than 30 ng/ml. A similar pattern was seen for elevated PTH.
Table 5.
Population-attributable risk reduction of all-cause mortality associated with the remediation of low 25(OH)D concentrations (<20 ng/ml or <30 ng/ml) and elevated PTH levels (≥70 pg/ml): the Health ABC study
| Population-attributable risk (95% CI)a |
|||
|---|---|---|---|
| Serum 25(OH)D |
PTH ≥70 pg/ml | ||
| <20 ng/ml | <30 ng/ml | ||
| Total sample | 13.1% (7.1–18.0%) | 19.5% (7.1–29.6%) | 6.5% (4.8–7.9%) |
| Whites | 8.9% (3.9–12.7%) | 11.1% (−2.7 to 22.0%) | 4.4% (2.4–5.8%) |
| Blacks | 16.4% (3.1–26.6%) | 37.7% (11.6–55.1%) | 8.8% (5.5–11.2%) |
Models adjusted for age, gender, race (for total sample only), education (less than high school, high school or more), season, and field center.
Predicted change in all-cause mortality by increments in 25(OH)D
To estimate the hypothetical reduction in total mortality for an increase in 25(OH)D, we refit the fully adjusted model in the combined sample with 25(OH)D represented by continuous linear and quadratic terms. Both the linear [β (se) −0.022 (0.006)] and quadratic [β (se) 1.06 × 10−4 (0.42 × 10−4)] coefficients for a 0.4 ng/ml (1 nmol/liter) 25(OH)D increments were significant, indicating that the predicted risk reduction depends on the initial concentration; specifically, the higher the starting concentration, the lower the expected benefit. Taking the starting concentration to be 10 ng/ml, the predicted reduction in all-cause mortality associated with a 5.6 ng/ml increment in 25(OH)D concentration, the expected increase associated with a daily supplemental vitamin D3 dose of 800 IU (22), was 19% [HR (95% CI) 0.81 (0.73–0.89)]. Taking the starting concentration to be 30 ng/ml, the estimated risk reduction was 6% [HR (95% CI) 0.94 (0.89–0.99)].
Discussion
In this biracial sample of older community-dwelling adults, 25(OH)D of less than 30 ng/ml was associated with significantly increased all-cause mortality. The association persisted after adjusting for health behaviors, physical function, disease biomarkers, and prevalent chronic conditions. Because 25(OH)D concentrations were considerably lower in Blacks compared with whites, the excess mortality attributable to low 25(OH)D was higher in Blacks. Elevated plasma PTH was also associated with significantly higher mortality. 25(OH)D and PTH independently predicted mortality, although 25(OH)D did not predict mortality in participants with PTH of 70 pg/ml or greater.
Whether the vitamin D-mortality relationship is similar across racial and/or ethnic groups is unclear. The bone mineral density/fracture literature suggests that Blacks are relatively insensitive to lower 25(OH)D concentrations than whites (13, 15). Other than NHANES (17, 23, 24), few studies include participants of non-European descent (25–27). Although there was no statistical interaction between race/ethnicity, the 25(OH)D-mortality relationship appeared blunted among Blacks in NHANES III (17). The combination of the strong risk gradient and the high prevalence of low 25(OH)D implies a high public health impact. In Health ABC, Blacks had, on average, 28% lower 25(OH)D concentrations than whites. Although this study cannot establish causality, a substantial proportion of mortality in both races may be attributable to low 25(OH)D concentrations. For a cut point of less than 20 ng/ml, the population attributable risks for all-cause mortality are 16.4% in Blacks and 8.9% in whites. When the cut point of less than 30 ng/ml is used, the population attributable risks are 37.7 and 11.1% in Blacks and whites, respectively. In NHANES III, the combination of low 25(OH)D and income entirely accounted for the excess CVD mortality in Blacks (24). In Health ABC, race was not a significant predictor of all-cause mortality after accounting for 25(OH)D.
In our combined sample, there was a graded inverse risk relationship below 30 ng/ml with a greater than 2-fold increase in mortality in those with 25(OH)D below 10 ng/ml. Most studies have found an inverse relationship between 25(OH)D and all-cause mortality, at least in minimally adjusted analyses (23, 25, 27–32), although in some cases the relationship is diminished substantially with covariate adjustment (9–11). In the combined sample, but most clearly in Blacks, 25(OH)D concentrations of 20 to less than 30 ng/ml were significantly associated with increased mortality compared with concentrations of 30 ng/ml or greater. This is above the 20 ng/ml target recently proposed by the Institute of Medicine (33). It is difficult to directly compare results across studies because of different 25(OH)D categorizations. However, an elevation in mortality in this range was observed in some (25, 32), but not all (9, 23, 26, 34), studies. High 25(OH)D concentrations (≥40 ng/ml) have also been associated with increased mortality in some studies (17, 34, 35). We further examined the association with mortality using 25(OH)D concentrations of 40 ng/ml or greater vs. 30 to less than 40 ng/ml and did not observe a significant increase in mortality for 25(OH)D concentrations above 40 ng/ml.
In Health ABC overall, there was a dose-response relationship between 25(OH)D and CVD, cancer, and noncancer, non-CVD mortality. With respect to CVD mortality, Health ABC's finding are consistent with the findings of several other cohort studies (2, 31), with some exceptions (26, 27, 34). For cancer mortality the data are inconsistent (3, 26, 34, 36). There was no association between 25(OH)D and cancer mortality in NHANES III (36). However, Michaelsson et al. (34) found both low and high 25(OH)D were associated with higher cancer mortality, whereas Cawthon et al. (26) found low 25(OH)D was associated with lower cancer mortality. In Health ABC, the relationship between 25(OH)D and cancer mortality was observed in whites but not in Blacks.
Elevated PTH (≥70 pg/ml) was associated with increased all-cause, CVD, and noncancer, non-CVD mortality in Health ABC. Several studies have reported associations with both total and cardiovascular mortality (26, 27, 37–39). A similar association with noncancer, non-CVD mortality, but not cancer mortality, was found in the Study of Osteoporotic Fractures in Men (26). In Health ABC, PTH was associated with cancer mortality in whites but not in Blacks and was associated with noncancer, non-CVD mortality more strongly in Blacks. The lack of consistency by race could be due to a relative insensitivity to PTH or low precision due to the limited number of deaths. Due to small number of deaths attributable to any single cause, it was not possible to analyze mortality by individual cancers or other subcategories.
Similar to findings from two other cohorts (28, 31), the Health ABC study found PTH and 25(OH)D to independently predict all-cause mortality. Chen et al. (11) found low 25(OH)D in the absence of elevated PTH was not associated with mortality; however, the study population had a high prevalence of secondary hyperparathyroidism (40%) and a low prevalence of normal 25(OH)D and PTH concentrations (14%). In Health ABC, 25(OH)D did not predict mortality in those with PTH concentrations ≥70 pg/ml or greater, which suggests that at least part of 25(OH)D's contribution to mortality is through a PTH-dependent pathway.
Because Health ABC is an observational study, we cannot determine whether the low 25(OH)D-mortality association is causal. In some studies the association between 25(OH)D and mortality was attenuated after the adjustment for clinical or frailty-related factors (9, 10). We have several reasons to believe that the associations observed in Health ABC were not confounded by these factors. The Health ABC cohort was recruited to be an initially well-functioning population; thus, the prevalence of persons with frailty or advanced clinical disease was low. We saw little attenuation of the results after adjusting for a variety of functional status indicators or comorbidities. The omission of deaths occurring early in the first 2 yr of follow-up (n = 109) did not appreciably affect the results (data not shown), suggesting that the results are not driven by mortality occurring early in the follow-up period, which might be expected if pathology present at the time of 25(OH)D measurement was associated with lower 25(OH)D concentrations. In a meta-analysis of vitamin D supplementation trials using doses ranging from 300 to 2000 IU, there was a statistically significant 7% reduction in all-cause mortality [relative risk (95% CI) 0.93 (0.87–0.99)] (8). We modeled the risk reduction associated with a difference of 5.6 ng/ml in 25(OH)D concentration [corresponding to the expected increase in 25(OH)D concentration with an 800 IU vitamin D3 dose, the recommended dietary allowance for adults aged older than 70 yr (33)] and found the predicted mortality reduction to range from 19 to 6% for initial 25(OH)D concentrations of 10 and 30 ng/ml, which is consistent with published clinical trial data.
The strengths of the Health ABC study are that it is a large, community-dwelling population, which was extensively characterized providing an unusually rich set of relevant covariates. The study retention was excellent with very few persons lost to follow-up. The population was selected to include well-functioning residents from two U.S. communities, somewhat limiting the generalizability of these results. Serum 25(OH)D standard reference materials were not available when 25(OH)D was measured, but 25(OH)D was measured in a laboratory meeting international certification standards. Serial measures of 25(OH)D and PTH are not available in Health ABC; thus, we are unable to account for changes in 25(OH)D and PTH over time and the observed association may underestimate the true magnitude of the association (7, 40). Although the prevalence of low 25(OH) has changed little over the past decade (12), this study was conducted when both the use of vitamin D supplements and the vitamin D content of multivitamin supplements were low, and therefore, the estimates of the population impact of low 25(OH)D may not accurately reflect the current situation.
In conclusion, both low 25(OH)D and high PTH were independently associated with increased all-cause and cause-specific mortality in both Black and white community-dwelling older adults. Although this study cannot establish causality, the potential impact of remediating low 25(OH)D concentrations is greater in Blacks than whites because low 25(OH)D concentrations are more common in Blacks. Randomized trials will be required to evaluate the ability of vitamin D supplementation to reduce mortality disparities between Black and white older adults.
Acknowledgments
This research was supported by the Intramural Research Program of the National Institutes of Health, National Institute on Aging, and National Institute on Aging Contracts N01-AG-6-2101, N01-AG-6-2103, and N01-AG-6-2106; National Institute on Aging Grants R01 AG029364 and R01 AG028050; National Institute of Nursing Research Grant R01 NR012459; and the Wake Forest University Claude D. Pepper Older Americans Independence Center Grant P30 AG021332.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- CES-D
- Center for Epidemiologic Studies Depression Scale
- CI
- confidence interval
- CVD
- cardiovascular disease
- Health ABC
- Health, Aging, and Body Composition study
- HR
- hazard ratio
- 3MS
- Modified Mini-Mental State Examination
- NHANES
- National Health and Nutrition Examination Surveys
- 25(OD)D
- 25-hydroxyvitamin D.
References
- 1. Yamshchikov AV, Desai NS, Blumberg HM, Ziegler TR, Tangpricha V. 2009. Vitamin D for treatment and prevention of infectious diseases: a systematic review of randomized controlled trials. Endocr Pract 15:438–449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Pittas AG, Chung M, Trikalinos T, Mitri J, Brendel M, Patel K, Lichtenstein AH, Lau J, Balk EM. 2010. Systematic review: vitamin D and cardiometabolic outcomes. Ann Intern Med 152:307–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Giovannucci E. 2009. Vitamin D and cancer incidence in the Harvard cohorts. Ann Epidemiol 19:84–88 [DOI] [PubMed] [Google Scholar]
- 4. Norman AW. 2008. From vitamin D to hormone D: fundamentals of the vitamin D endocrine system essential for good health. Am J Clin Nutr 88:491S–499S [DOI] [PubMed] [Google Scholar]
- 5. Holick MF. 2008. The vitamin D deficiency pandemic and consequences for nonskeletal health: mechanisms of action. Mol Aspects Med 29:361–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zittermann A, Iodice S, Pilz S, Grant WB, Bagnardi V, Gandini S. 2012. Vitamin D deficiency and mortality risk in the general population: a meta-analysis of prospective cohort studies. Am J Clin Nutr 95:91–100 [DOI] [PubMed] [Google Scholar]
- 7. Schottker B, Ball D, Gellert C, Brenner H. 17 February 2012. Serum 25-hydroxyvitamin D levels and overall mortality. A systematic review and meta-analysis of prospective cohort studies. Ageing Res Rev 10.1016/j.arr.2012.02.004 [DOI] [PubMed] [Google Scholar]
- 8. Autier P, Gandini S. 2007. Vitamin D supplementation and total mortality: a meta-analysis of randomized controlled trials. Arch Intern Med 167:1730–1737 [DOI] [PubMed] [Google Scholar]
- 9. Visser M, Deeg DJ, Puts MT, Seidell JC, Lips P. 2006. Low serum concentrations of 25-hydroxyvitamin D in older persons and the risk of nursing home admission. Am J Clin Nutr 84:616–622; quiz 671–672 [DOI] [PubMed] [Google Scholar]
- 10. Zittermann A, Schleithoff SS, Frisch S, Götting C, Kuhn J, Koertke H, Kleesiek K, Tenderich G, Koerfer R. 2009. Circulating calcitriol concentrations and total mortality. Clin Chem 55:1163–1170 [DOI] [PubMed] [Google Scholar]
- 11. Chen JS, Sambrook PN, March L, Cameron ID, Cumming RG, Simpson JM, Seibel MJ. 2008. Hypovitaminosis D and parathyroid hormone response in the elderly: effects on bone turnover and mortality. Clin Endocrinol (Oxf) 68:290–298 [DOI] [PubMed] [Google Scholar]
- 12. Looker AC, Johnson CL, Lacher DA, Pfeiffer CM, Schleicher RL, Sempos CT. 2011. Vitamin D status: United States, 2001–2006. NCHS Data Brief:1–8 [PubMed] [Google Scholar]
- 13. Gutiérrez OM, Farwell WR, Kermah D, Taylor EN. 2011. Racial differences in the relationship between vitamin D, bone mineral density, and parathyroid hormone in the National Health and Nutrition Examination Survey. Osteoporos Int 22:1745–1753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shea MK, Houston DK, Tooze JA, Davis CC, Johnson MA, Hausman DB, Cauley JA, Bauer DC, Tylavsky F, Harris TB, Kritchevsky SB. 2011. Correlates and prevalence of insufficient 25-hydroxyvitamin D status in Black and white older adults: the health, aging and body composition study. J Am Geriatr Soc 59:1165–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cauley JA, Danielson ME, Boudreau R, Barbour KE, Horwitz MJ, Bauer DC, Ensrud KE, Manson JE, Wactawski-Wende J, Shikany JM, Jackson RD. 2011. Serum 25-hydroxyvitamin D and clinical fracture risk in a multiethnic cohort of women: the Women's Health Initiative (WHI). J Bone Miner Res 26:2378–2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris SS. 2011. Does vitamin D deficiency contribute to increased rates of cardiovascular disease and type 2 diabetes in African Americans? Am J Clin Nutr 93:1175S–1178S [DOI] [PubMed] [Google Scholar]
- 17. Melamed ML, Michos ED, Post W, Astor B. 2008. 25-hydroxyvitamin D levels and the risk of mortality in the general population. Arch Intern Med 168:1629–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grant WB, Peiris AN. 2010. Possible role of serum 25-hydroxyvitamin D in Black-white health disparities in the United States. J Am Med Dir Assoc 11:617–628 [DOI] [PubMed] [Google Scholar]
- 19. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 20. Muntner P, Jones TM, Hyre AD, Melamed ML, Alper A, Raggi P, Leonard MB. 2009. Association of serum intact parathyroid hormone with lower estimated glomerular filtration rate. Clin J Am Soc Nephrol 4:186–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Natarajan S, Lipsitz SR, Rimm E. 2007. A simple method of determining confidence intervals for population attributable risk from complex surveys. Stat Med 26:3229–3239 [DOI] [PubMed] [Google Scholar]
- 22. Heaney RP. 2005. The vitamin D requirement in health and disease. J Steroid Biochem Mol Biol 97:13–19 [DOI] [PubMed] [Google Scholar]
- 23. Ginde AA, Scragg R, Schwartz RS, Camargo CA., Jr 2009. Prospective study of serum 25-hydroxyvitamin D level, cardiovascular disease mortality, and all-cause mortality in older U.S. adults. J Am Geriatr Soc 57:1595–1603 [DOI] [PubMed] [Google Scholar]
- 24. Fiscella K, Franks P. 2010. Vitamin D, race, and cardiovascular mortality: findings from a national U.S. sample. Ann Fam Med 8:11–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Semba RD, Houston DK, Ferrucci L, Cappola AR, Sun K, Guralnik JM, Fried LP. 2009. Low serum 25-hydroxyvitamin D concentrations are associated with greater all-cause mortality in older community-dwelling women. Nutr Res 29:525–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cawthon PM, Parimi N, Barrett-Connor E, Laughlin GA, Ensrud KE, Hoffman AR, Shikany JM, Cauley JA, Lane NE, Bauer DC, Orwoll ES, Cummings SR. 2010. Serum 25-hydroxyvitamin D, parathyroid hormone, and mortality in older men. J Clin Endocrinol Metab 95:4625–4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kestenbaum B, Katz R, de Boer I, Hoofnagle A, Sarnak MJ, Shlipak MG, Jenny NS, Siscovick DS. 2011. Vitamin D, parathyroid hormone, and cardiovascular events among older adults. J Am Coll Cardiol 58:1433–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dobnig H, Pilz S, Scharnagl H, Renner W, Seelhorst U, Wellnitz B, Kinkeldei J, Boehm BO, Weihrauch G, Maerz W. 2008. Independent association of low serum 25-hydroxyvitamin d and 1,25-dihydroxyvitamin d levels with all-cause and cardiovascular mortality. Arch Intern Med 168:1340–1349 [DOI] [PubMed] [Google Scholar]
- 29. Kuroda T, Shiraki M, Tanaka S, Ohta H. 2009. Contributions of 25-hydroxyvitamin D, co-morbidities and bone mass to mortality in Japanese postmenopausal women. Bone 44:168–172 [DOI] [PubMed] [Google Scholar]
- 30. Szulc P, Claustrat B, Delmas PD. 2009. Serum concentrations of 17β-E2 and 25-hydroxycholecalciferol (25OHD) in relation to all-cause mortality in older men—the MINOS study. Clin Endocrinol (Oxf) 71:594–602 [DOI] [PubMed] [Google Scholar]
- 31. Pilz S, Dobnig H, Nijpels G, Heine RJ, Stehouwer CD, Snijder MB, van Dam RM, Dekker JM. 2009. Vitamin D and mortality in older men and women. Clin Endocrinol (Oxf) 71:666–672 [DOI] [PubMed] [Google Scholar]
- 32. Semba RD, Houston DK, Bandinelli S, Sun K, Cherubini A, Cappola AR, Guralnik JM, Ferrucci L. 2010. Relationship of 25-hydroxyvitamin D with all-cause and cardiovascular disease mortality in older community-dwelling adults. Eur J Clin Nutr 64:203–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Institute of Medicine, Committee to Review Dietary Reference Intakes for Vitamin D and Calcium, Food and Nutrition Board. The National Academies Press 2011. Dietary reference intakes for calcium and vitamin D. Washington, D.C.: The National Academies Press [Google Scholar]
- 34. Michaelsson K, Baron JA, Snellman G, Gedeborg R, Byberg L, Sundström J, Berglund L, Arnlöv J, Hellman P, Blomhoff R, Wolk A, Garmo H, Holmberg L, Melhus H. 2010. Plasma vitamin D and mortality in older men: a community-based prospective cohort study. Am J Clin Nutr 92:841–848 [DOI] [PubMed] [Google Scholar]
- 35. Durup D, Jorgensen HL, Christensen J, Schwarz P, Heegaard AM, Lind B. 2012. A reverse J-shaped association of all-cause mortality with serum 25-hydroxyvitamin D in general practice, the CopD Study. J Clin Endocrinol Metab 97:2644–2652 [DOI] [PubMed] [Google Scholar]
- 36. Freedman DM, Looker AC, Abnet CC, Linet MS, Graubard BI. 2010. Serum 25-hydroxyvitamin D and cancer mortality in the NHANES III study (1988–2006). Cancer Res 70:8587–8597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Hagström E, Hellman P, Larsson TE, Ingelsson E, Berglund L, Sundström J, Melhus H, Held C, Lind L, Michaëlsson K, Arnlöv J. 2009. Plasma parathyroid hormone and the risk of cardiovascular mortality in the community. Circulation 119:2765–2771 [DOI] [PubMed] [Google Scholar]
- 38. Björkman MP, Sorva AJ, Tilvis RS. 2008. Elevated serum parathyroid hormone predicts impaired survival prognosis in a general aged population. Eur J Endocrinol 158:749–753 [DOI] [PubMed] [Google Scholar]
- 39. Pilz S, Tomaschitz A, Drechsler C, Ritz E, Boehm BO, Grammer TB, März W. 2010. Parathyroid hormone level is associated with mortality and cardiovascular events in patients undergoing coronary angiography. Eur Heart J 31:1591–1598 [DOI] [PubMed] [Google Scholar]
- 40. Grant WB. 2012. Effect of follow-up time on the relation between prediagnostic serum 25-hydroxyvitamin D and all-cause mortality rate. Dermato-Endocrinology 4:198–202 [DOI] [PMC free article] [PubMed] [Google Scholar]