Abstract
Context:
Idiopathic osteoporosis (IOP) affects otherwise healthy young individuals with intact gonadal function and no secondary cause of bone fragility. In premenopausal women with IOP, a low trauma fracture is evidence of impaired bone quality and strength. The extent to which low bone mineral density (BMD) by dual-energy x-ray absorptiometry (DXA) reflects low volumetric BMD, bone microstructure, and strength is uncertain in the absence of low trauma fracture.
Objective:
The objective of the study was to compare three-dimensional volumetric BMD and bone stiffness in premenopausal women with IOP based on fracture history, those with idiopathic low BMD (Z score ≤ −2.0) and no low trauma fracture, and normal age-matched controls.
Design:
We measured volumetric BMD and bone geometry by central quantitative computed tomography (cQCT) scans of the spine and hip and estimated bone stiffness by finite element analysis of cQCT data sets in 32 premenopausal women with IOP, 12 with idiopathic low BMD, and 34 controls.
Results:
Subjects had comparable decreases in total and trabecular volumetric BMD, cortical thickness, and whole-bone stiffness compared with controls, regardless of fracture history. These differences remained significant after controlling for age, body mass index, and bone size. The positive predictive values of a DXA Z score of −2.0 or less for a cQCT volumetric BMD Z score of −2.0 or less were 95% at the lumbar spine, 90% at the total hip, and 86% at the femoral neck.
Conclusion:
Women with idiopathic low BMD alone and those with low trauma fractures had comparable deficits in bone mass, structure, and stiffness. Low areal BMD by DXA is fairly accurate for predicting low volumetric BMD by cQCT. These results are consistent with three-dimensional bone imaging at the iliac crest, radius, and tibia in premenopausal IOP and suggest that the term osteoporosis may be appropriate in women with Z scores below −2.0, whether or not there is a history of fracture.
Idiopathic osteoporosis (IOP) is operationally defined as osteoporosis that affects otherwise healthy young individuals with intact gonadal function and no secondary cause of bone loss or bone fragility. Premenopausal women with IOP most commonly come to clinical attention after a fragility fracture. However, some affected women may present with very low bone mineral density (BMD) measured by dual-energy x-ray absorptiometry (DXA) and no history of fragility fractures. DXA is a two-dimensional technique that measures areal BMD and is affected by bone size. Areal BMD measurements may be artifactually low in individuals with smaller bones (1). Thus, it is unclear whether otherwise healthy young women with a low areal BMD measurement by DXA and no fractures truly have low volumetric BMD or reduced bone strength (2–6).
Quantitative computed tomography (QCT) is a three-dimensional imaging technique that provides a measurement of volumetric BMD that is independent of bone size. In addition, QCT data sets can be analyzed by finite element analysis to provide an estimate of bone stiffness or strength. In our study of premenopausal IOP, we originally hypothesized that women with IOP and low trauma fractures would have deficits in volumetric BMD, microarchitecture, and strength but that women with idiopathic low BMD (ILBMD), who were shorter and weighed less than controls (7), would have normal volumetric BMD and bone microarchitecture. To date, we have reported that, as anticipated, premenopausal women with IOP have lower volumetric BMD, thinner cortices, fewer, thinner, more widely separated, and heterogeneously distributed trabeculae, and reduced bone stiffness compared with controls, whether assessed by microcomputed tomography (μCT) of iliac crest bone biopsy samples (8) or by high-resolution peripheral quantitative computed tomography (HRpQCT) of the distal radius and tibia (9). Contrary to our hypothesis, however, we found that women with ILBMD also have severe deficits in volumetric BMD, microarchitecture, and stiffness at the iliac crest and distal radius and tibia that are comparable with those of women with IOP and fragility fractures (8, 9). To determine whether findings are similar at the central skeleton, we used QCT to measure volumetric BMD and stiffness of the lumbar spine and hip, important sites of osteoporotic fractures, in premenopausal women with IOP and fragility fractures and those with ILBMD.
Materials and Methods
Patient population
Premenopausal women, aged 18–48 yr, were recruited at Columbia University Medical Center (New York, NY) and Creighton University (Omaha, NE) by advertisement or self- or physician referral as previously described in detail (7). Included were women with a documented low-trauma fracture after age 18 yr (regardless of whether areal BMD was low; the IOP group) as well as women with low areal BMD (aBMD) by DXA (T score ≤−2.5 or Z score ≤−2.0) at the spine or hip without history of adult low trauma fracture (the ILBMD group). Fractures were ascertained by review of the radiographs or reports and categorized as low trauma (equivalent to a fall from a standing height or less) after review by physician panel (A.C., E.M.S., R.R.R., E.S.). Controls had normal aBMD by DXA (T score ≥−1.0 or Z score ≥−1.0) and no history of adult low trauma fractures.
Inclusion and exclusion criteria were previously reported (7). We defined premenopausal status as regular menses off hormonal contraception and early follicular phase FSH levels less than 20 mIU/ml. Secondary causes of osteoporosis were excluded in subjects and controls by detailed history and physical and biochemical evaluation (7). All subjects provided written informed consent. The institutional review boards of both institutions approved these studies.
Laboratory assessments
To assess for secondary causes of osteoporosis, fasting morning blood was drawn and a 24-h urine collected during the early follicular phase of the menstrual cycle on the participant's usual diet and supplement regimen and analyzed in a clinical laboratory (Quest Diagnostics, Madison, NJ). Additional serum was archived and stored at −80 C for batch analyses in the Bone Marker Laboratory of the Metabolic Bone Diseases Program and the Irving Institute for Clinical and Translational Research Biomarkers Core Laboratory at Columbia University Medical Center. Details regarding laboratory assessments have been previously published (7).
Areal BMD
aBMD was measured by DXA (QDR-4500; Hologic Inc., Walton, MA) at Columbia and Creighton University Medical Centers as previously described (7).
Central QCT (cQCT)
Subjects underwent helical (pitch = 1) QCT scanning of the proximal femur and spine (L1-L2) at 80 kVp, 2.5 mm slice thickness, at Columbia University Medical Center (GE CTi and GE Light Speed VCT 64; General Electric Medical Systems, Milwaukee WI) and Creighton University Medical Center (Siemens Somatom Plus 4 and GE LightSpeed DXi). All subjects were scanned together with a calibration phantom (3-Bar; Image Analysis, Columbia, KY) used to calibrate computed tomography (CT) images to equivalent concentration of calcium hydroxyapatite. A torso-simulating quality assurance phantom (Image Analysis), containing a circular insert of 100 mg/cm3, was used to determine the precise characteristics and track the performance of the CT scanners used at the two research sites.
Image data acquired at Columbia University and Creighton University were exported for structural and finite element analyses in the laboratory of Thomas Lang, Ph.D. Specialized image analysis software (10) was used to compute total, cortical, and trabecular volumetric density at the proximal femur and femoral neck bilaterally and total and (anterior vertebral body) trabecular volumetric density at the spine (mean of L1 and L2). Regions of cortical bone were determined by applying a threshold of 0.35 g/cm3 to the voxels falling outside the trabecular regions but within the integral regions. The spinal trabecular region of interest was a semicircular region encompassing the anterior vertebral body, centered on the midvertebral level, but encompassing 70% of the volume between the vertebral endplates. Trabecular bone regions at the proximal femur and femoral neck were determined by eroding the integral bone regions to produce regions of the same shape but fully contained within the medullary volumes. Detailed methods used to determine regions of interest have been previously published (10). Additionally, we calculated the area of the narrowest cross-section of the femoral neck (FNCS) as a measure of bone size, and the cortical index (C/I; cortical tissue volume/total integral tissue volume) as a measure of cortical width (10, 11). Both FNCS and C/I correlate with hip fracture risk in men and postmenopausal women (12, 13).
Finite element analyses for cQCT images of the L1 vertebra and proximal femur
All cQCT data sets were returned to Columbia University for finite element (FE) analyses (performed by X.S.L. and X.E.G.), according to previously described methods (14). Briefly, a continuum FE model was built for each vertebra and femur by converting each bone voxel to a 0.937 × 0.937 × 2.5 mm3 eight-node brick element as previously described (15–17). Hounsfield unit values were converted to equivalent mineral density values (grams per cubic centimeter) of calcium hydroxyapatite using linear regression of the calibration phantom images (Image Analysis). Vertebral bone tissue was assumed to be a transversely isotropic, linear elastic material (15, 17). Longitudinal elastic modulus (Ez, megapascal) was determined based on QCT mineral density values of each bone voxel (ρQCT, grams per cubic centimeter) using the experimentally determined correlation between Ez and ρQCT (17): Ez = −34.7 + 3230ρQCT. The other anisotropic engineering constants were then assigned using assumptions of transverse isotropy as previously described (15, 17). A thin layer of poly(methyl methacrylate) (2.5–5 mm thick, Young's modulus, E = 2.5 GPa, and Poisson's ratio υ = 0.3) was added on top of the endplates of the vertebra to facilitate applications of uniform displacement boundary conditions (15, 17). A uniaxial compression displacement boundary condition was applied to each model. Then the FE model of L1 was input into Abaqus 6.7 (SIMULIA, Providence, RI) to determine axial stiffness, as described by Crawford et al. (15).
For femoral bone tissue, ash density (ρash; grams per cubic centimeter) of each element was first calculated based on the calibrated relationship: ρash = 0.0633 + 0.887ρQCT (16). Femoral bone tissue was assumed to be an isotropic, linear elastic material. Material properties were assigned in an element-specific fashion by mapping the cQCT mineral density value of each element to an elastic modulus based on a previously established density-modulus relationship for femoral bone: E (MPa) = 14900ρash1.86 (16). A boundary condition simulating single-leg stance was applied to each femur model (16). The FE models of the proximal femur were input into Abaqus 6.7 (SIMULIA) to determine stiffness, as described by Keyak et al. (16).
Statistical analyses
Statistical analyses were performed using SAS software (SAS Institute, Cary, NC). Between-groups comparisons were conducted using Student's t tests. Because multiple CT scanners were used, data from scans of a single torso phantom (Image Analysis) on each scanner were used to calculate a linear conversion formula for each scanner. To derive the conversion factor for each scanner, BMD was measured in the torso phantom spine insert of known density (0.1 g/cm3) and in two water equivalent zero reference regions. Slope and intercept were calculated to relate the measured BMD to the known BMD of the phantom regions. The conversion formulae were applied to the values of each scanner, thus casting all CT results into torso phantom equivalent BMD values [torsoequivalent BMD = (slope measured BMD) + intercept]. Spearman correlation coefficients were calculated to test associations between variables. Multivariate linear regression analyses (generalized linear models) were used to control for covariates. All data are expressed as mean ± sd. Results were considered significant with P < 0.05.
Results
The cQCT scans of the lumbar spine were available in 34 controls and 44 subjects (32 IOP, 12 ILBMD) and the hip in 31 controls and 38 subjects (27 IOP, 11 ILBMD). Subjects and controls who chose to participate in the cQCT portion of the study represent a subset of our larger cohort of 64 affected women and 40 healthy controls. The 44 subjects who chose to participate did not differ from the 20 nonparticipators in terms of age, height, weight, body mass index (BMI), or number of fractures but had significantly higher BMD Z scores at the spine (−1.25 vs. −2.21; P = 0.0003) and femoral neck (−1.31 vs. −1.76 P = 0.048). Among the 32 subjects in the IOP group, the number of fractures per IOP subject ranged from one to seven; 17 subjects had multiple fractures, ranging from two to seven. Five subjects had vertebral, eight had rib, six had hip, one had pelvic, eight had forearm, three had humerus, three had lower leg, three had ankle, and seven had metatarsal fractures.
Characteristics of the control, IOP, and ILBMD subjects are shown in Table 1. Of note, both groups of subjects had lower BMD by DXA than controls. Additionally, ILBMD subjects were older and weighed less than both controls and IOP subjects.
Table 1.
Characteristics of the study groups
| Control (n = 34) | All affected subjects (n = 44) | IOP (n = 32) | ILBMD (n = 12) | |
|---|---|---|---|---|
| Age (yr) | 37.1 ± 8.6 | 38.3 ± 7.5 | 37.0 ± 8.3 | 41.7 ± 3.1a,b |
| Height (cm) | 165.4 ± 7.2 | 164.4 ± 5.8 | 165.2 ± 6.1 | 162.2 ± 4.4 |
| Weight (kg) | 70.0 ± 15.6 | 63.3 ± 14.4(0.05) | 65.8 ± 15.5 | 56.6 ± 7.9c,d |
| BMI (kg/m2) | 25.5 ± 4.7 | 23.4 ± 4.8(0.05) | 24.0 ± 5.2 | 21.5 ± 3.3a |
| BMD by DXA (g/cm2) | ||||
| LS | 1.092 ± 0.097 | 0.882 ± 0.133e | 0.912 ± 0.138e | 0.804 ± 0.081e,d |
| Total hip | 0.973 ± 0.070 | 0.788 ± 0.141e | 0.815 ± 0.150e | 0.719 ± 0.088e,d |
| Femoral neck | 0.858 ± 0.072 | 0.674 ± 0.137e | 0.699 ± 0.142e | 0.609 ± 0.100e |
| Distal radius | 0.721 ± 0.461 | 0.688 ± 0.055c | 0.698 ± 0.046(0.05) | 0.661 ± 0.065c,d |
| BMD by DXA (Z score) | ||||
| LS | 0.69 ± 0.91 | −1.25 ± 1.24e | −0.98 ± 1.28e | −1.95 ± 0.81e,d |
| Total hip | 0.40 ± 0.62 | −1.11 ± 1.14e | −0.90 ± 1.21e | −1.63 ± 0.71e |
| Femoral neck | 0.30 ± 0.72 | −1.31 ± 1.20e | −1.110 ± 1.261e | −1.82 ± 0.89e |
| Distal radius | 0.65 ± 0.84 | 0.24 ± 0.86a | 0.355 ± 0.826 | −0.08 ± 0.90a |
P < 0.05, comparisons vs. control.
P < 0.01, comparisons between IOP and ILBMD.
P < 0.01, comparisons vs. control.
P < 0.05, comparisons between IOP and ILBMD.
P < 0.001, comparisons vs. control.
cQCT measures
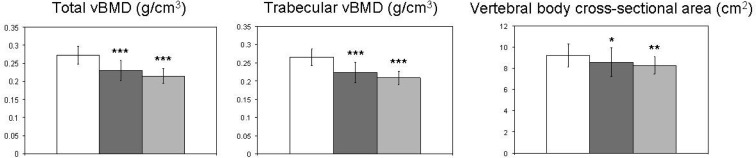
At the lumbar spine (Fig. 1), mean total and trabecular volumetric BMD were 16 and 22% lower than controls in the IOP and ILBMD groups, respectively. Vertebral size was smaller in both IOP and ILBMD groups, as evidenced by the vertebral body cross-sectional area (VBCSA) that was 7 and 10% lower in the IOP and ILBMD groups than controls, respectively. There were no significant differences between the IOP and ILBMD groups at the spine.
Fig. 1.
Compartmental vBMD and bone geometry at the lumbar spine in controls (white columns), IOP (dark gray columns), and ILBMD (light gray columns) subjects. Comparisons vs. controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
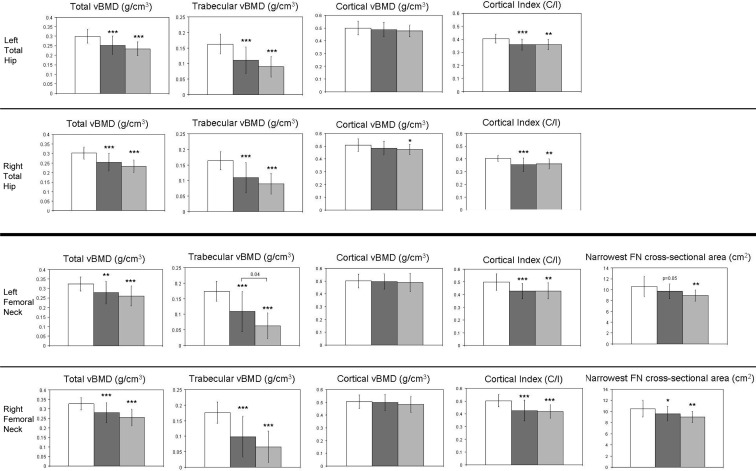
Because results for the left and right hip (Fig. 2) were similar, we present the results for the left hip. At the proximal femur, total volumetric BMD was 16 and 22% lower than controls in the IOP and ILBMD groups, respectively. At the femoral neck, total volumetric BMD was 14 and 20% lower in the IOP and ILBMD groups, respectively. Larger between-group differences were seen for trabecular volumetric BMD at the proximal femur and femoral neck, which were 32–37 and 45–64% lower than controls in the IOP and ILBMD groups, respectively. In contrast, there were no significant between-group differences for cortical volumetric BMD. However, C/I, an index of cortical thickness, was 11% lower than controls at the proximal femur and 14% lower than controls at the femoral neck, in both the IOP and ILBMD groups. There were no significant differences between the IOP and ILBMD groups for the majority of these measures at the hip, with the exception of trabecular vBMD at the left femoral neck, which was significantly lower in the ILBMD group. The area at the narrowest cross-section of the femoral neck (FNCS) was 8% (P = 0.05) and 16% (P = 0.007) lower than controls in the IOP and ILBMD groups, respectively, providing further evidence for smaller bone size in subjects than controls.
Fig. 2.
Compartmental vBMD and bone geometry at the proximal femur and femoral neck in controls (white columns), IOP (dark gray columns), and ILBMD (light gray columns) subjects. Comparisons vs. controls: *, P < 0.05; **, P < 0.01; ***, P < 0.001. Brackets show the P values for significant differences between IOP and ILBMD subjects.
FE analyses
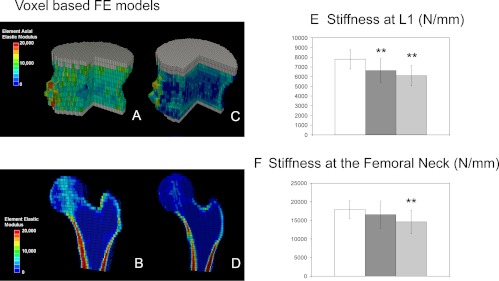
Voxel-based FE models were used to estimate bone stiffness at L1 and the femoral neck (Fig. 3). Bone stiffness at L1 was 15 and 22% lower than controls in the IOP and ILBMD groups, respectively. At the femoral neck, bone stiffness was significantly lower than controls only in the ILBMD group (by 19%).
Fig. 3.
cQCT-based FE models of the L1 vertebral body (A and C) and the proximal femur (B and D) of a control (A and B) and a subject (C and D). Distributions of elastic moduli of elements are indicated by different colors. Gray elements indicate poly(methyl methacrylate) layers. A portion of the vertebral body and half of the proximal femur have been removed for illustration purposes. E and F, Bone stiffness estimated from voxel-based FE models at L1 and at the femoral neck in controls (white columns), IOP (dark gray columns), and ILBMD (light gray columns) subjects. Comparisons vs. controls: **, P < 0.01.
Correlation analyses
Because very few differences were found between IOP and ILBMD subjects, all subjects were grouped together in the correlation analyses.
Because one goal of this study was to determine whether smaller bone size could account for the lower aBMD in our affected subjects and because the three-dimensional cQCT technique provides volumetric BMD (vBMD) data that are independent of bone size, we examined relationships between aBMD and vBMD in our subjects and controls. As expected, there were positive correlations between aBMD by DXA and cQCT measures of vBMD and bone size in the subjects and controls (Table 2). The somewhat stronger correlations in the subjects may be related to the higher variability of their aBMD. We also found significant correlations between cQCT measures, age, and anthropometric measures (Table 3). Age varied inversely with femoral neck trabecular vBMD only in controls. Weight and BMI correlated directly with total and trabecular vBMD of the proximal femur and femoral neck in the subjects, whereas weight correlated inversely with vBMD measures at the hip in the controls. Weight and BMI were not associated with cortical vBMD in either group. Height and weight correlated with FNCS and VBCSA, indices of bone size, in both groups.
Table 2.
Correlation between cQCT measures and aBMD by DXA in subjects and controls
| cQCT measures | Controls |
All affected subjects |
||||||
|---|---|---|---|---|---|---|---|---|
| LS aBMD | TH aBMD | FN aBMD | DR aBMD | LS aBMD | TH aBMD | FN aBMD | DR aBMD | |
| Spine | ||||||||
| LS total vBMD | 0.30 | 0.13 | −0.01 | 0.15 | 0.75a | 0.47b | 0.55b | 0.34c |
| LS Trabecular vBMD | 0.31 | 0.18 | 0.07 | 0.14 | 0.75a | 0.46b | 0.54b | 0.34c |
| VBCSA | 0.06 | 0.32 | 0.36c | 0.19 | 0.50b | 0.36c | 0.27 | 0.37c |
| Left hip | ||||||||
| PF total vBMD | 0.02 | 0.51b | 0.24 | 0.43c | 0.38c | 0.74a | 0.73a | 0.20 |
| PF trabecular vBMD | 0.10 | 0.36c | 0.20 | 0.17 | 0.45b | 0.84a | 0.81a | 0.30 |
| PF cortical vBMD | −0.05 | 0.38c | 0.18 | 0.48b | 0.29 | 0.26 | 0.29 | 0.05 |
| FN total vBMD | 0.06 | 0.30 | 0.21 | 0.31 | 0.38c | 0.64a | 0.67a | 0.13 |
| FN trabecular vBMD | 0.16 | 0.09 | 0.06 | 0.02 | 0.59b | 0.79a | 0.85a | 0.25 |
| FN cortical vBMD | 0.06 | 0.34 | 0.28 | 0.38c | 0.15 | 0.17 | 0.21 | −0.06 |
| FNCS | 0.36c | 0.24 | 0.43c | −0.06 | 0.57b | 0.43b | 0.38c | 0.48b |
| Right hip | ||||||||
| PF total vBMD | −0.08 | 0.40c | 0.11 | 0.32 | 0.32 | 0.75a | 0.69a | 0.17 |
| PF trabecular vBMD | 0.06 | 0.23 | 0.11 | 0.02 | 0.36c | 0.77a | 0.73a | 0.34c |
| PF Cortical vBMD | −0.12 | 0.32 | 0.07 | 0.39c | 0.19 | 0.22 | 0.21 | 0.04 |
| FN Total vBMD | −0.03 | 0.20 | 0.07 | 0.14 | 0.36c | 0.66a | 0.68a | 0.14 |
| FN trabecular vBMD | 0.21 | 0.12 | 0.10 | −0.11 | 0.48b | 0.73a | 0.75a | 0.24 |
| FN cortical vBMD | 0.02 | 0.28 | 0.19 | 0.27 | 0.14 | 0.15 | 0.16 | −0.01 |
| FNCS | 0.22 | 0.18 | 0.32 | −0.04 | 0.57b | 0.44b | 0.43c | 0.54b |
TH, Total hip; FN, femoral neck; DR, distal radius; PF, proximal femur.
Significant correlations are shown in bold.
P < 0.001.
P < 0.01.
P < 0.05.
Table 3.
Correlations between cQCT measures and anthropometric measures in subjects and controls
| cQCT measures | Controls |
All affected subjects |
||||||
|---|---|---|---|---|---|---|---|---|
| Age | Height | Weight | BMI | Age | Height | Weight | BMI | |
| Spine | ||||||||
| LS total vBMD | −0.12 | −0.25 | 0.00 | 0.16 | −0.03 | −0.07 | 0.26 | 0.33a |
| LS trabecular vBMD | −0.14 | −0.17 | 0.07 | 0.20 | −0.07 | −0.06 | 0.24 | 0.30a |
| VBCSA | 0.18 | 0.65b | 0.35a | 0.05 | 0.29 | 0.30a | 0.29 | 0.19 |
| Left hip | ||||||||
| PF total vBMD | −0.03 | −0.23 | −0.09 | 0.09 | −0.03 | 0.01 | 0.42c | 0.43c |
| PF trabecular vBMD | −0.02 | −0.07 | −0.19 | −0.07 | −0.10 | 0.11 | 0.58c | 0.60b |
| PF cortical vBMD | 0.01 | −0.29 | −0.10 | 0.10 | 0.13 | −0.11 | 0.01 | −0.00 |
| FN total vBMD | −0.13 | −0.11 | −0.23 | −0.11 | 0.02 | −0.10 | 0.34a | 0.39a |
| FN trabecular vBMD | −0.45a | 0.11 | −0.28 | −0.28 | −0.18 | 0.13 | 0.66b | 0.71b |
| FN cortical vBMD | 0.08 | −0.24 | −0.12 | 0.07 | 0.19 | −0.31 | −0.11 | −0.05 |
| FNCS | −0.15 | 0.40a | 0.44a | 0.20 | 0.07 | 0.53c | 0.51c | 0.39a |
| Right hip | ||||||||
| PF Total vBMD | 0.02 | −0.31 | −0.25 | −0.04 | 0.03 | −0.02 | 0.37a | 0.40a |
| PF Trabecular vBMD | −0.03 | −0.22 | −0.36a | −0.17 | 0.05 | 0.03 | 0.48c | 0.53c |
| PF Cortical vBMD | 0.06 | −0.29 | −0.21 | −0.01 | 0.22 | −0.20 | −0.09 | −0.08 |
| FN Total vBMD | −0.09 | −0.20 | −0.46c | −0.31 | 0.07 | −0.12 | 0.33a | 0.39a |
| FN Trabecular vBMD | −0.48c | 0.07 | −0.31 | −0.22 | −0.05 | 0.12 | 0.58c | 0.63b |
| FN cortical vBMD | 0.17 | −0.28 | −0.25 | −0.08 | 0.31 | −0.34a | −0.20 | −0.14 |
| FNCS | −0.09 | 0.51c | 0.41a | 0.15 | 0.04 | 0.59c | 0.53c | 0.37a |
FN, Femoral neck; PF, proximal femur.
Significant correlations are shown in bold.
P < 0.05.
P < 0.001.
P < 0.01.
Adjusted analyses
Because age, height, weight, and BMI differed between subjects and controls and correlated with cQCT measures, we adjusted for each of these variables. Between-group comparisons (controls vs. IOP and controls vs. ILBMD) for all trabecular vBMD measures shown in Figs. 1 and 3 were unchanged after adjustment for age, height, weight, and BMI. Between-group comparisons (controls vs. IOP and controls vs. ILBMD) for FNCS and VBCSA, measures of bone size, were somewhat attenuated after adjusting for weight (adjusted P values: 0.05–0.1 for controls vs. IOP; 0.08–0.1 for controls vs. ILBMD) and BMI (adjusted P values: 0.05–0.09 for controls vs. IOP; 0.02–0.03 for controls vs. ILBMD).
One goal of this study was to investigate whether bone size could account for low BMD, particularly in the ILBMD group, who had not had low trauma fractures. Adjusting for VBCSA did not change relationships between spine measures in Fig. 1 or 3. Similarly, adjusting for FNCS did not influence the findings for the hip measures in Fig. 2 or 3.
Analyses based on Z score
To assess whether low aBMD by DXA (defined as a Z score ≤−2.0) accurately predicted a low volumetric BMD (vBMD) by cQCT, we used cQCT results from the controls to calculate lumbar spine (LS), proximal femur, and femoral neck Z scores for the subjects. Total LS cQCT Z scores were −2.0 or less in 64% of subjects; 18 of 19 of subjects with LS Z scores −2.0 or less by DXA also had cQCT Z scores −2.0 or less. In contrast, only 15 of 25 subjects with LS aBMD Z scores −2.0 or greater also had LS cQCT vBMD Z score −2.0 or greater. Thus, the positive predictive value of an aBMD Z score −2.0 or less by DXA for a vBMD Z score −2.0 or less was 95%, whereas the negative predictive value was 60%. By cQCT, the vBMD Z scores were −2.0 or less in 50% of subjects at the right proximal femur and 47% at the femoral neck. The positive predictive value of DXA for a cQCT Z score −2.0 or less was 90% at the total hip and 86% at the femoral neck. In contrast, the negative predictive value of DXA for a Z score greater than −2.0 was only 65% at the total hip and 77% at the femoral neck.
Discussion
This is the first study to use cQCT and FE analyses of cQCT data sets to quantify cortical and trabecular vBMD and microarchitecture and estimated stiffness of the spine and hip in premenopausal women with unexplained low trauma fractures and/or low BMD and concurrently recruited normal women. We found that affected women have lower total and trabecular vBMD, cortical thickness, and whole-bone stiffness compared with controls, independent of age, BMI, and bone size. Moreover, women without a history of fragility fracture who were included on the basis of low aBMD by DXA had deficits in bone mass, structure, and stiffness that were just as profound, and in some cases more profound, than those included because of low trauma fractures.
DXA is a two-dimensional technique that may underestimate true volumetric bone density in individuals with smaller bones (1). At the outset of this study, we hypothesized that women in the ILBMD group might have low areal BMD by DXA because they had smaller skeletons but that measurement of volumetric BMD and microarchitecture by three-dimensional imaging techniques would reveal that their bone structure was more normal than women with fractures and comparable with controls. However, those in the ILBMD group had markedly lower three-dimensional volumetric BMD and stiffness than controls, even after controlling for their lower BMI and smaller bone size, thus definitively contradicting this hypothesis. The cQCT data are thus consistent with the results of the larger cross-sectional study of affected premenopausal women, which showed that women with fractures and those with low aBMD by DXA were also indistinguishable with regard to vBMD, microarchitecture, and stiffness measured by μCT at the iliac crest and HRpQCT at the distal radius and tibia (8, 9). Thus, our findings are consistent across central and peripheral skeletal sites and using three different CT imaging modalities. Moreover, although results from this small sample size are not conclusive, the positive predictive values of a DXA Z score of −2.0 or less for a cQCT Z score of −2.0 or less at the lumbar spine (95%), total hip (90%), and femoral neck (86%) suggests that a low areal BMD measurement on a DXA scan is fairly accurate for predicting a low volumetric BMD on cQCT.
Because fracture rates are orders of magnitude lower in premenopausal than postmenopausal women for any given areal BMD measurement (18), the predictive relationship between BMD and short-term fracture incidence is unclear in premenopausal women. Therefore, we have argued (2), as has the International Society for Clinical Densitometry (3) and others (4–6, 19–21), that young, otherwise healthy women should not be diagnosed with osteoporosis solely on the basis of low aBMD by DXA unless there is a history of fragility fracture or a secondary cause of osteoporosis, such as glucocorticoid exposure. However, taken together with our transiliac crest bone biopsy μCT and HRpQCT data, these cQCT results suggest that otherwise healthy women with low areal BMD by DXA and no history of fragility fracture have consistent cortical and trabecular microarchitectural deficits and reduced stiffness that are comparable to women with fractures. Although these consistent findings across multiple imaging platforms suggest that fracture history may not be required for the diagnosis of osteoporosis in premenopausal women with low aBMD, only a large population-based longitudinal study would be able to quantify fracture risk in premenopausal women with low aBMD. Because fracture is a relatively rare event in premenopausal women, such a large prospective study is not feasible.
This study has several limitations. The women with IOP and ILBMD comprised a subset of the full cohort. Although they did not differ from those in the full cohort who did not have cQCT scans, they had higher Z scores at the spine and femoral neck. However, if anything, this would bias against seeing any differences. Our younger subjects may not have reached peak bone mass. We may have misclassified fractures as low trauma. The overall sample size was quite small and may have limited our ability to distinguish between the groups. The number of subjects with low aBMD was very small and the women who volunteered for this study may have done so because of a family history of osteoporosis, high trauma, or childhood fractures, causing ascertainment bias. Although the ILBMD group did not have adult low trauma fractures, 26% reported childhood fractures, 16% reported high trauma fractures, and 84% had a family history of osteoporosis (7). Thus, our results may not be generalizable to all premenopausal women with low aBMD by DXA. We cannot determine whether microarchitectural abnormalities we detected resulted from ongoing bone loss or past insults now resolved. Assumptions of uniform bone mineralization are incorporated into FE analyses, which may or may not be appropriate for this cohort.
In summary, we found that premenopausal women with idiopathic fragility fractures and those with idiopathic low BMD have significantly lower total and trabecular volumetric BMD, cortical width, and estimated stiffness of the spine and hip than normal women. Those with and without fragility fractures were equally affected in terms of bone structure and stiffness and the differences persisted after adjusting for age, weight, BMI, and bone size. The results replicate those found by other three-dimensional imaging techniques in this population, specifically μCT of transiliac bone biopsy samples and HRpQCT of the radius and tibia (8, 9). Our findings also show that premenopausal women with DXA Z scores of −2.0 or less are highly likely to have decreased vBMD by cQCT. Thus, a low aBMD measurement by DXA in premenopausal women with or without fractures is likely to signify structurally and biomechanically compromised bone. Our results also suggest that the term osteoporosis may be appropriate in women with Z scores below −2.0, whether or not there is a history of fracture. We believe that the use of the term osteoporosis in such premenopausal women can provide a useful description of the microarchitectural defect present. However, because fracture risk depends greatly on age and because few studies have addressed risks and benefits of osteoporosis medications in premenopausal women, the diagnosis of osteoporosis solely on the basis of a low BMD should not be used to make treatment decisions in premenopausal women. In women with no history of low trauma fracture, we think that medications would be appropriate only in those rare cases in whom there is extremely low BMD and/or persistent bone loss.
Acknowledgments
This work was supported by National Institutes of Health Grants R01 AR049896 (to E.S.), K24AR05266 (to E.S.), and K23AR054127 (to A.C.).
Disclosure Summary: The authors have nothing to declare.
Footnotes
- aBMD
- Areal BMD
- BMD
- bone mineral density
- BMI
- body mass index
- C/I
- index of cortical tissue volume/total integral tissue volume
- cQCT
- central QCT
- CT
- computed tomography
- μCT
- micro-CT
- DXA
- dual-energy x-ray absorptiometry
- Ez
- elastic modulus
- FE
- finite element
- FNCS
- narrowest cross-section of the femoral neck
- HRpQCT
- high-resolution peripheral quantitative CT
- ILBMD
- idiopathic low BMD
- IOP
- idiopathic osteoporosis
- LS
- lumbar spine
- QCT
- quantitative CT
- ρQCT
- QCT mineral density values of each bone voxel
- VBCSA
- vertebral body cross-sectional area
- vBMD
- volumetric BMD.
References
- 1. Adams J, Bishop N. 2008. DXA in adults and children. In: Rosen CJ, ed. Primer on the metabolic bone diseases and disorders of mineral metabolism. Chap 29 Washington, DC: American Society for Bone and Mineral Research; 152–158 [Google Scholar]
- 2. Cohen A, Shane E. 2008. Premenopausal osteoporosis. In: Rosen CJ, ed. Primer on the metabolic bone diseases and other disorders of bone and mineral metabolism. Washington, DC: American Society for Bone and Mineral Research; 289–293 [Google Scholar]
- 3. Kahn AA, Syed Z. 2004. Bone densitometry in premenopausal women: synthesis and review. J Clin Densitom 7:85–92 [DOI] [PubMed] [Google Scholar]
- 4. Gourlay ML, Brown SA. 2004. Clinical considerations in premenopausal osteoporosis. Arch Intern Med 164:603–614 [DOI] [PubMed] [Google Scholar]
- 5. Lewiecki EM. 2004. Low bone mineral density in premenopausal women. South Med J 97:544–550 [DOI] [PubMed] [Google Scholar]
- 6. Lewiecki EM, Gordon CM, Baim S, Leonard MB, Bishop NJ, Bianchi ML, Kalkwarf HJ, Langman CB, Plotkin H, Rauch F, Zemel BS, Binkley N, Bilezikian JP, Kendler DL, Hans DB, Silverman S. 2008. International Society for Clinical Densitometry 2007 adult and pediatric official positions. Bone 43:1115–1121 [DOI] [PubMed] [Google Scholar]
- 7. Cohen A, Recker RR, Lappe J, Dempster DW, Cremers S, McMahon DJ, Stein EM, Fleischer J, Rosen CJ, Rogers H, Staron RB, Lemaster J, Shane E. 2012. Premenopausal women with idiopathic low-trauma fractures and/or low bone mineral density. Osteoporos Int 23:171–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Cohen A, Dempster DW, Recker RR, Stein EM, Lappe JM, Zhou H, Wirth AJ, van Lenthe GH, Kohler T, Zwahlen A, Müller R, Rosen CJ, Cremers S, Nickolas TL, McMahon DJ, Rogers H, Staron RB, LeMaster J, Shane E. 2011. Abnormal bone microarchitecture and evidence of osteoblast dysfunction in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 96:3095–3105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cohen A, Liu XS, Stein EM, McMahon DJ, Rogers HF, Lemaster J, Recker RR, Lappe JM, Guo XE, Shane E. 2009. Bone microarchitecture and stiffness in premenopausal women with idiopathic osteoporosis. J Clin Endocrinol Metab 94:4351–4360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lang T, LeBlanc A, Evans H, Lu Y, Genant H, Yu A. 2004. Cortical and trabecular bone mineral loss from the spine and hip in long-duration spaceflight. J Bone Miner Res 19:1006–1012 [DOI] [PubMed] [Google Scholar]
- 11. Lang TF, Leblanc AD, Evans HJ, Lu Y. 2006. Adaptation of the proximal femur to skeletal reloading after long-duration spaceflight. J Bone Miner Res 21:1224–1230 [DOI] [PubMed] [Google Scholar]
- 12. Black DM, Bouxsein ML, Marshall LM, Cummings SR, Lang TF, Cauley JA, Ensrud KE, Nielson CM, Orwoll ES. 2008. Proximal femoral structure and the prediction of hip fracture in men: a large prospective study using QCT. J Bone Miner Res 23:1326–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cheng X, Li J, Lu Y, Keyak J, Lang T. 2007. Proximal femoral density and geometry measurements by quantitative computed tomography: association with hip fracture. Bone 40:169–174 [DOI] [PubMed] [Google Scholar]
- 14. Liu XS, Cohen A, Shane E, Yin PT, Stein EM, Rogers H, Kokolus SL, McMahon DJ, Lappe JM, Recker RR, Lang T, Guo XE. 2010. Bone density, geometry, microstructure, and stiffness: relationships between peripheral and central skeletal sites assessed by DXA, HR-pQCT, and cQCT in premenopausal women. J Bone Miner Res 25:2229–2238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crawford RP, Cann CE, Keaveny TM. 2003. Finite element models predict in vitro vertebral body compressive strength better than quantitative computed tomography. Bone 33:744–750 [DOI] [PubMed] [Google Scholar]
- 16. Keyak JH, Kaneko TS, Tehranzadeh J, Skinner HB. 2005. Predicting proximal femoral strength using structural engineering models. Clin Orthop Relat Res:219–228 [DOI] [PubMed] [Google Scholar]
- 17. Kopperdahl DL, Morgan EF, Keaveny TM. 2002. Quantitative computed tomography estimates of the mechanical properties of human vertebral trabecular bone. J Orthop Res 20:801–805 [DOI] [PubMed] [Google Scholar]
- 18. Hui SL, Slemenda CW, Johnston CC., Jr 1988. Age and bone mass as predictors of fracture in a prospective study. J Clin Invest 81:1804–1809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Leib ES. 2005. Treatment of low bone mass in premenopausal women: when may it be appropriate? Curr Osteoporos Rep 3:13–18 [DOI] [PubMed] [Google Scholar]
- 20. Licata AA. 2000. “Does she or doesn't she … have osteoporosis?” The use and abuse of bone densitometry. Endocr Pract 6:336–337 [PubMed] [Google Scholar]
- 21. Lindsay R. 1994. Bone mass measurement for premenopausal women. Osteoporos Int 4:39–41 [DOI] [PubMed] [Google Scholar]