Abstract
Context:
In longitudinal studies of adults, elevated amino acid (AA) concentrations predicted future type 2 diabetes mellitus (T2DM).
Objective:
The aim of the present investigation was to examine whether increased plasma AA concentrations are associated with impaired β-cell function relative to insulin sensitivity [i.e. disposition index (DI)], a predictor of T2DM development.
Design, Setting, and Participants:
Metabolomic analysis for fasting plasma AAs was performed by tandem mass spectrometry in 139 normal-weight and obese adolescents with and without dysglycemia. First-phase insulin secretion was evaluated by a hyperglycemic (∼225 mg/dl) clamp and insulin sensitivity by a hyperinsulinemic-euglycemic clamp. DI was calculated as the product of first-phase insulin and insulin sensitivity.
Results:
DI was positively associated with branched-chain AAs (leucine/isoleucine and valine; r = 0.27 and 0.29, P = 0.001), neutrally transported AAs (phenylalanine and methionine; r = 0.30 and 0.35, P < 0.001), basic AAs (histidine and arginine; r = 0.28 and 0.23, P ≤ 0.007), serine (r = 0.35, P < 0.001), glycine (r = 0.26, P = 0.002), and branched-chain AAs-derived intermediates C3, C4, and C5 acylcarnitine (range r = 0.18–0.19, P ≤ 0.04).
Conclusion:
In youth, increased plasma AA concentrations are not associated with a heightened metabolic risk profile for T2DM; rather, they are positively associated with β-cell function relative to insulin sensitivity. These contrasting observations between adults and youth may be a reflection of developmental differences along the lifespan dependent on the combined impact of the aging process together with the impact of progressive obesity.
Obesity is associated with insulin resistance and hyperinsulinemia and is a major risk factor for type 2 diabetes mellitus (T2DM). Studies examining markers to predict future development of T2DM have used comprehensive metabolite profiling to identify perturbations in amino acid (AA) metabolism in obese and T2DM populations (1–3). These perturbations in AA metabolism are expressed as elevated blood concentrations of specific classes of AAs that are associated with the early manifestation of insulin resistance and T2DM. Specifically, strong negative associations between insulin sensitivity (IS) and the branched-chain AAs (BCAAs) (leucine, isoleucine, and valine) and the aromatic AAs (AAAs) (phenylalanine and tyrosine) are reported in adults (1–3). On the other hand, AAs act as direct insulin secretagogues (4), and chronic overexposure to elevated AAs enhances the expression of genes associated with insulin secretion in the β-cell (5). Recent longitudinal studies in adults demonstrated that high levels of circulating BCAAs and AAAs are predictive of the future development of T2DM (6). Based on such observations, it is speculated that high circulating AAs may have a pathophysiological role in T2DM (2). However, most studies are associative/correlative, and the field remains uncertain except that insulin sensitivity and diabetes impact AA metabolism (7). In youth, contrary to adults (1–3), we demonstrated that circulating AAs correlate positively, not negatively, with IS (8). Because both IS and β-cell function play a major role in the pathophysiology of T2DM (9, 10), we aimed to examine whether there is a negative relationship between β-cell function and AAs.
The disposition index (DI), a measure of β-cell function relative to IS, predicts the development of T2DM in longitudinal studies of adults (11). In cross-sectional studies, we have demonstrated significant impairment in DI in obese youth across the spectrum of glucose tolerance (10, 12–14). Furthermore, in obese adolescents, DI was shown to predict 2-h glucose after 2 yr and the development of dysglycemia (15). Therefore, the goal of the present investigation was to examine the relationships between β-cell function relative to IS, DI, and plasma AA concentrations in youth across the glucose tolerance spectrum.
Subjects and Methods
Subjects
The study sample consisted of 139 adolescents, 38 normal-weight (NW) [body mass index (BMI) < 85th percentile), and 101 obese (BMI ≥ 95th percentile). All participants were documented to be in good health, except for diabetes, by history, physical examination, and routine hematological and biochemical tests. Thirty-eight NW and 57 obese participants were classified to have normoglycemia [defined as a glycated hemoglobin (HbA1c) < 5.7% (n = 84), fasting glucose <100 mg/dl (n = 95), and/or normal glucose tolerance during an oral glucose tolerance test (n = 9)] and 44 obese youth to have dysglycemia [21 with impaired fasting glucose (≥100 mg/dl), six with impaired glucose tolerance (≥140 mg/dl), and 17 with T2DM]. The 17 T2DM youth, included in the dysglycemia group, were recruited from the Diabetes Center at Children's Hospital of Pittsburgh. Ten T2DM youth were treated with metformin plus lifestyle intervention, metformin plus insulin (n = 1), or insulin alone (n = 2), and four were treated with lifestyle alone. Metformin and long-acting insulin were discontinued 48 h before metabolic studies as detailed before (16). Nondiabetic youth were recruited through fliers posted on bus routes, on the university campus, in children's recreational areas, and through newspaper advertisements. Nondiabetic youth were excluded if they were taking medications known to affect substrate metabolism. Some participants were reported previously (8, 16). The study was approved by the University of Pittsburgh Institutional Review Board, and parental consent and participant assent were obtained before participation. Pubertal development was assessed using Tanner criteria (17).
Clamp studies
Subjects were admitted to the Pediatric Clinical and Translational Research Center twice within a 1- to 4-wk period to undergo a hyperglycemic clamp for assessment of insulin secretion and a hyperinsulinemic-euglycemic clamp for assessment of IS, in random order. Clamps were performed the morning after Pediatric Clinical and Translational Research Center admission after a 10- to 12-h overnight fast.
Hyperglycemic clamp
In vivo first-phase insulin secretion was evaluated during a 2-h hyperglycemic clamp as previously described (18). In brief, at time zero, plasma glucose was increased rapidly to 225 mg/dl with a bolus infusion of dextrose over 2 min and maintained by a variable-rate infusion of 20% dextrose solution for 120 min. Insulin concentrations were measured every 2.5 min from 2.5–12.5 min for determination of first-phase insulin secretion.
Hyperinsulinemic-euglycemic clamp
In vivo IS was evaluated during a 3-h hyperinsulinemic-euglycemic clamp as previously reported (14, 18). Intravenous insulin (Humulin; Lilly, Indianapolis, IN) was infused at a rate of 80 mU/m2 · min in obese individuals to suppress hepatic glucose production, and 40 mU/m2 · min in NW participants, and plasma glucose was clamped at 100 mg/dl (5.5 mmol/liter) with a variable-rate infusion of 20% dextrose based on arterialized plasma glucose determinations every 5 min.
Amino acid profiling
Archived fasting plasma samples (−80 C) were assessed for AA profiles and analyzed using tandem mass spectrometry of butyl derivatives as reported by us (8).
Body composition and abdominal adiposity
Dual-energy x-ray absorptiometry (DEXA) was used to determine fat mass, fat-free mass, and percent body fat. Subcutaneous and visceral adiposity were assessed using computed tomography at L4–L5 as reported (8). Data are not available for subjects whose weight/size exceeded the limit for DEXA (n = 13) or computed tomography scan (n = 8).
Biochemical measurements
Plasma glucose was determined with a glucose analyzer (Yellow Springs Instrument Co., Yellow Springs, OH) and insulin by commercially available RIA (Linco, St. Charles, MO; catalog item 1011) as before (8).
Calculations
In vivo IS was calculated by dividing insulin-stimulated glucose metabolism over the last 30 min of the clamp by the steady-state plasma insulin concentration, as reported (18). First-phase insulin was calculated as the mean of five insulin determinations every 2.5 min for the first 12.5 min of the clamp (18). The DI was calculated as the product of first-phase insulin and IS (13).
Statistical analyses
Data are presented as mean ± se. Statistical significance was set at P < 0.05. The statistical analyses were performed using PASW Statistics (version 18; SPSS Inc., Chicago, IL). Parametric or nonparametric statistics were applied when appropriate. ANOVA with Bonferroni post hoc correction for quantitative variables and χ2 test for categorical variables were used to examine subject characteristics among the three groups. Pearson correlations were used to determine the associations between plasma AAs (leucine/isoleucine, valine, methionine, alanine, serine, glycine, histidine, arginine, phenylalanine, citrulline, and tyrosine) and AA-associated acylcarnitines species (C3, C4, and C5 acylcarnitines) with DI. Partial correlations between AAs and DI were used to adjust for age, sex, race, Tanner stage, and BMI.
Results
Subject characteristics (Table 1)
Table 1.
Participants' physical and metabolic characteristics
| NW | Obese normoglycemic | Obese dysglycemic | P ANOVA | NW vs. obese normoglycemic | NW vs. obese dysglycemic | Obese normoglycemic vs. dysglycemic | |
|---|---|---|---|---|---|---|---|
| n | 38 | 57 | 44 | ||||
| Age (yr) | 13.0 ± 0.2 | 13.2 ± 0.2 | 14.1 ± 0.3 | 0.004 | NS | 0.007 | 0.018 |
| Sex (male/female) (%) | 50/50 | 54.4/45.6 | 34.1/65.9 | 0.04 | |||
| Race (Black/White) (%) | 42.1/57.9 | 50.9/49.1 | 52.3/47.7 | NS | |||
| Tanner stage (%) | |||||||
| II | 26.3 | 28.1 | 4.6 | 0.002 | |||
| III | 31.6 | 29.8 | 18.1 | ||||
| IV | 31.6 | 19.3 | 29.5 | ||||
| V | 10.5 | 22.8 | 47.7 | ||||
| BMI (kg/m2) | 18.9 ± 0.3 | 32.5 ± 0.9 | 35.5 ± 1.0 | <0.001 | <0.001 | <0.001 | NS |
| BMI percentile | 50.7 ± 4.0 | 97.0 ± 0.4 | 98.0 ± 0.4 | <0.001 | <0.001 | <0.001 | NS |
| Fat mass (kg) | 8.3 ± 0.7 | 32.1 ± 1.6 | 39.0 ± 1.8 | <0.001 | <0.001 | <0.001 | 0.036 |
| Body fat (%) | 17.8 ± 1.2 | 40.0 ± 1.2 | 43.3 ± 0.9 | <0.001 | <0.001 | <0.001 | NS |
| Visceral adipose tissue (cm2) | 16.7 ± 1.5 | 66.6 ± 5.3 | 77.1 ± 6.4 | <0.001 | <0.001 | <0.001 | NS |
| Subcutaneous adipose tissue (cm2) | 76.4 ± 8.2 | 452.1 ± 28.5 | 517.1 ± 23.2 | <0.001 | <0.001 | <0.001 | NS |
| Fasting glucose (mg/dl) | 94.8 ± 0.9 | 93.8 ± 0.5 | 113.4 ± 2.8 | <0.001 | NS | <0.001 | <0.001 |
| Fasting insulin (μU/ml) | 19.6 ± 1.4 | 34.6 ± 2.4 | 46.7 ± 5.7 | <0.001 | <0.001 | <0.001 | 0.001 |
| HbA1c (%) | 5.3 ± 0.1 | 5.2 ± 0.1 | 5.9 ± 0.1 | <0.001 | NS | <0.001 | <0.001 |
| First-phase insulin (μU/ml) | 119.0 ± 11.6 | 237.7 ± 16.0 | 159.0 ± 26.2 | <0.001 | <0.001 | NS | <0.001 |
| IS (mg/kg · min per μU/ml) | 9.8 ± 0.6 | 3.5 ± 0.4 | 1.9 ± 0.2 | <0.001 | <0.001 | <0.001 | 0.019 |
| DI (mg/kg · min) | 1038.3 ± 88.9 | 684.3 ± 56.2 | 299.9 ± 48.0 | <0.001 | <0.001 | <0.001 | <0.001 |
| Leucine/isoleucine (μm) | 341.2 ± 16.6 | 324.7 ± 16.0 | 264.1 ± 11.0 | 0.001 | NS | 0.002 | 0.012 |
| Phenylalanine (μm) | 64.0 ± 2.9 | 60.7 ± 2.4 | 53.5 ± 2.2 | 0.019 | NS | 0.021 | NS |
| Methionine (μm) | 46.3 ± 2.6 | 45.5 ± 1.4 | 38.0 ± 1.0 | 0.001 | NS | 0.002 | 0.004 |
| Histidine (μm) | 327.8 ± 22.5 | 283.4 ± 12.2 | 239.9 ± 11.1 | <0.001 | NS | <0.001 | NS |
| Arginine (μm) | 110.2 ± 5.0 | 99.6 ± 3.9 | 88.5 ± 3.9 | 0.004 | NS | 0.003 | NS |
| Serine (μm) | 37.0 ± 1.8 | 33.6 ± 1.9 | 27.6 ± 1.1 | 0.001 | NS | 0.001 | 0.04 |
| Glycine (μm) | 176.1 ± 11.6 | 170.6 ± 7.5 | 145.1 ± 6.6 | 0.03 | NS | NS | 0.05 |
Two subjects did not have HbA1c, 13 subjects did not have DEXA data, and eight subjects did not have visceral or sc adipose tissue data. AA concentrations were derived from archived fasting plasma samples. NS, Not significant.
As expected, obese normoglycemic and dysglycemic youth had significantly higher adiposity parameters than NW youth. Obese dysglycemic youth were slightly older with more advanced puberty, had higher fasting glucose and HbA1c, and lower IS and DI compared with the other two groups. Significant differences in AA concentrations among the groups are presented in Table 1, showing lower concentrations in general in obese dysglycemic youth compared with the other two groups.
Associations between amino acid concentrations and β-cell function
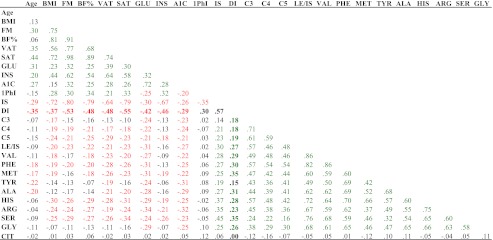
Table 2 provides a full picture of the correlation matrix between various AAs, clinical parameters, and metabolic parameters. DI correlated positively with all AAs except tyrosine and citrulline. It also correlated positively with BCAA-derived intermediates C3, C4, and C5 acylcarnitine. After adjusting for BMI, age, sex, race, and Tanner stage, the significant associations between DI and C3, C4, and C5 acylcarnitine and between IS and C4 and C5 acylcarnitine, methionine and tyrosine disappeared. None of the AAs showed negative associations with DI or IS in the total group or separately in each of the normoglycemic or the dysglycemic groups. In obese youth with normoglycemia, DI correlated with leucine/isoleucine (r = 0.30; P = 0.03), valine (r = 0.37; P = 0.004), serine (r = 0.45; P < 0.001), and phenylalanine (r = 0.28; P = 0.03). In obese youth with dysglycemia, DI correlated with C5 acylarnitine (r = 0.35; P = 0.005), methionine (r = 0.44; P = 0.002), alanine (r = 0.31; P = 0.02), glycine (r = 0.30; P = 0.03), histidine (r = 0.35; P = 0.005), and citrulline (r = 0.30; P = 0.03). In males, DI was significantly correlated with leucine/isoleucine (r = 0.26, P = 0.04), valine (r = 0.31; P = 0.01), phenylalanine (r = 0.31; P = 0.01), methionine (r = 0.48, P < 0.001), alanine (r = 0.32, P = 0.009), histidine (r = 0.31, P = 0.01), arginine (r = 0.26, P = 0.04), and serine (r = 0.40, P = 0.001). In females, DI correlated with leucine/isoleucine (r = 0.30, P = 0.01), valine (r = 0.27, P = 0.02), phenylalanine (r = 0.29, P = 0.01), alanine (r = 0.29, P = 0.01), serine (r = 0.28, P = 0.02), glycine (r = 0.28, P = 0.02), and C3 acylcarnitine (r = 0.27, P = 0.02).
Table 2.
Correlation matrix in the total group of participants, NW, obese normoglycemic, and obese dysglycemic youth
Correlation coefficients are presented. Significant associations are presented in color (P < 0.05). Red indicates negative associations. Green indicates positive associations. AA concentrations were derived from archived fasting plasma samples. A1C, Glycated hemoglobin; ALA, alanine; ARG, arginine; BF%, percent body fat; C3, C3 acylcarnitine; C4, C4 acylcarnitine; C5, C5 acylcarnitine; CIT, citrulline; FM, fat mass; GLU, fasting glucose; GLY, glycine; HIS, histidine; INS, fasting insulin; LE/IS, leucine/isoleucine; MET, methionine; PHE, phenylalanine; 1PhI, first-phase insulin; SAT, sc adipose tissue; SER, serine; TYR, tyrosine; VAL, valine; VAT, visceral adipose tissue.
Discussion
This investigation demonstrates that plasma AA concentrations are positively associated with β-cell function relative to IS in adolescents. Our findings are in contrast to the adult literature showing a positive association between elevated blood AAs and risk of T2DM (1–3, 6). This adult vs. pediatric contrast was also present in our previous publication of positive associations between IS and several AAs and acylcarnitine in youth (8) in distinction to adults (1–3, 19). The reasons behind such divergent observations remain unknown but may stem from developmental differences across the human lifespan, adolescence vs. adulthood, and the impact of aging and continued escalating obesity on metabolic risk, IS, β-cell function, and T2DM.
Metabolomic profiling of adults shows elevated plasma AA concentrations in obese compared with lean men and women (2, 3) and in lean insulin-resistant compared with lean insulin-sensitive men (1). Furthermore, strong negative associations between IS and a combination of BCAAs, the AAAs, methionine, glutamate/glutamine, and C3 and C5 acylcarnitines are observed in obese adults (with and without T2DM) and in insulin-resistant lean men (1–3, 19). Furthermore, many of these AAs are associated with a 5- to 7-fold higher risk of developing T2DM (6).
In contrast to the reported associations between elevated blood AA concentrations and development of T2DM in adults, the present study illustrates a positive relationship between AA concentrations and β-cell function relative to IS in youth, reflective of a reduced metabolic risk for T2DM. Thus, the higher the AA concentrations the better β-cell function relative to IS, a pathophysiological marker of T2DM and its prediction. These findings that plasma AAs are positively associated with β-cell function relative to IS in youth may be related to AA stimulation of insulin secretion. Indeed, several AAs (leucine, isoleucine and arginine) are recognized as direct insulin secretagogues (4). Because AAs can further regulate β-cell gene expression responsible for metabolism, β-cell's response to nutrient availability and functional integrity (5), chronic exposure, in an otherwise healthy β-cell, may cause eventual deleterious effects. Although further investigations are warranted to understand the mechanisms of these adult/youth disparities, it is plausible that chronic, low-level AA stimulation of the β-cell, with progressive obesity, induces both mitochondrial damage and altered gene expression, akin to lipotoxicity (20), ultimately resulting in β-cell failure. This time-dependent failure of β-cell function could lead to the negative relationship between AA concentrations and T2DM risk reported in adults (1–3). Alternatively, plasma AA concentrations may be a reflection of intracellular alterations in the oxidative metabolism of these AAs. In that regard, we recently demonstrated that T2DM youth have lower fatty acid-derived medium- to short-chain acylcarnitine species, consistent with enhanced, rather than reduced, mitochondrial use of these intermediates, despite having similar inputs into β-oxidation to their lean counterparts (8). Similar results were observed in obese and lean young adults (mean age 22 yr) (21). Moreover, although diabetic Zucker fatty rats initially up-regulate their mitochondrial oxidative capacity, over time, this mechanism fails to halt the transition to hyperglycemia (22). On the other hand, circulating AA concentrations may reflect reduced catabolism and thus reduced generation of the metabolites. Our current data showing overall lower AA concentrations in obese youth may be a reflection of either decreased proteolysis or increased protein synthesis potentially driven by hyperinsulinemia. Conversely, Zeng et al. (23) demonstrated similar AA concentrations in obese compared with NW prepubertal youth. However, Zeng et al. (23) support our current findings in that the adult patterns of AAs is not clearly recapitulated in youth at risk for T2DM or insulin resistance. Another interesting distinction between adults and youth is that the associations of AAs with IS, β-cell function, and T2DM in youth involve more AAs, i.e. less “selective” associations vs. what is observed in older individuals (typically BCAAs and AAAs are the clearest). The reason(s) for this remain unknown but could be speculated to be due to decreased protein degradation that is characteristic of puberty (24), a period of remarkably accelerated physiological growth in humans. Despite several unknowns that remain to be investigated, the data collectively suggest that mitochondrial adaptation may occur early in the course of obesity, lessening short-term diabetes risk. However, with the continued juxtaposition of time and aging against the backdrop of progressive obesity, mitochondrial overload likely ensues.
The strengths of this investigation are, first, lack of such studies in pediatrics or adults; second, that we included a large number of obese youth spanning the glucose tolerance spectrum; and third, the application of the gold-standard hyperglycemic clamp and hyperinsulinemic-euglycemic clamp to assess β-cell function relative to IS. Potential weaknesses are some of the missing body composition data due to participant weight/size exceeding the machine limit and the slightly older age and maturational stage of the dysglycemic group that we adjusted for in the statistics.
In summary, our results demonstrate that in youth, higher plasma AA concentrations are not associated with a heightened metabolic risk profile for T2DM; rather, they are positively associated with in vivo β-cell function relative to IS. Whether or not these positive associations, observed in childhood early in the course of obesity, disintegrate over time with aging and progressive obesity can be examined only in longitudinal studies from youth to adulthood.
Acknowledgments
Our sincere apologies to investigators whose original scientific contributions to this area were not referenced due to limited space. We express our gratitude to all the children and their parents who participated in this study, without whom science would not advance. We are grateful to the nursing staff of the Pediatric Clinical and Translational Research Center for their outstanding care of the participants and meticulous attention to the research, to Nancy Guerra (Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center) for her assistance with clamp experiments and to Resa Stauffer (Children's Hospital of Pittsburgh of University of Pittsburgh Medical Center) for her laboratory analytical contributions.
The project described was supported by the National Institutes of Health through Grants UL1 RR024153 and UL1TR000005, DK54936 (to J.V.), R01 HD-27503 (to S.A.A.), K24 HD-01357 (to S.A.A.), ROI DK-78775 (to J.V.), Richard L. Day Endowed Chair (to S.A.A.), and Department of Defense (to S.A.A., S.J.M., L.A.S., and S.L.).
S.F.M. analyzed and interpreted the data and wrote the manuscript; L.A.S. contributed to data interpretation and reviewing/editing the manuscript; S.J.M. interpreted metabolomics data and reviewed/edited the manuscript; S.L. and F.B. contributed participants and data to the research project and contributed laboratory/analytical tools; D.H.C. instituted the tandem mass spectrometry methodology and interpretation and with V.R.D.J. and S.J.M. analyzed the tandem mass spectrometry samples; J.V. provided financial support; S.A.A. provided the study concept and design, acquired data, obtained funding, provided administrative and technical and material support, supervised the study, and critically reviewed/edited the manuscript.
Disclosure Summary: None of the authors report any conflict of interest or financial interest with respect to this work. The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Footnotes
- AA
- Amino acid
- AAA
- aromatic AA
- BCAA
- branched-chain AA
- BMI
- body mass index
- DEXA
- dual-energy x-ray absorptiometry
- DI
- disposition index
- HbA1c
- glycated hemoglobin
- IS
- insulin sensitivity
- NW
- normal-weight
- T2DM
- type 2 diabetes mellitus.
References
- 1. Tai ES, Tan ML, Stevens RD, Low YL, Muehlbauer MJ, Goh DL, Ilkayeva OR, Wenner BR, Bain JR, Lee JJ, Lim SC, Khoo CM, Shah SH, Newgard CB. 2010. Insulin resistance is associated with a metabolic profile of altered protein metabolism in Chinese and Asian-Indian men. Diabetologia 53:757–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Newgard CB, An J, Bain JR, Muehlbauer MJ, Stevens RD, Lien LF, Haqq AM, Shah SH, Arlotto M, Slentz CA, Rochon J, Gallup D, Ilkayeva O, Wenner BR, Yancy WS, Jr, Eisenson H, Musante G, Surwit RS, Millington DS, Butler MD, Svetkey LP. 2009. A branched-chain amino acid-related metabolic signature that differentiates obese and lean humans and contributes to insulin resistance. Cell Metab 9:311–326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kim JY, Park JY, Kim OY, Ham BM, Kim HJ, Kwon DY, Jang Y, Lee JH. 2010. Metabolic profiling of plasma in overweight/obese and lean men using ultra performance liquid chromatography and Q-TOF mass spectrometry (UPLC-Q-TOF MS). J Proteome Res 9:4368–4375 [DOI] [PubMed] [Google Scholar]
- 4. Bolea S, Pertusa JA, Martín F, Sanchez-Andrés JV, Soria B. 1997. Regulation of pancreatic β-cell electrical activity and insulin release by physiological amino acid concentrations. Pflugers Arch 433:699–704 [DOI] [PubMed] [Google Scholar]
- 5. Newsholme P, Brennan L, Bender K. 2006. Amino acid metabolic, β-cell function, and diabetes. Diabetes 55(Suppl. 2):S39–S47 [Google Scholar]
- 6. Wang TJ, Larson MG, Vasan RS, Cheng S, Rhee EP, McCabe E, Lewis GD, Fox CS, Jacques PF, Fernandez C, O'Donnell CJ, Carr SA, Mootha VK, Florez JC, Souza A, Melander O, Clish CB, Gerszten RE. 2011. Metabolite profiles and the risk of developing diabetes. Nat Med 17:448–453 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Adams SH. 2011. Emerging perspectives on essential amino acid metabolism in obesity and the insulin-resistant state. Adv Nutr 2:445–456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Mihalik SJ, Michaliszyn SF, de las Heras J, Bacha F, Lee S, Chace DH, DeJesus VR, Vockley J, Arslanian SA. 2012. Metabolomic profiling of fatty acid and amino acid metabolism in youth with obesity and type 2 diabetes: evidence for enhanced mitochondrial oxidation. Diabetes Care 35:605–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gungor N, Bacha F, Saad R, Janosky J, Arslanian S. 2005. Youth type 2 diabetes: insulin resistance, beta-cell failure, or both? Diabetes Care 28:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Bacha F, Lee S, Gungor N, Arslanian SA. 2010. From pre-diabetes to type 2 diabetes in obese youth: pathophysiological characteristics along the spectrum of glucose dysregulation. Diabetes Care 33:2225–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lyssenko V, Almgren P, Anevski D, Perfekt R, Lahti K, Nissén M, Isomaa B, Forsen B, Homström N, Saloranta C, Taskinen MR, Groop L, Tuomi T. 2005. Predictors of and longitudinal changes in insulin sensitivity and secretion preceding onset of type 2 diabetes. Diabetes 54:166–174 [DOI] [PubMed] [Google Scholar]
- 12. Burns SF, Bacha F, Lee SJ, Tfayli H, Gungor N, Arslanian SA. 2011. Declining β-cell function relative to insulin sensitivity with escalating OGTT 2-h glucose concentrations in the nondiabetic through the diabetic range in overweight youth. Diabetes Care 34:2033–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sjaarda LG, Bacha F, Lee S, Tfayli H, Andreatta E, Arslanian S. 2012. Oral disposition index in obese youth from normal to prediabetes to diabetes: relationship to clamp disposition index. J Pediatr 161:51–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tfayli H, Lee S, Arslanian S. 2010. Declining β-cell function relative to insulin sensitivity with increasing fasting glucose levels in the nondiabetic range in children. Diabetes Care 33:2024–2030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giannini C, Weiss R, Cali A, Bonadonna R, Santoro N, Pierpont B, Shaw M, Caprio S. 2012. Evidence for early defects in insulin sensitivity and secretion before the onset of glucose dysregulation in obese youths: a longitudinal study. Diabetes 61:606–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bacha F, Gungor N, Lee S, Arslanian SA. 2009. In vivo insulin sensitivity and secretion in obese youth: what are the differences between normal glucose tolerance, impaired glucose tolerance, and type 2 diabetes? Diabetes Care 32:100–105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tanner JM. 1981. Growth and maturation during adolescence. Nutr Rev 39:43–55 [DOI] [PubMed] [Google Scholar]
- 18. Tfayli H, Bacha F, Gungor N, Arslanian S. 2009. Phenotypic type 2 diabetes in obese youth: insulin sensitivity and secretion in islet cell antibody-negative versus -positive patients. Diabetes 58:738–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mihalik SJ, Goodpaster BH, Kelley DE, Chace DH, Vockley J, Toledo FG, DeLany JP. 2010. Increased levels of plasma acylcarnitines in obesity and type 2 diabetes and identification of a marker of glucolipotoxicity. Obesity (Silver Spring) 18:1695–1700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Unger RH. 1995. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes 44:863–870 [DOI] [PubMed] [Google Scholar]
- 21. Boyle KE, Canham JP, Consitt LA, Zheng D, Koves TR, Gavin TP, Holbert D, Neufer PD, Ilkayeva O, Muoio DM, Houmard JA. 2011. A high-fat diet elicits differential responses in genes coordinating oxidative metabolism in skeletal muscle of lean and obese individuals. J Clin Endocrinol Metab 96:775–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lenaers E, De Feyter HM, Hoeks J, Schrauwen P, Schaart G, Nabben M, Nicolay K, Prompers JJ, Hesselink MK. 2010. Adaptations in mitochondrial function parallel, but fail to rescue, the transition to severe hyperglycemia and hyperinsulinemia: a study in Zucker diabetic fatty rats. Obesity (Silver Spring) 18:1100–1107 [DOI] [PubMed] [Google Scholar]
- 23. Zeng M, Liang Y, Li H, Wang M, Wang B, Chen X, Zhou N, Cao D, Wu J. 2010. Plasma metabolic fingerprinting of childhood obesity by GC/MS in conjunction with multivariate statistical analyses. J Pharm Biomed Anal 52:265–472 [DOI] [PubMed] [Google Scholar]
- 24. Arslanian SA, Kalhan SC. 1996. Protein turnover during puberty in normal children. Am J Physiol 270:E79–E84 [DOI] [PubMed] [Google Scholar]