Abstract
Context:
Hyperthyroidism is associated with severe comorbidity, such as stroke, and seems to confer increased mortality. However, it is unknown whether this increased mortality is explained by hyperthyroidism per se, comorbidity, and/or genetic confounding.
Objective:
The objective of the study was to investigate whether hyperthyroidism is associated with an increased mortality and, if so, whether the association is influenced by comorbidity and/or genetic confounding.
Methods:
This was an observational cohort study using record-linkage data from nationwide Danish health registers. We identified 4850 singletons and 926 twins from same-sex pairs diagnosed with hyperthyroidism. Each case was matched with four controls for age and gender. The Charlson score was calculated from discharge diagnoses on an individual level to measure comorbidity. Cases and controls were followed up for a mean of 10 yr (range 0–31 yr), and the hazard ratio (HR) for mortality was calculated using Cox regression analyses.
Results:
In singletons there was a significantly higher mortality in individuals diagnosed with hyperthyroidism than in controls [HR 1.37; 95% confidence interval (CI) 1.30–1.46]. This persisted after adjustment for preexisting comorbidity (HR 1,28; 95% CI 1.21–1.36). In twin pairs discordant for hyperthyroidism (625 pairs), the twin with hyperthyroidism had an increased mortality compared with the corresponding cotwin (HR 1.43; 95% CI 1.09–1.88). However, this was found only in dizygotic pairs (HR 1.80; 95% CI 1.27–2.55) but not in monozygotic pairs (HR 0.95; 95% CI 0.60–1.50).
Conclusions:
Hyperthyroidism is associated with an increased mortality independent of preexisting comorbidity. The study of twin pairs discordant for hyperthyroidism suggests that genetic confounding influences the association between hyperthyroidism and mortality.
Hyperthyroidism is a common disease, with a lifetime risk of 2–5% (1). The condition affects the metabolism of every organ system and in addition to the classical symptoms hyperthyroidism has been linked with a number of potentially lethal conditions such as atrial fibrillation (2), pulmonary embolism (3), stroke, and coagulopathy (4, 5). In line with this, an association between hyperthyroidism and excess mortality has been reported in many previous studies (6–11), although there are some exceptions (12, 13). Our recent meta-analysis, based on these studies (6–13), concluded that hyperthyroidism is associated with a 21% increased risk in all-cause mortality (14). In contrast to hypothyroidism (15), there is little doubt that hyperthyroidism is linked to mortality, but a key question remains. Is it the hyperthyroid condition per se, or other factors such as comorbidity and/or genetic confounding, which cause the observed association between hyperthyroidism and mortality.
Hyperthyroidism (16, 17), cardiovascular disease (18), and stroke as well as life span demonstrate familial and to some degree individual clustering (19–21). If not adequately controlled for, preexisting comorbidity, such as cardiovascular disease and genetic factors, could hamper the interpretation of mortality data. Twin studies allow control for genetic confounding (22–24). The twin approach exploits the fact that twin individuals within each monozygotic (MZ) twin pair carry identical genes, whereas individuals within dizygotic (DZ) pairs share, on average, as ordinary siblings, 50% of their nuclear DNA. It follows that differences in mortality within MZ pairs are presumed to be due to environmental and/or stochastic factors. In contrast, the differences in mortality within DZ pairs are due to a combination of genetic and environmental factors as well as stochasticity.
In the present study, we used nationwide data from various Danish health registers to investigate whether hyperthyroidism is associated with an increased mortality and, if so, whether the association can, at least partly, be explained by comorbidity before the diagnosis of hyperthyroidism and/or by genetic confounding. In the case of the latter, we would expect to find an attenuated association between hyperthyroidism and mortality within disease discordant DZ pairs (genetic confounding is only partially controlled) and no association within disease discordant MZ pairs.
Materials and Methods
Data sources
The calendar period covered by the individual data sources are shown in Fig. 1.
Fig. 1.
Calendar period covered by different data sources.
The Danish Civil Registration System (DCRS) and the Danish Demographic Database cover information on demographics, vital status, date of death, and residence of all persons living or having lived in Denmark from 1968 and until 2008 (25). We have identified a random 5% sample of the Danish background population covering the birth cohorts 1870–2001 from the DCRS.
The Danish Twin Registry is a nationwide and population-based registry, established in 1954, and comprises nearly 75,000 twin pairs born in Denmark from 1870 until 2001 (26). All twins in the registry are ascertained independently of zygosity and disease.
The Danish National Patient Registry (DNPR) includes registrations of admissions to hospitals (both primary and secondary diagnoses) since January 1, 1977. Outpatient admissions have been registered separately since January 1, 1995 (25). All registrations are according to the International Classification of Disease (ICD). The validity of the DNPR is very high, and misclassification of hyperthyroidism has been shown to occur in less than 2% of cases (27).
The Danish National Prescription Registry (DNPrR) provides information on all prescriptions of drugs dispensed from Danish pharmacies since 1995 (25). Coding for medical products is according to the Anatomic Therapeutical Chemical (ATC) classification system. In addition to the ATC code, the DNPrR register covers information on date of dispensing, strength, and quantity (in defined daily doses). In Denmark the national health security system covers all inhabitants and partially reimburses drug expenses. Data from the DNPrR are transmitted directly from the cash register in the pharmacy and is used in the calculation (made on an individual level) of the expenses reimbursed. In addition, antithyroid drugs are sold solely as prescribed drugs. This, for our purpose, like in other Danish surveys makes the registry very valid (28).
All described databases are hosted at Statistics Denmark. The DCRS is based on a unique 10-digit personal identification number (CPR number) assigned to all persons living or having lived in Denmark. The CPR number allows record linkage between all the mentioned databases on an individual level.
Diagnosis of hyperthyroidism
Information on thyroid status was drawn from the DNPR or DNPrR (25). To be classified as having hyperthyroidism, subjects should be recorded in at least one of these registers. In the DNPR, hyperthyroidism was defined by ICD-8 codes 242.00–242.99 (1977–1994) and the ICD-10 codes E05-E05.9 (1995–2008). In the DNPrR hyperthyroidism was defined by at least two dispensed prescriptions of antithyroid medication (ATC = H03B). The date for diagnosis with hyperthyroidism, was chosen as the first date of registration with a hyperthyroid diagnosis in the DNPR or the first date of collecting antithyroid medication registered in the DNPrR. For individuals identified with hyperthyroidism both from the DNPR and DNPrR, the first of these dates was chosen.
Study population
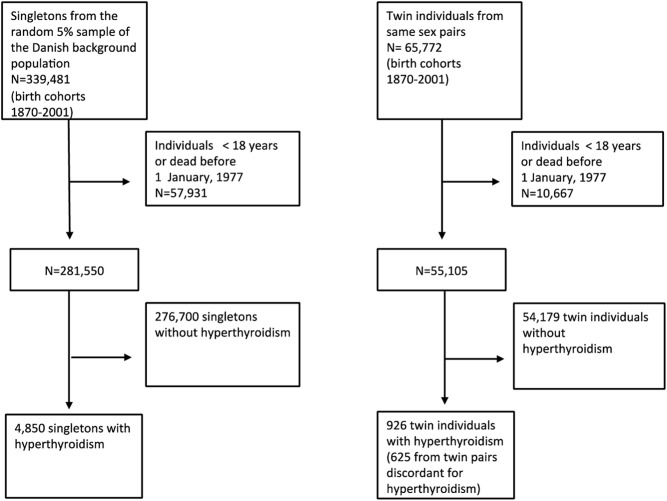
The study populations were identified from the DCRS and the Danish Twin Registry (25). Participants were ascertained as shown in Fig. 2. After excluding all individuals younger than 18 yr of age, 4850 singletons from the random 5% sample of the Danish background population and 926 twins from same-sex pairs were identified with hyperthyroidism. All participants were followed up until death or December 31, 2008.
Fig. 2.
Selection of study populations.
Mortality
Data on mortality came from the DCRS and Danish Demographic Database (25).
Comorbidity
The Charlson score (CS) accounts for 19 disease groups (myocardial infarction, heart failure, vascular disease, cerebrovascular disease, dementia, chronic lung disease, rheumatic disease, gastric ulcer, liver disease, diabetes mellitus without complications, diabetes mellitus with complications, hemiplegia, kidney disease, cancer, cancer with metastases, lymphoma, leukemia, liver failure, and AIDS) by creating a weighted score on an individual level to optimize the prediction of the 1-yr mortality risk within each disease category (29). The CS was originally constructed to estimate 1-yr mortality in patients with breast cancer but has subsequently been validated and used in different phenotypes including nonmalignant diseases (30, 31). As an example, myocardial infarction equals 1 point in the CS, whereas diabetes with complications equals 2 points. The 1-yr mortality rates have been estimated to be 12, 26, 52, and 85% for a CS of 0, 1–2, 3–4, and 5 or more, respectively (29). In both cases and controls, the CS was calculated from individual records in the DNPR using relevant ICD codes and therefore covers all inpatient and outpatient treatments in a hospital setting.
Data analysis
Group frequencies were compared with the Pearson χ2 test, whereas group means and medians were compared by a t test and the Mann-Whitney test, respectively. In the case of paired comparisons, the paired t test was used.
The relationship between hyperthyroidism and mortality was evaluated by the Cox regression model. Age was chosen as the underlying time variable. In both cases and controls, person-years of follow-up were accumulated from the date of the diagnosis of hyperthyroidism in the case and were terminated at the date of death or end of follow-up (December 31, 2008), whichever came first. In the analysis of the random 5% sample of the background population, four control subjects were randomly selected for each case, after the principles of density sampling, and matched for age and gender (32). To evaluate whether the twin population was representative of the singletons in the random 5% sample of the background population, we also performed Cox regression analyses by considering the population of twins as single individuals. In these analyses the hyperthyroid twin was matched with four unrelated nonhyperthyroid twins with respect to age, gender, and zygosity. To explore the impact of possible genetic confounding, we additionally performed intrapair analyses, in which the twin with hyperthyroidism was matched with the euthyroid cotwin (discordant twin pairs). In all analyses the variable pair was used as a stratum variable, fixing the baseline hazard within a matched pair, while at the same time allowing this baseline hazard to vary freely between pairs. Subsequently, all analyses were adjusted for the degree of comorbidity preceding the diagnosis of hyperthyroidism using the CS. For subjects with hyperthyroidism, the CS reflects the time period from the first of January 1977 (start of the DNPR) until the date of diagnosis of hyperthyroidism. In controls, the CS covers the time period from the start of the DNPR until the date of the diagnosis of hyperthyroidism in the corresponding case.
Significant differences were defined as a P < 0.05 using two-tailed tests. All analyses were conducted using STATA version 11.0 (2009; Stata Corp., College Station, TX).
Results
Basic characteristics of the study population
Characteristics of the cases from the random 5% sample of the background population as well as the cases from the twin cohort are presented in Table 1. There was less than 1-yr difference in the age of twins and singletons, with the twins being younger. There was no significant age difference between MZ and DZ twins (69 vs. 69 yr, P = 0.91). There was no difference in gender distribution and comorbidity between the two study populations (singleton and twin cases). However, as expected, subjects with hyperthyroidism had significantly higher comorbidity than the controls (data not shown).
Table 1.
Basic characteristics of the study population identified with hyperthyroidism
| Random 5% background population | Twins from same-sex pairs | P value | |
|---|---|---|---|
| Number | 4,850 | 926 | |
| Mean age (yr) | 70 | 69 | 0.09 |
| Female (%) | 82.6 | 83.0 | 0.75 |
| Age at diagnosis of hyperthyroidism (yr) | 60 | 59 | 0.00 |
| Study time prevalence of hyperthyroidism recorded from DNPR | 3,493 of 281,550 (1.2%) | 674 of 55,105 (1.2%) | 0.73 |
| Study time prevalence of hyperthyroidism recorded from DNPrR | 2,983 of 281,550 (1.1%) | 571 of 55,105 (1.0%) | 0.62 |
| Study time prevalence of hyperthyroidism recorded from DNPR or DNPrR | 4,850 of 281,550 (1.7%) | 926 of 55,105 (1.7%) | 0.48 |
| Number of cases with a CS of 0 | 2,065 (42.6%) | 413 (44.6%) | 0.25 |
A total of 4,850 singletons from the background population and 926 twin individuals from same-sex pairs were identified with hyperthyroidism, reflecting a prevalence of 1.7% (4,850 of 281,550) and 1.7% (926 of 55,105), respectively (P = 0.48). The majority of singleton cases (3493 of 4850) as well as twin cases (674 of 926) were identified from the DNPR. About one third of the singletons (1626 of 4850) and twins (319 of 926) were registered in both the DNPR and the DNPrR. Two thousand sixty-five of 4850 of the singleton cases and 413 of 926 of the twin cases had no measured comorbidity based on the CS. The mean follow-up time was 10.5 yr in twins (range 0–32) and 10 yr in singletons (range 0–32).
Standard Cox regression analyses
In singletons from the random 5% sample of the Danish background population, individuals with hyperthyroidism had an increased mortality compared with the control individuals, as reflected by a hazard ratio (HR) of 1.37 [95% confidence interval (CI) 1.30–1.46]. As evident from Table 2, stratification for gender and adjustment for comorbidity did not significantly change this finding. In the twin population, analyzed as singletons, we found essentially similar results (HR 1.35; 95% CI 1.20–1.52).
Table 2.
Hazard ratio of mortality in singletons with hyperthyroidism
| Study population | Sex | Number |
HR |
||
|---|---|---|---|---|---|
| Hyperthyroidism | Controls | Nonadjusted | Adjusteda | ||
| Random 5% sample of the background population | All | 4,850 | 19,400b | 1.37 (1.30–1.46) | 1.28 (1.21–1.36) |
| Male | 843 | 3,372b | 1.31 (1.14–1.49) | 1.12 (0.98–1.29) | |
| Female | 4,007 | 1,628b | 1.39 (1.31–1.48) | 1.32 (1.24–1.41) | |
Adjusted for degree of comorbidity, prior to the diagnosis of hyperthyroidism, using the CS.29
Each case is matched with four unrelated controls with respect to age and gender.
In the singleton population, the risk estimates were smaller for cases ascertained from the DNPrR compared with the cases identified from the DNPR (HR 1.09, 95% CI 1.01–1.18, and HR 1.29, 95% CI 1.21–1.32, respectively). This picture remained after adjustment for the degree of comorbidity (HR 1.05, 95% CI 0.97–1.14, and HR 1.17, 95% CI 1.10–1.24 when ascertained from the DNPrR and the DNPR, respectively). Differentiating between individuals diagnosed before or after January 1, 1995 (outpatient treatment has been registered since that date), in the DNPR did not change the overall findings of an increased mortality in subjects with hyperthyroidism (HR1.27, 95% CI 1.17–1.37, and HR 1.33, 95% CI 1.19–1.48, respectively).
To evaluate the impact of comorbidity on the observed associations between hyperthyroidism and mortality, we reanalyzed data stratified for comorbidity. When restricting the analyses to those subjects without comorbidity (defined as a CS = 0), hyperthyroidism remained associated with an increased mortality among the singletons (HR 1.20; 95% CI 1.12–1.31).
Intrapair Cox regression analyses
The risk estimates from the intrapair analyses of 625 (207 MZ and 418 DZ) twins from same-sex pairs, discordant for hyperthyroidism, are presented in Table 3. Overall, irrespective of gender and zygosity, the twin with hyperthyroidism had an increased mortality compared with the corresponding euthyroid cotwin (HR 1.43; 95% CI 1.09–1.88). Mortality was significantly increased in the DZ twins affected with hyperthyroidism as compared with the respective healthy cotwin (HR 1.80; 95% CI 1.27–2.55). Stratification for gender did not change the overall result for DZ females (HR 1.88; 95% CI 1.28–2.75) and for DZ males (HR 1.44; 95% CI 0.62–3.38). In contrast, the association between hyperthyroidism and mortality disappeared within MZ twin pairs discordant for hyperthyroidism (HR 0.95; 95% CI 0.60–1.50).
Table 3.
Hazard ratio of mortality in twin pairs discordant for hyperthyroidism
| Zygosity | Number of twin pairs | Hazard Ratio |
|---|---|---|
| DZ and MZ pairs | 625 | 1.43 (1.09–1.88) |
| DZ pairs | 418 | 1.80 (1.27–2.55) |
| MZ pairs | 207 | 0.95 (0.60–1.50) |
When restricting the analyses to twin pairs concordant for no comorbidity (defined as a CS = 0) before the diagnosis of hyperthyroidism, the twin with hyperthyroidism had a nonsignificantly higher mortality risk (HR 1.28; 95% CI 0.84–1.93). After stratification for zygosity, hyperthyroidism remained associated with an increased mortality in DZ pairs (HR 1.86; 95% CI 1.11–3.13) but not in MZ twin pairs (HR 0.56; 95% CI 0.26–1.20).
Discussion
A number of studies have demonstrated a relationship between hyperthyroidism and mortality (6–11, 14). In the present study, besides replicating these findings in a population-based nationwide study, our focus was to explore whether such an association might be confounded by preexisting comorbidity and/or genetic factors. To verify previous findings, we first examined the association between hyperthyroidism and mortality in a random 5% sample of the Danish background population and in a population-based cohort of Danish twins. In this part of our study, the twins were analyzed as an ordinary cohort of singletons. Both in singletons and in the twin cohort, we found a significant association between hyperthyroidism and mortality, which is of the same magnitude, a 21% increased mortality, as demonstrated in our recent meta-analysis based on the pooled data from seven previous studies (14).
Comorbidity, especially cardiovascular disease, has a major impact on mortality or may even facilitate the development of hyperthyroidism due to treatment with iodine containing drugs. However, available studies are inconsistent when controlling for comorbidity. Some studies have controlled for cardiovascular diseases (9, 12), some for diabetes (9, 12, 13), and some control for cancer (6). In the present study, we were able to obtain more extensive coverage of comorbidity from discharge diagnoses. Ours is thus the first study to use a standardized and validated scale to measure comorbidity and thereby allows estimation of the independent association of hyperthyroidism and mortality. Surprisingly, we found that preexisting comorbidity had only a minor impact on the overall association between mortality and hyperthyroidism. The fact that subjects without comorbidity before the diagnosis of hyperthyroidism had a significantly increased risk of mortality indicates a direct association between hyperthyroidism and mortality.
To evaluate whether the association between hyperthyroidism and mortality was causal or could be explained by the presence of genetic confounding, we additionally analyzed the association between hyperthyroidism and mortality within twin pairs discordant for hyperthyroidism. In these pairs the twin with hyperthyroidism had an increased risk of mortality compared with the corresponding nonhyperthyroid cotwin, indicating an independent association between hyperthyroidism and mortality. However, zygosity had a major impact on the result. The association between hyperthyroidism and mortality was significant within the DZ pairs, whereas the relationship vanished within MZ pairs. According to the well-accepted and classical interpretation of twin data, our findings suggest that genetic factors may totally account for the apparent association between hyperthyroidism and mortality (22). However, the confidence interval for MZ pairs contains the overall estimates from the singletons analyses, and consequently, there is no significant difference between these estimates. In addition, the estimates even increased within DZ pairs in comparison with the singleton population, whereas the lower values were to be expected if genetic confounding is present. Therefore, we cautiously interpret our findings as being in line, with the association between hyperthyroidism and mortality being explained, at least to some degree, by genetic factors. Although specific genes that might affect both the development of hyperthyroidism and mortality have not been identified, it is likely that a wide range of genes play a role in both pathways. As an example, genetic confounding has recently been suggested in the association between obesity and mortality (33). In this case a variant of the FTO gene could be an example of a potentially interesting genetic component because it is a common genotype both linked to obesity and type 2 diabetes mellitus (34).
Intuitively, more severe conditions of hyperthyroidism should be linked with a worse prognosis, in this case a higher mortality. Indeed, severe hyperthyroidism, as seen in thyrotoxic crisis, can be lethal (35). Two meta-analyses have shown an increased risk of mortality associated with subclinical hyperthyroidism as well (36, 37). However, at least in subclinical hyperthyroidism, the risk of mortality does not increase with declining TSH levels (37). Based on the above facts and the lack of systematic evaluation, it is unclarified whether there is a relationship between the severity of hyperthyroidism and mortality (14). However, assuming that hyperthyroid patients seen at a hospital have more severe disease compared with the group of nonreferred hyperthyroid patients, the existing literature indicates a possible dose-response relationship. As shown by Flynn et al. (12), there was no increased mortality in patients treated in primary care. In contrast, Goldman et al. (6) found a 40–50% increase in mortality in patients treated at a hospital. In support, our data show a higher risk estimate for mortality when using data from the DNPR, which represents treatment at hospitals than when using data from the DNPrR, which represents patients treated in primary care. However, after adjustment for the degree of comorbidity, the association in the DNPrR population attenuated, indicating that both the higher burden of comorbidity in patients treated at a hospital but also the severity of the disease explain the higher risk in patients treated at a hospital.
Although the validity of the DNPR is high, the criteria for diagnosis of hyperthyroidism have changed considerably during the period of study. However, this is true for any study and not unique to ours. Moreover, the fact that outpatient treatment has been recorded in the DNPR only since 1995 implies that we cannot rule out that there are differences in the prognosis of outpatients and inpatients, due to either more pronounced hyperthyroidism or increased risk of comorbidity. However, the lack of difference in the risk estimates when comparing individuals ascertained before or after outpatient treatment was registered in the DNPR suggests that this kind of selection bias is not critical.
The strengths of our study include a large sample size, ascertainment of participants from nationwide population-based registers, use of standardized and validated procedures for evaluating the degree of comorbidity, and a relatively long period of follow-up. In addition, our study is very robust because the twin design allows optimal control for genetic factors affecting both hyperthyroidism and mortality. On the other hand, the lack of information regarding the cause of hyperthyroidism, type of therapy, effect of treatment, lack of biochemical data, and cause of death constitute, in line with most other studies, weaknesses in the present study. Even had such data been available, patients would not have been randomized to divergent therapy options but have been assigned this based on, for example, the severity of disease and patient/physician preference. Therefore, differences in mortality would not necessarily pertain to differences in type of therapy but to a multitude of known and unknown parameters. Importantly, it seems counterintuitive that patients are left untreated. Therefore, we assume that all individuals are treated for hyperthyroidism. The fact that others have compared different treatment modalities like radioiodine and surgery (38) and found no difference in mortality in relation to the mode of therapy suggests that lacking such information is not crucial to our overall conclusions. Although the diagnosis of hyperthyroidism based on data from the DNPR and DNPrR has been shown to be robust, we cannot entirely rule out the possibility that our sample includes a few individuals with subclinical hyperthyroidism (due to lack of biochemical data). However, this misclassification ought to affect cases and controls alike and thus most likely does not affect our relative risk estimates.
In conclusion, our study raises the possibility that hyperthyroidism per se may be associated with increased mortality, independent of preexisting comorbidity. However, data based on the study of same-sex twins discordant for hyperthyroidism indicate that the association between hyperthyroidism and mortality is influenced by shared genetic determinants and may be entirely due to such genetic factors. Future studies, accepting that such data are not easy to obtain, should focus on the importance of subtype and severity of hyperthyroidism, treatment options for hyperthyroidism, and cause of death for the association of hyperthyroidism and mortality.
Acknowledgments
This work was supported by Grant P01 AG08761 from the National Institute on Aging.
Disclosure Summary: D.A., K.C., A.G., and T.H.B. have nothing to declare. F.B. is enrolled as a P.h.D. student financed by The School of Endocrinology, University of Southern Denmark in Odense. He has also received funding from the Danish Thyroid Patient Organization. L.H. is the recipient of an unrestricted research grant from the Novo Nordisk Foundation.
Footnotes
- ATC
- Anatomic Therapeutical Chemical
- CI
- confidence interval
- CS
- The Charlson score
- DCRS
- The Danish Civil Registration System
- DNPR
- The Danish National Patient Registry
- DNPrR
- The Danish National Prescription Registry
- DZ
- dizygotic twins from same sex pairs
- HR
- hazard ratio
- ICD
- International Classification of Disease
- MZ
- monozygotic twins.
References
- 1. Bülow Pedersen I, Laurberg P, Knudsen N, Jørgensen T, Perrild H, Ovesen L, Rasmussen LB. 2006. Increase in incidence of hyperthyroidism predominantly occurs in young people after iodine fortification of salt in Denmark. J Clin Endocrinol Metab 91:3830–3834 [DOI] [PubMed] [Google Scholar]
- 2. Klein I, Danzi S. 2007. Thyroid disease and the heart. Circulation 116:1725–1735 [DOI] [PubMed] [Google Scholar]
- 3. Lin HC, Yang LY, Kang JH. 2010. Increased risk of pulmonary embolism among patients with hyperthyroidism: a 5-year follow-up study. J Thromb Haemost 8:2176–2181 [DOI] [PubMed] [Google Scholar]
- 4. Sheu JJ, Kang JH, Lin HC, Lin HC. 2010. Hyperthyroidism and risk of ischemic stroke in young adults: a 5-year follow-up study. Stroke 41:961–966 [DOI] [PubMed] [Google Scholar]
- 5. Erem C, Ucuncu O, Yilmaz M, Kocak M, Nuhoglu İ, Ersoz HO. 2010. Increased thrombin-activatable fibrinolysis inhibitor and decreased tissue factor pathway inhibitor in patients with hyperthyroidism. Endocrine 36:473–478 [DOI] [PubMed] [Google Scholar]
- 6. Goldman MB, Monson RR, Maloof F. 1990. Cancer mortality in women with thyroid disease. Cancer Res 50:2283–2289 [PubMed] [Google Scholar]
- 7. Metso S, Jaatinen P, Huhtala H, Auvinen A, Oksala H, Salmi J. 2007. Increased cardiovascular and cancer mortality after radioiodine treatment for hyperthyroidism. J Clin Endocrinol Metab 92:2190–2196 [DOI] [PubMed] [Google Scholar]
- 8. Hall P, Lundell G, Holm LE. 1993. Mortality in patients treated for hyperthyroidism with iodine-131. Acta Endocrinol (Copenh) 128:230–234 [DOI] [PubMed] [Google Scholar]
- 9. Osman F, Franklyn JA, Holder RL, Sheppard MC, Gammage MD. 2007. Cardiovascular manifestations of hyperthyroidism before and after antithyroid therapy: a matched case-control study. J Am Coll Cardiol 49:71–81 [DOI] [PubMed] [Google Scholar]
- 10. Bauer DC, Rodondi N, Stone KL, Hillier TA. 2007. Thyroid hormone use, hyperthyroidism and mortality in older women. Am J Med 120:343–349 [DOI] [PubMed] [Google Scholar]
- 11. Franklyn JA, Maisonneuve P, Sheppard MC, Betteridge J, Boyle P. 1998. Mortality after the treatment of hyperthyroidism with radioactive iodine. N Engl J Med 338:712–718 [DOI] [PubMed] [Google Scholar]
- 12. Flynn RW, Macdonald TM, Jung TR, Morris AD, lease GP. 2006. Mortality and vascular outcomes in patients treated for thyroid dysfunction. J Clin Endocrinol Metab 91:2159–2164 [DOI] [PubMed] [Google Scholar]
- 13. Nyirenda MJ, Clark DN, Finlaysen AR, Read J, Elders A, Bain M, Fox KA, Toft AD. 2005. Thyroid disease and increased cardiovascular risk. Thyroid 15:718–725 [DOI] [PubMed] [Google Scholar]
- 14. Brandt F, Green A, Hegedüs L, Brix TH. 2011. A critical review and meta-analysis of the association between overt hyperthyroidism and mortality. Eur J Endocrinol 165:491–497 [DOI] [PubMed] [Google Scholar]
- 15. Thvilum M, Brandt F, Brix TH, Hegedüs L. 2012. A review of the evidence for and against increased mortality in hypothyroidism. Nat Rev Endocrinol 8:417–424 [DOI] [PubMed] [Google Scholar]
- 16. Brix TH, Kyvik KO, Hegedüs L. 1998. What is the evidence of genetic factors in the etiology of Graves' disease? A brief review. Thyroid 8:727–734 [DOI] [PubMed] [Google Scholar]
- 17. Brix TH, Kyvik KO, Christensen K, Hegedüs L. 2001. Evidence for a major role of heredity in Graves' disease: a population-based study of two Danish twin cohorts. J Clin Endocrinol Metab 86:930–934 [DOI] [PubMed] [Google Scholar]
- 18. Acton RT, Go RC, Roseman JM. 2004. Genetics and cardiovascular disease. Ethn Dis 14:S2–S16 [PubMed] [Google Scholar]
- 19. Bak S, Gaist D, Sindrup SH, Skytthe A, Christensen K. 2002. Genetic liability in stroke: a long-term follow-up study of Danish twins. Stroke 33:769–774 [DOI] [PubMed] [Google Scholar]
- 20. Herskind AM, McGue M, Holm NV, Sørensen TI, Harvald B, Vauoel JW. 1996. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet 97:319–323 [DOI] [PubMed] [Google Scholar]
- 21. Skytthe A, Pedersen NL, Kaprio J, Stazi MA, Hjelmborg JV, Iachine I, Vaupel JW, Christensen K. 2003. Longevity studies in GenomEUtwin. Twin Res 6:448–454 [DOI] [PubMed] [Google Scholar]
- 22. McGue M, Osler M, Christensen K. 2010. Causal inference and observational research: the utility of twins. Persepect Psychol Sci 5:546–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Brix TH, Hegedüs L. 2012. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf) 76:457–464 [DOI] [PubMed] [Google Scholar]
- 24. Brix TH, Hegedüs L. 2011. Twins as a tool for evaluating the influence of genetic susceptibility in thyroid autoimmunity. Ann Endocrinol (Paris) 72:103–107 [DOI] [PubMed] [Google Scholar]
- 25. Thygesen LC, Daasnes C, Thaulow I, Brønnum-Hansen H. 2011. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation and archiving. Scand J Public Health 39:12–16 [DOI] [PubMed] [Google Scholar]
- 26. Skytthe A, Kyvik KO, Holm NV, Christensen K. 2011. The Danish Twin Registry. Scand J Public Health 39:75–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Thomsen AF, Kvist TK, Andersen PK, Kessing LV. 2005. Increased risk of affective disorder following hospitalisation with hyperthyroidism—a register based study. Eur J Endocrinol 152:535–543 [DOI] [PubMed] [Google Scholar]
- 28. Cerqueira C, Knudsen N, Ovesen L, Perrild H, Rasmussen LB, Laurberg P, Jørgensen T. 2009. Association of iodine fortification with incident use of antithyroid medication—a Danish nationwide study. J Clin Endocrinol Metab 94:2400–2405 [DOI] [PubMed] [Google Scholar]
- 29. Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383 [DOI] [PubMed] [Google Scholar]
- 30. Albertsen PC, Moore DF, Shih W, Lin Y, Li H, Lu-Yao GL. 2011. Impact of comorbidity on survival among men with localized prostate cancer. J Clin Oncol 29:1335–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Almagro P, Salvadó M, Garcia-Vidal C, Rodríguez-Carballeira M, Cuchi E, Torres J, Ll Heredia J. 2012. Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration 84:36–43 [DOI] [PubMed] [Google Scholar]
- 32. Rothman XJ, Greenland S, Lash TL. 2008. Modern epidemiology: case control studies. Philadelphia: Lippincott Williams, Wilkins [Google Scholar]
- 33. Carlsson S, Andersson T, de Faire U, Lichtenstein P, Michaëlsson K, Ahlbom A. 2011. Body mass index and mortality: is the association explained by genetic factors? Epidemiology 22:98–103 [DOI] [PubMed] [Google Scholar]
- 34. Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, Perry JR, Elliott KS, Lango H, Rayner NW, Shields B, Harries LW, Barrett JC, Ellard S, Groves CJ, Knight B, Patch AM, Ness AR, Ebrahim S, Lawlor DA, Ring SM, Ben-Shlomo Y, Jarvelin MR, Sovio U, Bennett AJ, Melzer D, Ferrucci L, Loos RJ, Barroso I, Wareham NJ, Karpe F, Owen KR, Cardon LR, Walker M, Hitman GA, Palmer CN, Doney AS, Morris AD, Smith GD, Hattersley AT, McCarthy MI. 2007. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science 316:889–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burch HB, Wartofsky L. 1993. Life-threatening thyrotoxicosis. Thyroid storm. Endocrinol Metab Clin North Am 22:263–277 [PubMed] [Google Scholar]
- 36. Yang LB, Jiang DQ, Qi WB, Zhang T, Feng YL, Gao L, Zhao J. 2012. Subclinical hyperthyroidism and the risk of cardiovascular events and all-cause mortality: an updated meta-analysis of cohort studies. Eur J Endocrinol 167:75–84 [DOI] [PubMed] [Google Scholar]
- 37. Collet TH, Jacobijn G, Bauer DC, den Elzen WPJ, Cappola AR, Balmer P, Iervasi G, Åsvold BO, Sgarbi JA, Völzke H, Gencer B, Maciel RMB, Molinaro S, Bremner A, Luben RN, Maisonneuve P, Cornuz J, Newman AB, Khaw KT, Westendrop RGJ, Franklyn JA, Vittinghoff E, Walsh JP, Rodondi N. 2012. Subclinical Hyperthyroidism and the Risk of Coronary Heart Disease and Mortality. Arch Intern Med 172:799–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hoffman DA, McConahey WM, Diamond EL, Kurland LT. 1982. Mortality in women treated for hyperthyroidism. Am J Epidemiol 115:243–254 [DOI] [PubMed] [Google Scholar]