Abstract
Context:
Recent studies have suggested that obesity in men is associated with increased fracture risk. Obesity in men is also associated with dysregulation of the GH/IGF-I and gonadal steroid axes, important regulators of bone homeostasis.
Objective:
The aim of the study was to investigate body composition and endocrine determinants of bone microarchitecture and mechanical properties in obese men.
Design and Setting:
We conducted a cross-sectional study at a clinical research center.
Participants:
Thirty-five obese men (mean age, 33.8 ± 6.4 yr; mean body mass index, 36.5 ± 5.8 kg/m2) participated in the study.
Outcome Measures:
Distal radius microarchitecture and mechanical properties were measured by three-dimensional high-resolution peripheral quantitative computed tomography and microfinite element analysis; body composition by computed tomography; bone marrow fat by proton magnetic resonance spectroscopy; total and free estradiol and testosterone; IGF-I; peak glucagon-stimulated GH; 25-hydroxyvitamin D.
Results:
Men with high visceral adipose tissue (VAT) had impaired mechanical properties compared to men with low VAT (P < 0.05), despite comparable body mass index. VAT was inversely associated and thigh muscle was positively associated with mechanical properties (P < 0.05). Bone marrow fat was inversely associated with cortical parameters (P ≤ 0.02). Free estradiol was positively associated with total density (P = 0.05). Free testosterone was positively associated with trabecular thickness and inversely with trabecular number (P ≤ 0.05). Peak stimulated GH was positively associated with trabecular thickness, as was IGF-I with cortical area (P ≤ 0.04).
Conclusion:
VAT and bone marrow fat are negative predictors and muscle mass is a positive predictor of microarchitecture and mechanical properties in obese men. Testosterone, estradiol, and GH are positive determinants of trabecular microarchitecture, and IGF-I is a positive determinant of cortical microarchitecture. This supports the notion that VAT is detrimental to bone and that decreased GH and testosterone, characteristic of male obesity, may exert deleterious effects on microarchitecture, whereas higher estradiol may be protective.
Obesity and osteoporosis are major public health concerns. More than 50% of adults in the United States are overweight or obese, and it is estimated that three of four Americans will be overweight or obese by 2020 (1, 2). Osteoporosis, a disorder characterized by reduced bone quantity and quality and increased fracture risk, is of similar socioeconomic impact (3). Currently, more than 20 million adults over age 50, corresponding to one third of the women and one fifth of the men, meet the criteria for osteoporosis treatment (4). Despite being a risk factor for cardiovascular disease and diabetes, obesity has been thought to protect against osteoporosis, via mechanical loading and adipocyte-derived hormones (5, 6). However, strong evidence now links accumulation of fat, particularly in the abdominal depot, with bone loss (7–11). Results from the Osteoporotic Fractures in Men Study (MrOS) indicate that obesity in men is associated with increased fracture risk (12), and a recent position paper on male osteoporosis emphasized the important public health implications of fractures in obese men (13). However, no data have been published that have investigated bone microarchitecture and strength and their determinants in obese men.
Obesity is associated with dysregulation of the GH/IGF-I and gonadal steroid axes (14, 15), both important regulators of bone homeostasis (16). GH stimulates differentiation of pluripotent stem cells into the osteoblast lineage at the expense of adipogenesis (17), and IGF-I is critical to cortical bone structure, a major determinant of bone strength (18). The GH/IGF-I axis also plays a significant role in modulating body composition, decreasing abdominal adiposity and increasing muscle mass (19).
Testosterone, a positive determinant of bone mineral density (BMD) and muscle mass, is reduced in male obesity (15), whereas estrogen production is increased in proportion to body weight (20).
Vitamin D, a regulator of bone metabolism, is inversely associated with abdominal adiposity, and vitamin D deficiency is emerging as a risk factor for the metabolic syndrome (21, 22).
Several studies have suggested that alterations in bone microarchitecture could explain bone fragility and fracture risk independent of BMD (23, 24). In addition, it has been suggested that vertebral bone marrow fat determined by proton magnetic resonance spectroscopy (1H-MRS), in combination with BMD, is of significance in evaluating skeletal integrity and that increased bone marrow fat is associated with impaired bone health (25).
The purpose of our study was to determine hormonal and body composition predictors of bone microarchitecture and mechanical properties in otherwise healthy, young, abdominally obese men. We evaluated the effects of different abdominal fat depots, muscle mass and composition, bone marrow fat, and hormonal determinants, such as GH, IGF-I, testosterone, estradiol, and vitamin D on bone microarchitecture and estimated mechanical properties in obese men. We hypothesized that obese men with abdominal adiposity would have impaired bone microarchitecture and strength and increased bone marrow fat associated with dysregulation of the GH/IGF-I and gonadal steroid axes.
Subjects and Methods
The study was approved by Partners Healthcare Institutional Review Board and complied with Health Insurance Portability and Accountability Act guidelines. Written informed consent was obtained from all subjects after the nature of the procedures had been fully explained.
Subjects
Subjects were recruited from the community through advertisements. Inclusion criteria were male gender, age 18 to 45 yr, body mass index (BMI) of at least 25 kg/m2, and waist circumference greater than 102 cm (26). Exclusion criteria included smoking, hypothalamic or pituitary disorders, diabetes mellitus or other chronic illnesses, glucocorticoid use, use of statins, antihypertensives, or medications that are known to affect bone metabolism or bone density, or regular use of aspirin.
We studied 35 healthy obese men with a mean age of 33.8 ± 6.4 yr and a mean BMI of 36.5 ± 5.8 kg/m2. To determine the effect of visceral adipose tissue (VAT) on bone microarchitecture and mechanical properties, subjects were divided into two groups for comparison based on VAT, as determined by computed tomography (CT). The men with VAT below the median for the group were grouped into a low VAT group, and the men with VAT greater than the median were grouped into a high VAT group as previously described (8, 14).
Subjects were admitted to the Clinical Research Center at the Massachusetts General Hospital, where testing was performed. Each participant underwent three-dimensional high-resolution peripheral quantitative CT (HR-pQCT) of the distal radius; CT of the abdomen and thigh; dual-energy x-ray absorptiometry (DXA) of the lumbar spine, hip, and distal radius; and 1H-MRS of the fourth lumbar vertebra. Fasting blood tests and a GH glucagon stimulation test were performed after the im administration of 1 mg (if <90 kg) or 1.5 mg (if >90 kg) glucagon (GlucaGen; Novo Nordisk, Bagsvaerd, Denmark), followed by blood draw every 30 min for 4 h in 33 subjects.
Endocrine testing
Subjects underwent the following fasting blood tests: IGF-I (ng/ml), GH (ng/ml), SHBG (nmol/liter), total and free testosterone (ng/dl), total and free estradiol (pg/ml), and 25-hydroxyvitamin D [25(OH)D] (ng/ml).
IGF-I was measured using an Immulite 2000 Immunoassay System (Siemens Medical Systems, Erlangen, Germany) by a solid-phase enzyme-labeled chemiluminescent immunometric assay with a coefficient of variation (CV) below 5%. GH levels were measured using a quantikine immunoassay (R&D Systems, Inc., Minneapolis, MN), with a minimum detection limit of 0.64 pg/ml and an intraassay CV below 4%. Serum testosterone was measured by electrochemiluminescence immunoassay (Roche Diagnostics GmbH, Mannheim, Germany) with a within-run CV of 1.2–10.2% for concentrations of 4.5–1450 ng/dl. SHBG and estradiol were measured using a chemiluminescent microparticle immunoassay kit from Architect (Abbott Laboratories, Abbott Park, IL) with a within-run CV of 4.78–5.24% and an analytical sensitivity no greater than 0.1 nmol/liter for SHBG and a within-run CV of 1.5–6.4% for concentrations of 45–192 pg/ml and a functional sensitivity no greater than 25 pg/ml for estradiol. Free testosterone was calculated from total testosterone and SHBG by the laws of mass action. Free estradiol was calculated from total estradiol and SHBG by the laws of mass action. 25(OH)D was measured by liquid chromatography/tandem mass spectrometry with a minimum detection limit of 2 ng/ml and a between-run CV of 7.5%.
HR-pQCT for bone microarchitecture and mechanical properties
All subjects underwent HR-pQCT of the nondominant distal radius (XtremeCT; Scanco Medical AG, Bassersdorf, Switzerland) as previously described (23) with an isotropic voxel size of 82 μm3 and precision of 0.7–1.5% for density and 2.5–4.4% for trabecular and cortical architecture. All data were of diagnostic quality. At the distal radial metaphysis, 110 CT slices were obtained, delivering a three-dimensional representation of approximately 9 mm in the axial direction. The volume of interest was then automatically separated into a cortical and trabecular region using a threshold-based algorithm. The threshold used for separation of trabecular and cortical bone was set to one third of the apparent cortical density value. The following parameters were determined: average volumetric BMD [mg hydroxyapatite (HA)/cm3] for total (Dtot), trabecular (Dtrab), and cortical (Dcort) regions; cortical thickness (mm), cortical area (CtAr) (mm2), and trabecular area (mm2); as well as trabecular number (TbN) (mm−1), thickness (TbTh) (mm), and separation (TbSp) (mm).
Microfinite element analysis (μFEA) was performed as previously described (24) using software provided by Scanco by converting each voxel to an equal-sized brick element (27). Elements representing cortical bone and trabecular bone were assigned a Young's modulus of 20 and 17.5 GPa, respectively, and a Poisson's ratio of 0.3. Boundary conditions were set to simulate axial compression. The estimated stiffness (kN/mm) and failure load (N) were determined.
1H-MRS of bone marrow
Twenty-nine subjects underwent 1H-MRS of the fourth lumbar vertebral body to determine bone marrow lipid content using a 3.0 T magnetic resonance imaging system (Siemens Trio; Siemens Medical Systems) as previously described (28). All data were of diagnostic quality. Fitting of all 1H-MRS data was performed using LCModel (version 6.1–4A; Stephen Provencher, Oakville, Canada). LCModel bone marrow lipid estimates were automatically scaled to unsuppressed water peak (4.7 ppm) and expressed as lipid to water ratio (%).
CT and DXA for body composition analysis and lumbar spine BMD
Thirty-four subjects underwent single-slice CT of the abdomen at the level of L4 and the left mid-thigh as previously described (8). Abdominal sc adipose tissue (SAT), VAT, and abdominal total adipose tissue (TAT) areas were determined (cm2). Paraspinous muscle areas (cm2) were determined as the sum of erector spinae, psoas major, and quadratus lumborum muscles. In the left thigh, muscle and thigh SAT areas (cm2), and mean attenuation coefficient, measured in Hounsfield units of thigh muscle, as a measure of fatty infiltration, were determined. Lumbar spine, hip, and distal radius BMD were measured by DXA (Hologic QDR-4500; Hologic Inc., Waltham, MA).
Statistical analysis
JMP Statistical Database Software (version 5.0.1; SAS Institute, Cary, NC) was used for statistical analyses. The study was powered to detect a difference in failure load between the low- and high-VAT groups. With 34 evaluable subjects, we had an 80% probability of detecting a difference in failure load between the two groups at a two-sided 0.05 significance level if the true difference between the groups was 566.35 N. This is based on the assumption that the sd of the response variable is 570, as derived from Boutroy et al. (24). Variables were tested for normality of distribution using the Wilk-Shapiro test. Variables that were not normally distributed were log-transformed. Groups were compared using the Student's t test. Multivariate standard least squares regression modeling was performed to control for BMI and BMD. Linear regression analysis was performed. Forward stepwise regression modeling was performed to determine predictors of microarchitecture and mechanical properties. P ≤ 0.05 was used to denote significance, and P > 0.05 and ≤0.1 was used to denote a trend. Data are presented as mean ± sd.
Results
Clinical and endocrine characteristics of study subjects
Clinical characteristics of the study group are shown in Table 1. Six men (17%) had testosterone levels below normal for age. Six men (17%) had estradiol levels above normal. There was no overlap between the men with testosterone levels below normal and those with estradiol levels above normal. One man was vitamin D deficient [25(OH)D < 20 ng/ml], and 26 men (74%) were vitamin D insufficient [25(OH)D < 30 ng/ml]. Free testosterone was inversely associated with BMI (r = −0.50; P = 0.003), abdominal TAT (r = −0.45; P = 0.01), and VAT (r = −0.36; P = 0.04). Peak stimulated GH was inversely associated with BMI (r = −0.65; P < 0.0001), abdominal TAT (r = −0.54; P = 0.002), and VAT (r = −0.55; P = 0.001). IGF-I was inversely associated with abdominal TAT (r = −0.49; P = 0.006). There were no association between total and free estradiol and 25(OH)D and measures of body composition.
Table 1.
Characteristics of subjects with low and high VAT
| Obese men | Low VAT | High VAT | Pa | |
|---|---|---|---|---|
| n | 35 | 17 | 17 | |
| Age (yr) | 33.8 ± 6.4 | 33.5 ± 5.8 | 33.9 ± 7.3 | 0.9 |
| Weight (kg) | 118.2 ± 21.7 | 113.2 ± 20.4 | 124.8 ± 22.1 | 0.1 |
| BMI (kg/m2) | 36.5 ± 5.8 | 35.0 ± 5.6 | 38.4 ± 5.6 | 0.09 |
| IGF-I (ng/ml) | 126.8 ± 42.4 | 138.4 ± 42.0 | 112.6 ± 40.6 | 0.08 |
| IGF-I SDS | −1.8 ± 0.5 | −1.7 ± 0.5 | −1.9 ± 0.5 | 0.2 |
| Peak stimulated GH (ng/ml) | 5.1 ± 6.4 | 7.4 ± 8.1 | 2.3 ± 2.0 | 0.02 |
| Estradiol (pg/ml) | 34.5 ± 13.0 | 38.4 ± 15.6 | 31.6 ± 8.0 | 0.1 |
| Testosterone (ng/dl) | 380.3 ± 132.6 | 422.6 ± 134.0 | 327.4 ± 111.9 | 0.03 |
| 25(OH)D (ng/ml) | 24.1 ± 9.2 | 23.8 ± 9.2 | 25.3 ± 8.9 | 0.6 |
| Thigh muscle area (cm2) | 214.3 ± 32.1 | 221.4 ± 38.7 | 207.3 ± 22.8 | 0.2 |
| Parasp muscle area (cm2) | 133.8 ± 13.4 | 132.3 ± 15.6 | 135.2 ± 11.2 | 0.5 |
| Dtot (mg HA/cm3) | 349.8 ± 56.3 | 368.6 ± 51.2 | 331.8 ± 58.3 | 0.06 |
| Dtrab (mg HA/cm3) | 215.9 ± 28.6 | 229.8 ± 21.5 | 206.0 ± 26.4 | 0.007 |
| TbTh (mm) | 0.080 ± 0.01 | 0.085 ± 0.01 | 0.076 ± 0.01 | 0.02 |
| TbSp (mm) | 0.367 ± 0.047 | 0.361 ± 0.042 | 0.365 ± 0.037 | 0.8 |
| TbN (1/mm) | 2.26 ± 0.24 | 2.27 ± 0.23 | 2.29 ± 0.21 | 0.7 |
| Dcort (mg HA/cm3) | 837.9 ± 60.5 | 845.1 ± 69.8 | 826.0 ± 51.2 | 0.4 |
| Cortical thickness (mm) | 0.89 ± 0.21 | 0.93 ± 0.21 | 0.84 ± 0.21 | 0.2 |
| CtAr (mm2) | 76.1 ± 15.7 | 78.9 ± 14.5 | 72.2 ± 16.4 | 0.2 |
| Trabecular area (mm2) | 330.5 ± 99.7 | 326.6 ± 113.0 | 335.6 ± 90.8 | 0.8 |
| Stiffness (kN/mm) | 124.6 ± 21.0 | 132.4 ± 22.1 | 116.8 ± 17.8 | 0.03 |
| Failure load (N) | 6326.8 ± 1018.5 | 6693.5 ± 1074.5 | 5965.7 ± 876.3 | 0.04 |
| L-spine BMD (g/cm2) | 1.13 ± 0.14 | 1.16 ± 0.03 | 1.10 ± 0.03 | 0.2 |
| L-spine T-score | 0.39 ± 1.27 | 0.71 ± 1.32 | 0.11 ± 1.19 | 0.2 |
| Hip BMD (g/cm2) | 1.17 ± 0.12 | 1.21 ± 0.13 | 1.13 ± 0.10 | 0.06 |
| Hip T-score | 0.90 ± 0.82 | 1.19 ± 0.84 | 0.66 ± 0.64 | 0.06 |
| Distal radius BMD (g/cm2) | 0.71 ± 0.06 | 0.73 ± 0.06 | 0.68 ± 0.05 | 0.03 |
| Distal radius T-score | 0.37 ± 1.08 | 0.78 ± 1.11 | −0.06 ± 0.92 | 0.03 |
Data are presented as mean ± sd. parasp, Paraspinous; L-spine, lumbar spine.
Low vs. high VAT groups.
Thigh and paraspinous muscles correlated positively with L-spine BMD (r = 0.45, P = 0.01; and r = 0.44, P = 0.01, respectively). There was trend toward an inverse association between abdominal TAT with L-spine BMD (r = −0.33; P = 0.08) and of a positive association between IGF-I and L-spine BMD (r = 0.31; P = 0.08). There was no association between L-spine BMD and 25(OH)D.
Characteristics of subjects with high and low VAT
Clinical and microarchitecture characteristics of the low VAT and high VAT groups are shown in Table 1. Subjects with high and low VAT were of comparable age. Subjects with high VAT had significantly lower peak stimulated GH levels and lower testosterone levels compared with subjects with low VAT. Subjects with high VAT had lower Dtrab, TbTh, and decreased μFEA-estimated stiffness and failure load compared with subjects with low VAT (Fig. 1), despite similar lumbar spine and hip BMD determined by DXA.
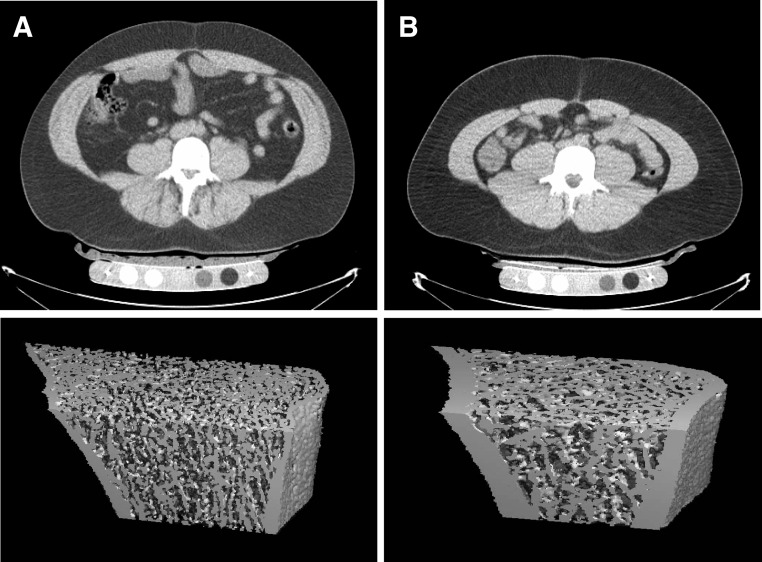
Fig. 1.
HR-pQCT of the distal radius in two representative obese men with comparable BMI but with high (A) and low (B) visceral fat (A, BMI, 32 kg/m2, and visceral fat, 181 cm2; B, BMI, 32 kg/m2, and visceral fat, 48.3 cm2). The subject with high visceral fat (A) has lower average, trabecular, and cortical bone density and trabecular and cortical thickness and cortical area than the subject with low visceral fat (B): average bone density, 251 vs. 450 mg HA/cm3; trabecular bone density, 183 vs. 218 mg HA/cm3; cortical density, 728 vs. 916 mg HA/cm3; trabecular thickness, 0.064 vs. 0.1 mm; cortical thickness, 0.4 vs. 1.18 mm; and cortical area, 42.5 vs. 83.5 mm2.
Hormonal predictors of microarchitecture and mechanical properties
Associations between hormones and microarchitecture are shown in Table 2. Free testosterone was positively associated with TbTh; however, the association lost significance after controlling for BMI (P = 0.3). There was a trend toward a positive correlation between free testosterone and Dtot. After excluding the six subjects with low testosterone levels, the correlation between free testosterone and TbTh remained significant (r = 0.47; P = 0.01).
Table 2.
Associations between microarchitecture and mechanical properties of the distal radius and body composition, GH/IGF-1 axis, gonadal steroids in obese men
| Dtot |
Dtrab |
Dcort |
TbN |
TbTh |
Log TbSp |
CtAr |
Stiffness |
Failure load |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | r | p | |
| BMI | 0.39 | 0.02 | −0.40 | 0.02 | −0.34 | 0.05 | −0.27 | 0.1 | ||||||||||
| Abdominal TAT | 0.36 | 0.05 | −0.37 | 0.04 | −0.32 | 0.08 | −0.42 | 0.02 | −0.39 | 0.03 | ||||||||
| Log abdominal SAT | 0.32 | 0.08 | −0.32 | 0.08 | −0.33 | 0.07 | −0.31 | 0.09 | ||||||||||
| VAT | −0.31 | 0.08 | −0.36 | 0.04 | −0.50 | 0.002 | −0.23 | 0.1 | −0.45 | 0.008 | −0.42 | 0.01 | ||||||
| Log parasp muscle | 0.29 | 0.09 | 0.28 | 0.1 | 0.31 | 0.07 | ||||||||||||
| Thigh muscle | 0.39 | 0.02 | −0.40 | 0.02 | 0.35 | 0.04 | 0.39 | 0.02 | ||||||||||
| Bone marrow fat | −0.49 | 0.007 | −0.42 | 0.02 | −0.38 | 0.03 | −0.45 | 0.01 | ||||||||||
| Log peak GH | 0.43 | 0.01 | ||||||||||||||||
| IGF-I | 0.36 | 0.04 | 0.26 | 0.1 | ||||||||||||||
| Free testosterone | 0.29 | 0.09 | 0.34 | 0.04 | ||||||||||||||
| Free estradiol | 0.32 | 0.05 | 0.28 | 0.1 | ||||||||||||||
parasp, Paraspinous.
Free estradiol was positively associated with Dtot, which remained significant after controlling for BMI (P = 0.04), and there was a trend toward a positive correlation with Dtrab. After excluding the six subjects with elevated estradiol levels, there remained a trend between free estradiol and Dtot (r = 0.28; P = 0.1).
IGF-I was positively associated with CtAr, which remained significant after controlling for BMI (P = 0.04), and peak stimulated GH was positively associated with TbTh, which lost significance after controlling for BMI (P = 0.2). There was a trend toward a positive correlation between IGF-I and stiffness.
There were no significant associations between 25(OH)D and microarchitecture or mechanical properties (P = 0.2 to 0.8).
When TbTh was entered as a dependent variable and peak stimulated GH, IGF-I, estradiol, and testosterone as independent variables in a forward stepwise regression model, GH and estradiol were significant predictors of TbTh (P = 0.02 and P = 0.04, respectively); GH explained 18% and estradiol 11% of the variability of TbTh.
When CtAr was entered as a dependent variable and peak stimulated GH, IGF-I, estradiol, and testosterone as independent variables in a forward stepwise regression model, IGF-I was a significant predictor of CtAr (P = 0.04); IGF-I explained 13% of the variability of CtAr.
Body composition predictors of microarchitecture and mechanical properties
Associations between body composition and microarchitecture are shown in Table 2.
Predictors of microarchitecture
BMI was positively associated with TbN and inversely associated with TbSp and TbTh.
Thigh muscle was positively associated with TbN and inversely associated with TbSp. There was a trend toward a positive correlation between paraspinous muscle area and TbN.
VAT was inversely associated with Dtrab and TbTh, and there was a trend toward an inverse association with Dtot and CtAr. Abdominal TAT was positively associated with TbN and inversely with TbTh and TbSp (trend). There was a trend toward a positive correlation between abdominal SAT and TbN and an inverse correlation with TbSp.
Bone marrow fat was inversely associated with Dtot, Dcort, TbTh, cortical thickness, and CtAr. All associations remained significant after controlling for lumbar spine BMD by DXA (P < 0.04).
There were no associations between age and microarchitecture parameters.
Predictors of mechanical properties
Thigh muscle was positively associated with μFEA-estimated stiffness and failure load, and there was a trend toward a positive correlation between paraspinous muscle area and both stiffness and failure load. Thigh muscle density correlated positively with stiffness (r = 0.34; P = 0.05), and there was a trend toward a positive correlation with failure load (r = 0.31; P = 0.07).
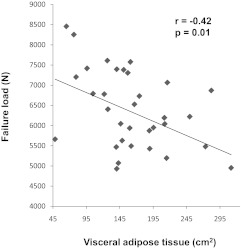
VAT and abdominal TAT were inversely associated with stiffness and failure load (Fig. 2). These associations remained significant after controlling for thigh muscle (P < 0.01).
Fig. 2.
Regression analysis of VAT and mechanical properties. There is an inverse association between VAT and failure load.
When failure load was entered as a dependent variable and thigh muscle area, thigh muscle density, and VAT as independent variables in a forward stepwise regression model, VAT and thigh muscle area were significant predictors of stiffness (P = 0.01 and P = 0.02, respectively); VAT explained 17% and thigh muscle area explained 14% of the variability of failure load, the effect of VAT being negative and of thigh muscle being positive.
When stiffness was entered as a dependent variable and thigh muscle area, muscle density, and VAT as independent variables in a forward stepwise regression model, VAT and thigh muscle area were significant predictors of stiffness (P = 0.008 and P = 0.04, respectively); VAT explained 20% and thigh muscle area explained 10% of the variability of stiffness, the effect of VAT being negative and of thigh muscle being positive.
Discussion
Our study demonstrates that men with large VAT depots have impaired bone microarchitecture and mechanical properties compared with overweight and obese men with smaller VAT depots, supporting the notion that excessive VAT accumulation is detrimental to bone health. In addition, bone marrow fat of the lumbar spine was a negative predictor of total bone density and cortical microarchitecture. Our study also showed that in obese men, muscle areas, free testosterone, free estradiol, and GH are positive determinants of trabecular microarchitecture, and IGF-I is a positive determinant of cortical microarchitecture. This suggests that decreased GH, IGF-I, and testosterone, characteristic of male obesity, may exert deleterious effects on skeletal microarchitecture, whereas higher estradiol levels may be relatively protective.
Recent studies have shown that obesity, especially abdominal adiposity, is detrimental to bone health and correlates inversely with BMD (7, 8, 10, 11). However, no studies have been performed evaluating the effects of detailed measures of body composition on microarchitecture and mechanical properties of bone in obese men. Assessment of bone microarchitecture and mechanical properties using HR-pQCT and μFEA have been shown to improve the discrimination of subjects with prior fragility fracture compared with BMD measurements alone (24, 29). In our study, obese men with larger amounts of VAT had impaired microarchitecture and estimated mechanical properties at the distal radius compared with obese men with lower amounts of VAT, despite similar BMI. In addition, VAT was a negative predictor of total and trabecular density and trabecular thickness by HR-pQCT. Potential explanations raised for the detrimental effects of VAT on bone health include the release of proinflammatory cytokines secreted by adipocytes, such as IL-6, TNF-α, and adipokines, such as E-selectin, stimulating osteoclast activity (11, 30).
Visceral obesity is also associated with dysregulation of the GH/IGF-I and the gonadal steroid axes, both of which are important regulators of bone homeostasis (14, 15, 31). Visceral obesity is a state of relative GH and IGF-I deficiency (14) and GH deficiency in patients with pituitary disease is associated with reduced bone mass and increased fracture risk (32). We have previously demonstrated a positive association between IGF-I and BMD and a negative association between IGF-I and vertebral bone marrow fat in premenopausal women ranging from lean to obese, supporting the role of the GH/IGF-I axis as an important regulator of skeletal integrity (7, 8). In our current study, peak stimulated GH was a positive predictor of trabecular thickness, and IGF-I was a positive predictor of cortical area. This is concordant with studies in mice that have shown that IGF-I is a major determinant of cortical bone structure (33, 34). GH has also been shown to increase muscle mass, an important positive predictor of bone formation (19). In our study, thigh muscle area and density were strong positive predictors of distal radius bone microarchitecture and mechanical properties.
The gonadal steroid axis also plays a crucial role in the maintenance of skeletal health in men and is dysregulated in obesity. Obese men have a progressive decrease in testosterone levels with increasing body weight (15), and there is a concurrent decrease in SHBG production (35). In our study, 17% of these obese but otherwise healthy men had testosterone deficiency. Estrogen production increases with increasing body weight (20). In our study, 17% of obese men had elevated estradiol levels. Although testosterone has traditionally been implicated in the regulation of skeletal health in men, clinical observations and animal studies have demonstrated that estrogens may be even more important for optimal bone acquisition and maintenance of skeletal health in men (36, 37). Testosterone is the most abundant circulating androgen in men and can act directly on the androgen receptor in osteoblasts, stimulating trabecular bone growth (38). In addition, testosterone can be converted to estradiol in peripheral tissues, mostly in fat, and subsequently activate the estrogen receptor in osteoblasts. Studies have suggested that estradiol is a better predictor of BMD than testosterone and the best predictor of an increase in BMD in young men and a decrease in BMD in elderly men (36, 37). In our study in young obese men, free estradiol was a positive predictor of total bone density and trabecular microarchitecture, and free testosterone was a positive predictor of trabecular bone parameters.
Vitamin D levels are reduced in patients with obesity (7, 22) due to sequestration of vitamin D, a fat-soluble molecule, in adipose tissue. Over 75% of the obese men in our study were vitamin D insufficient or deficient. We did not observe a significant association between 25(OH)D and microarchitecture, consistent with previous reports in adolescents and young adults (7, 39). A possible explanation for these findings might be that the detrimental effects of vitamin D deficiency on bone may not yet be present in young obese men. Alternatively, the effects of vitamin D levels on bone density in this population may not be strong enough to be detected in a small number of study subjects.
An emerging area of intense interest is the role of the common mesenchymal stem cell and its differentiation into the osteoblast and adipocyte lineage. Increased bone marrow fat is seen in subjects with morphological evidence of bone weakness (25). Therefore, an understanding of the factors that influence the development of bone marrow fat and stem cell differentiation is of clinical importance. Obesity is emerging as an important factor that influences this differentiation pathway toward the adipocyte lineage (40). We have demonstrated an inverse association between bone marrow fat and BMD, and a positive association between bone marrow fat and visceral fat in obese premenopausal women (8). In our current study, vertebral bone marrow fat was a negative predictor of trabecular and cortical microarchitecture, supporting the detrimental effects of bone marrow fat on bone integrity.
A limitation of our study is the cross-sectional study design, which limits our ability to prove causality. Second, our relatively small numbers may have limited our ability to detect all endocrine and body composition determinants of bone microarchitecture and mechanical properties. This is an exploratory study, which suggests interesting associations and lays the foundation for future, larger, definitive studies. Third, the resolution of the HR-pQCT of 82 μm3 is not sufficient to resolve thinner trabeculae and introduces a partial volume effect. In addition, the scanning length of 110 images only covers an area of approximately 9 mm of the distal radial metaphysis and does not allow for evaluation of the entire radial metaphysis. Fourth, our study was focused on obese men, and our findings cannot be extrapolated to normal-weight men or women. Strengths of our study include the detailed evaluation of bone microarchitecture and mechanical properties as well as the evaluation of bone marrow fat content, the detailed evaluation of body composition using state-of-the-art radiological techniques, and inclusion of hormonal determinants such as glucagon-stimulated GH levels, IGF-I, and gonadal steroids, including free hormone concentrations and vitamin D.
In conclusion, our study shows that VAT and bone marrow fat are negative predictors, and muscle is a positive predictor of microarchitecture and mechanical properties in obese men, supporting the notion that VAT and bone marrow fat are detrimental to bone strength. We also showed that testosterone, estradiol, and GH are positive determinants of trabecular microarchitecture and IGF-I is a positive determinant of cortical microarchitecture in obese men. This suggests that decreased GH and testosterone, characteristic of male obesity, may exert deleterious effects on skeletal microarchitecture, whereas higher estradiol levels may be relatively protective. Estradiol may be a more important determinant of bone microarchitecture than testosterone. The potential differential effects of GH and IGF-I on trabecular vs. cortical microarchitecture warrant further study.
Acknowledgments
This work was supported in part by National Institutes of Health Grants R01 HL-077674, UL1 RR-025758, and K23 RR-23090.
Disclosure Summary: All authors state that they have no conflicts of interest.
Footnotes
- BMD
- Bone mineral density
- BMI
- body mass index
- CT
- computed tomography
- CtAr
- cortical area
- CV
- coefficient of variation
- Dcort
- cortical BMD
- Dtot
- total BMD
- Dtrab
- trabecular BMD
- DXA
- dual-energy x-ray absorptiometry
- μFEA
- microfinite element analysis
- HA
- hydroxyapatite
- 1H-MRS
- proton magnetic resonance spectroscopy
- HR-pQCT
- high-resolution peripheral quantitative CT
- 25(OH)D
- 25-hydroxyvitamin D
- SAT
- sc adipose tissue
- TAT
- total adipose tissue
- TbN
- trabecular number
- TbSp
- trabecular separation
- TbTh
- trabecular thickness
- VAT
- visceral adipose tissue.
References
- 1. Wang YC, McPherson K, Marsh T, Gortmaker SL, Brown M. 2011. Health and economic burden of the projected obesity trends in the USA and the UK. Lancet 378:815–825 [DOI] [PubMed] [Google Scholar]
- 2. Wyatt HR. 2003. The prevalence of obesity. Prim Care 30:267–279 [DOI] [PubMed] [Google Scholar]
- 3. Cole ZA, Dennison EM, Cooper C. 2008. Osteoporosis epidemiology update. Curr Rheumatol Rep 10:92–96 [DOI] [PubMed] [Google Scholar]
- 4. Dawson-Hughes B, Looker AC, Tosteson AN, Johansson H, Kanis JA, Melton LJ., 3rd 2012. The potential impact of the National Osteoporosis Foundation guidance on treatment eligibility in the USA: an update in NHANES 2005–2008. Osteoporos Int 23:811–820 [DOI] [PubMed] [Google Scholar]
- 5. Albala C, Yáñez M, Devoto E, Sostin C, Zeballos L, Santos JL. 1996. Obesity as a protective factor for postmenopausal osteoporosis. Int J Obes Relat Metab Disord 20:1027–1032 [PubMed] [Google Scholar]
- 6. Klein KO, Larmore KA, de Lancey E, Brown JM, Considine RV, Hassink SG. 1998. Effect of obesity on estradiol level, and its relationship to leptin, bone maturation, and bone mineral density in children. J Clin Endocrinol Metab 83:3469–3475 [DOI] [PubMed] [Google Scholar]
- 7. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Harrington LM, Breggia A, Rosen CJ, Miller KK. 2011. Determinants of bone mineral density in obese premenopausal women. Bone 48:748–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bredella MA, Torriani M, Ghomi RH, Thomas BJ, Brick DJ, Gerweck AV, Rosen CJ, Klibanski A, Miller KK. 2011. Vertebral bone marrow fat is positively associated with visceral fat and inversely associated with IGF-1 in obese women. Obesity (Silver Spring) 19:49–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Di Iorgi N, Mittelman SD, Gilsanz V. 2008. Differential effect of marrow adiposity and visceral and subcutaneous fat on cardiovascular risk in young, healthy adults. Int J Obes (Lond) 32:1854–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gilsanz V, Chalfant J, Mo AO, Lee DC, Dorey FJ, Mittelman SD. 2009. Reciprocal relations of subcutaneous and visceral fat to bone structure and strength. J Clin Endocrinol Metab 94:3387–3393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Russell M, Mendes N, Miller KK, Rosen CJ, Lee H, Klibanski A, Misra M. 2010. Visceral fat is a negative predictor of bone density measures in obese adolescent girls. J Clin Endocrinol Metab 95:1247–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nielson CM, Marshall LM, Adams AL, LeBlanc ES, Cawthon PM, Ensrud K, Stefanick ML, Barrett-Connor E, Orwoll ES. 2011. BMI and fracture risk in older men: the Osteoporotic Fractures in Men Study (MrOS). J Bone Miner Res 26:496–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kanis JA, Bianchi G, Bilezikian JP, Kaufman JM, Khosla S, Orwoll E, Seeman E. 2011. Towards a diagnostic and therapeutic consensus in male osteoporosis. Osteoporos Int 22:2789–2798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Miller KK, Biller BM, Lipman JG, Bradwin G, Rifai N, Klibanski A. 2005. Truncal adiposity, relative growth hormone deficiency, and cardiovascular risk. J Clin Endocrinol Metab 90:768–774 [DOI] [PubMed] [Google Scholar]
- 15. Pasquali R. 2006. Obesity and androgens: facts and perspectives. Fertil Steril 85:1319–1340 [DOI] [PubMed] [Google Scholar]
- 16. Mora S, Pitukcheewanont P, Nelson JC, Gilsanz V. 1999. Serum levels of insulin-like growth factor I and the density, volume, and cross-sectional area of cortical bone in children. J Clin Endocrinol Metab 84:2780–2783 [DOI] [PubMed] [Google Scholar]
- 17. Kassem M, Mosekilde L, Eriksen EF. 1994. Growth hormone stimulates proliferation of normal human bone marrow stromal osteoblast precursor cells in vitro. Growth Regul 4:131–135 [PubMed] [Google Scholar]
- 18. Ohlsson C, Mellström D, Carlzon D, Orwoll E, Ljunggren O, Karlsson MK, Vandenput L. 2011. Older men with low serum IGF-1 have an increased risk of incident fractures: the MrOS Sweden study. J Bone Miner Res 26:865–872 [DOI] [PubMed] [Google Scholar]
- 19. Baum HB, Biller BM, Finkelstein JS, Cannistraro KB, Oppenhein DS, Schoenfeld DA, Michel TH, Wittink H, Klibanski A. 1996. Effects of physiologic growth hormone therapy on bone density and body composition in patients with adult-onset growth hormone deficiency. A randomized, placebo-controlled trial. Ann Intern Med 125:883–890 [DOI] [PubMed] [Google Scholar]
- 20. Glass AR. 1989. Endocrine aspects of obesity. Med Clin North Am 73:139–160 [DOI] [PubMed] [Google Scholar]
- 21. Martini LA, Wood RJ. 2006. Vitamin D status and the metabolic syndrome. Nutr Rev 64:479–486 [DOI] [PubMed] [Google Scholar]
- 22. Rajakumar K, de las Heras J, Chen TC, Lee S, Holick MF, Arslanian SA. 2011. Vitamin D status, adiposity, and lipids in black American and Caucasian children. J Clin Endocrinol Metab 96:1560–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Boutroy S, Bouxsein ML, Munoz F, Delmas PD. 2005. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab 90:6508–6515 [DOI] [PubMed] [Google Scholar]
- 24. Boutroy S, Van Rietbergen B, Sornay-Rendu E, Munoz F, Bouxsein ML, Delmas PD. 2008. Finite element analysis based on in vivo HR-pQCT images of the distal radius is associated with wrist fracture in postmenopausal women. J Bone Miner Res 23:392–399 [DOI] [PubMed] [Google Scholar]
- 25. Schellinger D, Lin CS, Hatipoglu HG, Fertikh D. 2001. Potential value of vertebral proton MR spectroscopy in determining bone weakness. AJNR Am J Neuroradiol 22:1620–1627 [PMC free article] [PubMed] [Google Scholar]
- 26. Han TS, van Leer EM, Seidell JC, Lean ME. 1995. Waist circumference action levels in the identification of cardiovascular risk factors: prevalence study in a random sample. BMJ 311:1401–1405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. van Rietbergen B, Weinans H, Huiskes R, Odgaard A. 1995. A new method to determine trabecular bone elastic properties and loading using micromechanical finite-element models. J Biomech 28:69–81 [DOI] [PubMed] [Google Scholar]
- 28. Bredella MA, Fazeli PK, Miller KK, Misra M, Torriani M, Thomas BJ, Ghomi RH, Rosen CJ, Klibanski A. 2009. Increased bone marrow fat in anorexia nervosa. J Clin Endocrinol Metab 94:2129–2136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sornay-Rendu E, Boutroy S, Munoz F, Delmas PD. 2007. Alterations of cortical and trabecular architecture are associated with fractures in postmenopausal women, partially independent of decreased BMD measured by DXA: the OFELY study. J Bone Miner Res 22:425–433 [DOI] [PubMed] [Google Scholar]
- 30. Cartier A, Lemieux I, Alméras N, Tremblay A, Bergeron J, Després JP. 2008. Visceral obesity and plasma glucose-insulin homeostasis: contributions of interleukin-6 and tumor necrosis factor-α in men. J Clin Endocrinol Metab 93:1931–1938 [DOI] [PubMed] [Google Scholar]
- 31. Giustina A, Mazziotti G, Canalis E. 2008. Growth hormone, insulin-like growth factors, and the skeleton. Endocr Rev 29:535–559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Rosén T, Wilhelmsen L, Landin-Wilhelmsen K, Lappas G, Bengtsson BA. 1997. Increased fracture frequency in adult patients with hypopituitarism and GH deficiency. Eur J Endocrinol 137:240–245 [DOI] [PubMed] [Google Scholar]
- 33. Yakar S, Canalis E, Sun H, Mejia W, Kawashima Y, Nasser P, Courtland HW, Williams V, Bouxsein M, Rosen C, Jepsen KJ. 2009. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J Bone Miner Res 24:1481–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu JL, Ooi GT, Setser J, Frystyk J, Boisclair YR, LeRoith D. 2002. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest 110:771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zumoff B, Strain GW, Miller LK, Rosner W, Senie R, Seres DS, Rosenfeld RS. 1990. Plasma free and non-sex-hormone-binding-globulin-bound testosterone are decreased in obese men in proportion to their degree of obesity. J Clin Endocrinol Metab 71:929–931 [DOI] [PubMed] [Google Scholar]
- 36. Bouillon R, Bex M, Vanderschueren D, Boonen S. 2004. Estrogens are essential for male pubertal periosteal bone expansion. J Clin Endocrinol Metab 89:6025–6029 [DOI] [PubMed] [Google Scholar]
- 37. Szulc P, Munoz F, Claustrat B, Garnero P, Marchand F, Duboeuf F, Delmas PD. 2001. Bioavailable estradiol may be an important determinant of osteoporosis in men: the MINOS study. J Clin Endocrinol Metab 86:192–199 [DOI] [PubMed] [Google Scholar]
- 38. Vanderschueren D, Vandenput L, Boonen S, Lindberg MK, Bouillon R, Ohlsson C. 2004. Androgens and bone. Endocr Rev 25:389–425 [DOI] [PubMed] [Google Scholar]
- 39. Kristinsson JO, Valdimarsson O, Sigurdsson G, Franzson L, Olafsson I, Steingrimsdottir L. 1998. Serum 25-hydroxyvitamin D levels and bone mineral density in 16–20 years-old girls: lack of association. J Intern Med 243:381–388 [DOI] [PubMed] [Google Scholar]
- 40. Rosen CJ, Bouxsein ML. 2006. Mechanisms of disease: is osteoporosis the obesity of bone? Nat Clin Pract Rheumatol 2:35–43 [DOI] [PubMed] [Google Scholar]