In this edition of the JCEM, Farr et al. (1) explore the role of the sympathetic nervous system (SNS) in the pathophysiology of osteoporosis. They hypothesized that sympathetic tone would be increased in postmenopausal women and that this rise would be associated with a lower trabecular bone volume fraction in the radius, as well as decreased serum amino-terminal propeptide of type I procollagen (PINP), a marker of bone formation and osteopontin (1). To test their hypothesis, the investigators employed a well-recognized, albeit invasive method to measure sympathetic tone, i.e. intraarterial catheterization and microneurography of the peroneal nerve (2). Quantification of sympathetic activity was regressed against parameters of skeletal microarchitecture measured by high-resolution peripheral quantitative computed tomography (HRpQCT) as well as by indices of bone turnover; the results were then adjusted for age because both pre- and postmenopausal women were analyzed. Their results point to a potentially important inverse relationship between SNS output and trabecular bone mass in the radius, although not in the tibia, particularly in postmenopausal women (1). Cautiously, the authors call for more studies to definitively assess the importance of the SNS in modulating bone turnover.
How is the SNS involved in bone turnover? The SNS regulates multiple homeostatic mechanisms by innervating virtually every organ, although it is best known for mediating the “fight or flight” response. There are two operative circuits in the SNS—one that is direct through postganglionic neuronal norepinephrine release, and the other from preganglionic fibers that secrete acetylcholine, which then stimulates epinephrine and norepinephrine from the adrenal medulla (Fig. 1). Catecholamines activate three β-adrenergic receptors (β1AR, β2AR, and β3AR) that are distributed selectively in different tissues. The β1AR is found principally in cardiac muscle. β2AR is expressed in osteoblasts and is the principal mediator of catecholamine activation in the bone-remodeling unit. In contrast, β2AR and β3AR are expressed in white adipocytes, including marrow fat cells, and serve as inducers of lipolysis. The β3AR is the central mediator of catecholamine action in brown adipose tissue (BAT) to initiate the thermogenic program for lipolysis and heat generation. It is also operative in “brown-like” white adipose cells.
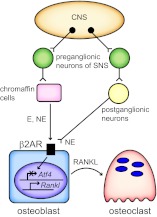
Fig. 1.
The SNS and bone remodeling. Impulses from the central nervous system (CNS) activate preganglionic neurons of the SNS, which then can stimulate peripheral catecholamine release (E, epinephrine; NE, norepinephrine) from postganglionic neurons and from the adrenal medulla. These mediators then bind to the β2AR to suppress osteoblast transcription factors such as ATF4, and enhance RANKL expression, which then enhances osteoclast differentiation.
How does the SNS regulate bone remodeling? To answer this question one has to consider the evolution of our thinking regarding skeletal remodeling and ultimately metabolic bone diseases, because this question would never have been asked a decade ago. For virtually all of the last century, bone was considered as an isolated organ that served as a target of both calciotropic hormones and sex steroids to maintain calcium balance. In that context, there is no question that major advances have been made in our understanding of both the bone-remodeling unit and a host of hormones and growth factors that regulate bone formation and bone resorption. The prototypic example of these efforts has been our experience with PTH, a hormone that we continue to appreciate not only because of its bone-resorbing properties, but also because of its anabolic activities on osteoblasts and osteocytes (3). Ten years of clinical experience with teriparatide represent a wonderful example of the remarkable nature of translating basic skeletal studies to successful bedside treatment of osteoporosis (4).
Notwithstanding those advances, a new frontier in skeletal research opened a decade ago, one that ultimately would change our perspective of bone from that of solely a calcium reservoir to a metabolic integrator. The Karsenty laboratory, using the ob/ob and db/db mice, reported that leptin, a cytokine released from adipocytes, regulated skeletal remodeling through its actions in the central nervous system (5). Relatively soon thereafter, Takeda et al. (6) and then Elefteriou et al. (7), working in the Karsenty group, showed that the SNS modulated the central inhibitory effects of leptin on skeletal remodeling through its actions on CART and ATF4 (6, 7). Subsequent studies detailed the molecular mechanisms inherent in that process, beginning with a careful characterization of the β2AR on osteoblasts, which had been identified a decade earlier (7, 8). Global deletion of three βAR genes in mice reinforced their importance in bone formation (9). More recently, a report from the Karsenty laboratory (10) showed that conditional deletion of the β2AR in osteoblasts led to very high bone mass and established the essential nature of the SNS in regulating skeletal turnover (Fig. 1).
Despite advances in our understanding of murine remodeling and its modulation by the SNS, considerable skepticism persists as to whether sympathetic activity plays a major role in regulating skeletal remodeling in humans. There are several reasons for this. First, although the periosteum is rich in nerve endings, identifying sympathetic nerve fibers in trabecular bone has been much more difficult. Second, studies of β-blockers and bone mineral density or fractures led to conflicting results, despite the fact that selective and nonselective β-blockers have been widely prescribed for nearly four decades. Although long-term observational data from very large cohorts were easy to find, none turned out to be definitive in respect to the skeletal protective effects from β-blockers. Indeed, one randomized placebo-controlled trial with 160 mg of propranolol per day for 3 months showed virtually no effect of this agent on markers of bone turnover (11). However, it should be noted that there was significant heterogeneity among those studies, including a wide range of β-blocking agents and different cohorts with varying observational times and distinct dosing schedules (11–13).
Another aspect that bred skepticism about the SNS-skeletal relationship was the real difficulty in measuring sympathetic output in humans. Although microneurography had been employed previously, it remains invasive, and most clinical investigators are not familiar with its use. In fact, clinicians who investigated the role of the SNS in chronic diseases faced major challenges in obtaining any data on sympathetic tone outside of neurography. Indeed, many of the β-blocker studies were longitudinal analyses of cohorts, rather than interventional trials; hence, there was no likelihood that an additional phenotype could have been added retrospectively to define an effect from these agents. Increased catecholamine production with subsequent activation of βARs accelerates heart rates, constricts blood vessels, widens bronchial passages, and alters rapid eye movement (REM) sleep. Except for heart rate, tools to measure those changes chronically are often not very precise or readily available. Notwithstanding, in one analysis from the Study of Osteoporotic Fractures, increased heart rate was a major risk factor for hip fracture in elderly women (14).
A third difficulty related to the measurement of sympathetic tone is the circadian variation that occurs in both heart rate and blood pressure. Autonomic function is modulated by the central clock that is entrained by the light-dark cycle in the suprachiasmatic nuclei of the hypothalamus. Both epinephrine and norepinephrine, as well as the enzymes that regulate their disposition, are produced in a circadian fashion (15). Hence, time of day is critical, as is the 24-h phase cycle, such that some individuals, particularly night-shift workers, may have significant alterations or prolongation in that cycle. Intriguingly, most circulating markers of bone remodeling including osteocalcin, PINP, C-telopeptide, and alkaline phosphatase exhibit a diurnal variation in synthesis, as do marrow stem cells that enter the circulation in response to adrenergic stimulation (16). It should be noted that the essential clock genes that keep cellular time such as Bmal1, Per, and Clock, are also expressed in osteoblasts supporting the tenet that a peripheral clock influences bone remodeling (17). In sum, time is a critical variable that must be considered in firmly establishing the link between the SNS and bone turnover.
Understanding the regulation of sympathetic tone will provide insight into the finding of Farr et al. (1) that postmenopausal women have higher bursts of activity/heartbeat than do premenopausal individuals. As noted previously, increased sympathetic tone is a hallmark of the “fight or flight” response. Another strong stimulus for SNS activity is cold temperature. In rodents, as well as neonates and most young adults, a cold stimulus induces uncoupling protein 1 (UCP-1) in brown and “brown-like” fat cells. UCP-1 is essential for uncoupling the electron transport chain in mitochondria and generating heat in a more efficient manner than shivering thermogenesis from muscle tissue. In fact, Ucp1 expression in interscapular brown fat (BAT) of mice is a reliable marker of SNS activity. However, brown fat function declines with age such that the BAT present in humans and rodents begins to take on the appearance of white fat. This age-associated dysfunction has not been well delineated, but based on positron emission tomography-computed tomography and other preliminary studies, likely represents a transitional period in midlife where preformed BAT cannot trap glucose and use fatty acids for thermogenesis (18). Intriguingly, the body attempts to compensate for these age-associated changes through the induction of “brown-like” adipocytes in peripheral tissues such as the inguinal and sc depots in mice. In animal models where preformed interscapular BAT is dysfunctional or nonexistent, brown-like adipogenesis, under the influence of the SNS, is quite pronounced. It is conceivable, but not proven, that menopause, or another signal during this time period, stimulates SNS activity. In light of a possible age-related dysfunction in BAT, the peripheral system of “brown-like” or “bright” cells would then take over. In our laboratory, C57BL6 mice that are ovariectomized show a dramatic increase in Ucp1 and Pgc1a expression within the inguinal fat depots (V. DeMambro, personal communication). Although ovariectomy does not quite model menopause, these data would suggest that body composition changes in women, including loss of bone mineral density, may be associated with enhanced SNS activity. Moreover, it is possible although not shown, that peripheral clock changes occur during menopause in bone-remodeling cells; these too may influence overall bone turnover as well as body composition.
Nonetheless, it still is surprising that there are no previous data relating sympathetic tone to skeletal remodeling in humans. Part of the problem relates, as noted earlier, to phenotyping for SNS tone. But it is also conceivable that any previous efforts to study bone mass were negative because they used dual-energy x-ray absorptiometry (DXA) measurements, which assess bone mineral density but are not very sensitive to changes in trabecular architecture. On the other hand, Farr et al. (1) used HRpQCT to examine skeletal microstructure and trabecular indices of bone strength (1, 19). They found a very strong negative relationship between trabecular bone volume (as well as thickness, connectivity, and strength) and SNS bursts in the distal radius, but they found no correlation between SNS activity and areal bone mineral density by DXA or HRpQCT determinants of cortical bone. Surprisingly, although the trends were similar in the tibia, they were not statistically significant, suggesting that it is conceivable that a more loaded bone, such as the tibia, can blunt the negative effects of SNS activity. Indeed, in mouse studies from our laboratory and others, cortical bone is not dramatically affected by higher SNS tone, consistent with the hypothesis that a more loaded compartment may not have the same skeletal responsiveness to β-adrenergic activation. This could also explain why some studies that used DXA to determine the effects of β-blockers were negative.
As this area of examination proceeds beyond a single but novel study, many questions still need to be answered. First, is estrogen deprivation a signal to up-regulate sympathetic tone? Second, what is the status of BAT during menopause, and if it is dysfunctional, does this contribute to the enhancement in SNS activity observed by Farr et al.? Third, is the uncoupling of bone-remodeling units due to suppression in bone formation, up-regulation in bone resorption, or a combination of the two? In the current study, formation, as measured by PINP was significantly and negatively related to SNS bursts, whereas receptor activator of nuclear factor-κB ligand (RANKL) and osteoprotegerin were not. Understanding the dynamics within the bone-remodeling unit will be essential to determining novel therapeutic targets for SNS-mediated bone loss. Finally, could chronic cold exposure up-regulate SNS activity enough to promote bone loss in northern latitude populations? More studies are needed to understand how the recently cloned cold receptor, TRPM8, is regulated and how activation of this receptor affects skeletal and adipose tissue through activation of UCP-1 (20).
In summary, the data in this paper by Farr et al. (1) are all about time!! A recent cohort study, also published in this edition of the JCEM (21), demonstrating that pheochromocytoma patients have high bone resorption that normalizes after surgery, makes it clear that a new chapter in skeletal investigations has begun.
Acknowledgments
This work was supported by U.S. Public Health Service-National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant AR45433 and National Institute of Diabetes and Digestive and Kidney Diseases Grant 092759.
Disclosure Summary: The authors have nothing to declare.
For articles see pages 4219 and E2093
- βAR
- β-Adrenergic receptor
- BAT
- Brown adipose tissue
- DXA
- dual-energy x-ray absorptiometry
- HRpQCT
- high-resolution peripheral quantitative computed tomography
- PINP
- amino-terminal propeptide of type I procollagen
- RANKL
- receptor activator of nuclear factor-κB ligand
- SNS
- sympathetic nervous system
- UCP-1
- uncoupling protein 1.
References
- 1. Farr JN, Charkoudian N, Barnes JN, Monroe DN, McCready LK, Atkinson EJ, Amin S, Melton J, III, Joyner MJ, Khosla S. 2012. Relationship of sympathetic activity to bone microstructure, turnover, and plasma osteopontin levels in women. J Clin Endocrinol Metab 97:4219–4227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sundlöf G, Wallin BG. 1977. The variability of muscle nerve sympathetic activity in resting recumbent man. J Physiol 272:383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marie PJ. 2012. Signaling pathways affecting skeletal health. Curr Osteoporos Rep 10:190–198 [DOI] [PubMed] [Google Scholar]
- 4. Cusano NE, Bilezikian JP. 2012. Combination anabolic and antiresorptive therapy for osteoporosis. Endocrinol Metab Clin North Am 41:643–654 [DOI] [PubMed] [Google Scholar]
- 5. Ducy P, Amling M, Takeda S, Priemel M, Schilling AF, Beil FT, Shen J, Vinson C, Rueger JM, Karsenty G. 2000. Leptin inhibits bone formation through a hypothalamic relay: a central control of bone mass. Cell 100:197–207 [DOI] [PubMed] [Google Scholar]
- 6. Takeda S, Elefteriou F, Levasseur R, Liu X, Zhao L, Parker KL, Armstrong D, Ducy P, Karsenty G. 2002. Leptin regulates bone formation via the sympathetic nervous system. Cell 111:305–317 [DOI] [PubMed] [Google Scholar]
- 7. Elefteriou F, Ahn JD, Takeda S, Starbuck M, Yang X, Liu X, Kondo H, Richards WG, Bannon TW, Noda M, Clement K, Vaisse C, Karsenty G. 2005. Leptin regulation of bone resorption by the sympathetic nervous system and CART. Nature 434:514–520 [DOI] [PubMed] [Google Scholar]
- 8. Moore RE, Smith CK, 2nd, Bailey CS, Voelkel EF, Tashjian AH., Jr 1993. Characterization of β-adrenergic receptors on rat and human osteoblast-like cells and demonstration that β-receptor agonists can stimulate bone resorption in organ culture. Bone Miner 23:301–315 [DOI] [PubMed] [Google Scholar]
- 9. Bouxsein ML, Devlin MJ, Glatt V, Dhillon H, Pierroz DD, Ferrari SL. 2009. Mice lacking β-adrenergic receptors have increased bone mass but are not protected from deleterious skeletal effects of ovariectomy. Endocrinology 150:144–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kajimura D, Hinoi E, Ferron M, Kode A, Riley KJ, Zhou B, Guo XE, Karsenty G. 2011. Genetic determination of the cellular basis of the sympathetic regulation of bone mass accrual. J Exp Med 208:841–851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Reid IR, Lucas J, Wattie D, Horne A, Bolland M, Gamble GD, Davidson JS, Grey AB. 2005. Effects of a β-blocker on bone turnover in normal postmenopausal women: a randomized controlled trial. J Clin Endocrinol Metab 90:5212–5216 [DOI] [PubMed] [Google Scholar]
- 12. Wiens M, Etminan M, Gill SS, Takkouche B. 2006. Effects of antihypertensive drug treatments on fracture outcomes: a meta-analysis of observational studies. J Intern Med 260:350–362 [DOI] [PubMed] [Google Scholar]
- 13. Levasseur R, Dargent-Molina P, Sabatier JP, Marcelli C, Bréart G. 2005. β-Blocker use, bone mineral density, and fracture risk in older women: results from the Epidemiologie de l'Osteoporose prospective study. J Am Geriatr Soc 53:550–552 [DOI] [PubMed] [Google Scholar]
- 14. Kado DM, Lui LY, Cummings SR. 2002. Study Of Osteoporotic Fractures Research Group. Rapid resting heart rate: a simple and powerful predictor of osteoporotic fractures and mortality in older women. J Am Geriatr Soc 50:455–460 [DOI] [PubMed] [Google Scholar]
- 15. Curtis AM, Cheng Y, Kapoor S, Reilly D, Price TS, Fitzgerald GA. 2007. Circadian variation of blood pressure and the vascular response to asynchronous stress. Proc Natl Acad Sci USA 104:3450–3455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mechanism of circadian variation in bone resorption Bjarnason NH, Henriksen EE, Alexandersen P, Christgau S, Henriksen DB, Christiansen C. 2002. Mechanism of circadian variation in bone resorption. Bone 30:307–313 [DOI] [PubMed] [Google Scholar]
- 17. Fu L, Patel MS, Bradley A, Wagner EF, Karsenty G. 2005. The molecular clock mediates leptin regulated bone formation. Cell 122:803–815 [DOI] [PubMed] [Google Scholar]
- 18. Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerbäck S, Nuutila P. 2009. Functional brown adipose tissue in healthy adults. N Engl J Med 360:1518–1525 [DOI] [PubMed] [Google Scholar]
- 19. Kazakia GJ, Hyun B, Burghardt AJ, Krug R, Newitt DC, de Papp AE, Link TM, Majumdar S. 2008. In vivo determination of bone structure in postmenopausal women: a comparison of HR-pQCT and high-field MR imaging. J Bone Miner Res 23:463–474 [DOI] [PubMed] [Google Scholar]
- 20. Ma S, Yu H, Zhao Z, Luo Z, Chen J, Ni Y, Jin R, Ma L, Wang P, Zhu Z, Li L, Zhong J, Liu D, Nilius B, Zhu Z. 2012. Activation of the cold-sensing TRPM8 channel triggers UCP1-dependent thermogenesis and prevents obesity. J Mol Cell Biol 4:88–96 [DOI] [PubMed] [Google Scholar]
- 21. Veldhuis-Vlug AG, El Mahdiuin M, Endert E, Heijboer AC, Fliers E, Bisschop PH. 2012. Bone resorption is increased in pheochromocytoma patients and normalizes following adrenalectomy. J Clin Endocrinol Metab 97:E2093–E2097 [DOI] [PubMed] [Google Scholar]