Abstract
Context:
Vitamin D deficiency and insufficiency occur frequently in youth with HIV infection, particularly among those receiving the antiretroviral drug efavirenz. Optimal vitamin D dosing for treatment is unclear.
Objective:
Our objective was to evaluate safety and measure change in 25-hydroxyvitamin D (25-OHD) concentration from baseline to study wk 4 and 12 during treatment with vitamin D3, 50,000 IU monthly.
Design, Setting, and Participants:
We conducted a randomized double-blind, placebo-controlled multicenter trial of HIV-infected youth ages 18–24 yr, with viral load below 5000 copies/ml, on stable antiretroviral therapy.
Intervention:
Intervention included vitamin D3, 50,000 IU (n = 102), or matching placebo (n = 101) administered in three directly observed oral doses at monthly intervals.
Results:
At baseline, mean (sd) age was 20.9 (2.0) yr; 37% were female and 52% African-American, and 54% were vitamin D deficient/insufficient (25-OHD < 20 ng/ml), with no randomized group differences. Of evaluable participants vitamin D deficient/insufficient at baseline who were administered vitamin D, 43 of 46 (93%) had sufficient 25-OHD by wk 12. Vitamin D supplementation increased 25-OHD serum concentration from a baseline of 21.9 (13.3) to 35.9 (19.1) ng/ml at wk 12 (P < 0.001) with no change for placebo. Although use of the antiretroviral efavirenz was associated with lower baseline 25-OHD concentration, efavirenz did not diminish the response to vitamin D supplementation. There was no treatment-related toxicity.
Conclusions:
Supplementation with vitamin D3 50,000 IU monthly for three doses was safe. Increases in 25-OHD occurred in treated participants regardless of antiretroviral regimen.
Vitamin D deficiency is common in youth with HIV-1 infection in the United States (1, 2). Recommendations for correction of vitamin D deficiency vary widely (3–12), but vitamin D2 or D3 50,000 IU weekly for 5 (13) to 8 (14) weeks is commonly recommended. Although the only 50,000-IU preparation available by prescription from pharmacies in the United States is vitamin D2, vitamin D3 is more potent than vitamin D2 in raising serum 25-hydroxyvitamin D (25-OHD) concentrations (12, 15–18) .
The Institute of Medicine (IOM) (19, 20) suggests that a 25-OHD serum concentration of 20 ng/ml (50 nmol/liter) is adequate to meet the needs of 97.5% of the general population (21), although others suggest that higher levels [28–30 ng/ml (70–75 nmol/liter)] might be optimal for bone health (13, 22). The IOM report states that 25-OHD serum concentrations greater than 50 ng/ml (125 nmol/liter) may raise concern for potential adverse effects (19). This identifies a potential target serum concentration for 25-OHD between 20 and 30 ng/ml, not to exceed 50 ng/ml (21).
Persons with HIV infection who are treated with combination antiretroviral therapy (cART) that contains efavirenz have lower 25-OHD serum concentrations than those treated with cART that does not contain efavirenz (23–25). There are no prospective data available on the ability of vitamin D treatment to overcome the apparent suppressive effect of efavirenz on 25-OHD concentrations.
Adherence to antiretroviral regimens is difficult for adolescents and young adults with HIV (26), and daily dosing of vitamin D supplements may further threaten antiretroviral adherence, because an increase in pill burden is associated with decline in medication adherence (27). We designed this study to use a once-monthly vitamin D dosing strategy to avoid an increase in pill burden and to allow directly observed therapy.
If the goal of correction of vitamin D deficiency/insufficiency is to rapidly reach a concentration in the identified target range, the optimal dose needed to treat vitamin D deficiency/insufficiency may vary by patient, because the change in serum 25-OHD in response to supplementation is inversely proportional to the starting serum vitamin D concentration (5, 12, 28).
Our randomized trial of vitamin D supplementation in youth with HIV infection, in which we enrolled participants without regard to baseline 25-OHD serum concentration, offered the opportunity to measure the change in serum vitamin D concentration after monthly directly observed administration of vitamin D3 50,000 IU.
Materials and Methods
The Adolescent Medicine Trials Network for HIV/AIDS Intervention (ATN) study 063 was a 12-wk randomized, double-blind, placebo-controlled, multicenter study carried out between November 2007 and April 2010 at 35 ATN or International Maternal Pediatric Adolescent AIDS Clinical Trials Group clinical sites in the United States and Puerto Rico. The study was approved by the local Institutional Review Board of each participating center. Subjects were enrolled only after written informed consent was obtained. Details of the study design and primary outcomes are reported elsewhere (29).
We enrolled persons with HIV-1 infection, ages 18–24 yr, treated with unchanged cART with at least three antiretroviral drugs for at least 90 d, and with HIV-1 plasma RNA (viral load) below 5000 copies/ml within 60 d before entry. Enrollment was stratified based on inclusion of tenofovir disoproxil fumarate in the cART, because tenofovir use is associated with decreases in bone density (30–32). Participants were excluded for hypercalcemia or hypercalciuria at screening; renal diseases, current or recent pregnancy or lactation, and use of vitamin D supplements exceeding 400 IU/d. There was no exclusion criterion based on pretreatment serum 25-OHD concentration or on use of a cART regimen that included efavirenz.
Randomization and treatment
Within antiretroviral treatment groups (tenofovir or no tenofovir in the regimen), participants were randomly assigned in fixed blocks of four by gender to treatment with vitamin D3, 50,000 IU (Bio-Tech Pharmacal, Fayetteville, AR) or matching placebo gelatin capsule. Capsule content was confirmed by independent analysis before use in the study and at the time medication lots were changed. All study participants and personnel except the site pharmacist were blinded to treatment assignment, with unblinding only after prespecified data analysis. Directly observed oral treatment was administered at the study site at baseline and wk 4 and 8 for a cumulative vitamin D3 dose of 150,000 IU.
Measurements
At baseline and wk 4 and 12, the main outcome variables were measured by batch analysis of stored samples at the U.S. Department of Agriculture Agricultural Research Service, Western Human Nutrition Research Center, Davis, CA. All samples were collected after at least 4 h fasting and before administration of study medication at each visit.
Serum 25-OHD was measured by RIA (25-OHD 125I RIA kit; DiaSorin, Stillwater, MN). Vitamin D status was categorized as deficient for 25-OHD below 12 ng/ml (<30 nmol/liter), insufficient for 25-OHD from 12–20 ng/ml (30–50 nmol/liter), sufficient for 25-OHD more than 20–50 ng/ml (>50–125 nmol/liter), and excess for 25-OHD higher than 50 ng/ml (>125 nmol/liter), based on U.S. IOM 2010 criteria (20).
Serum 1,25-dihydroxyvitamin D [1,25-(OH)2D] was measured by RIA [1,25-(OH)2D 125I RIA kit; IDS, Inc., Fountain Hills, AZ], with normal range of 48–150 pmol/liter.
Urine calcium (UCa), urine creatinine (UCr), and serum calcium (SCa) were measured using standard laboratory methods. Estimated glomerular filtration rate (milliliters per minute per 1.73 m2) was calculated by the modification of diet in renal disease formula (33). The UCa to UCr ratio (milligrams per milligram) was used to monitor for hypercalciuria secondary to hypercalcemia or hypervitaminosis D, with normal no higher than 0.20 mg/mg.
Calcium and vitamin D intake from diet and supplements were measured by self-report at baseline and wk 12 using the Block Calcium/Vitamin D Screener (Nutritionquest, Berkeley, CA) (34).
Safety
Measurements of SCa and UCa/UCr were performed at baseline and every 4 wk in the clinical laboratory at each study site and monitored by the study team. For participants with SCa above the upper limit of normal or UCa/UCr higher than 0.20 mg/mg, repeat testing was required, and study drug was discontinued for those with persistent SCa above the upper limit of normal or UCa/UCr higher than 0.20 mg/mg.
Statistical considerations
Data are presented as mean (sd) or median (range) for continuous variables and frequency and percentage for categorical variables. The study was designed to have 80 evaluable subjects in each randomized arm (29). Baseline analyses in all participants for whom data were available compared cross-sectional differences between randomized groups (vitamin D vs. placebo). The primary outcome measures for this report were changes from baseline to wk 4 and 12 in 25-OHD and 1,25-(OH)2D concentrations. Measurements of change from baseline to wk 4 or 12 were based on data from fully evaluable subjects, i.e. those who completed all follow-up visits and had no missed doses of vitamin D. To determine the effect of baseline vitamin D status on the response to vitamin D supplementation, we evaluated the change in serum 25-OHD concentrations by baseline vitamin D status (grouped in IOM categories). Multivariable models were used to measure the main effects of vitamin D supplementation on change in vitamin D serum concentration and to measure the effect of potential confounding variables including baseline 25-OHD serum concentration (nanograms per milliliter), latitude of residence (<35° N, 35° to <40° N, or >40° N), season of the year at the time of the baseline visit, body weight (kilograms), race (African-American vs. other), and use of tenofovir and/or efavirenz in cART. Statistical significance of differences was determined using the Pearson χ2 (or Fisher's exact) test for categorical variables and the Wilcoxon rank sum test for continuous variables. Generalized linear models were used for the univariate and multivariable models.
Results
Of 217 subjects screened and eligible for randomization, 207 were randomized, 203 completed the baseline visit, and 169 completed 12 wk of study with fully evaluable data and no missed doses of vitamin D. The vitamin D randomized group had 102 participants at randomization (88 fully evaluable at wk 12) and 101 were randomized to placebo (81 fully evaluable at wk 12). There were no clinically important statistically significant differences between participants in the fully evaluable subset (n = 169) compared with the not-fully-evaluable subset (n = 34) except in body mass index (BMI) [25.2 (sd 6.9) vs. 27.8 (7.2) kg/m2, respectively]. Although supplemental vitamin D intake was higher in the evaluable subset compared with the not-fully-evaluable subset [376 (96) vs. 267 (200) IU, respectively], only 34 evaluable and nine not-fully-evaluable group members took supplements.
Baseline data
At baseline (Table 1), there were no statistically significant differences between randomized groups in age, sex, racial distribution, BMI, HIV-related factors including the use of tenofovir and/or efavirenz, renal function, calcium or vitamin D intake, or 25-OHD or 1,25-(OH)2D levels. In the group as a whole, a total of 54% of all participants had 25-OHD levels in the insufficient or deficient range at baseline. Daily calcium intake was inadequate for age in 74% of participants, and vitamin D intake was below the recommended level of 600 IU/d in 95%.
Table 1.
Characteristics of the study population by randomization group (vitamin D or placebo): baseline population
| Characteristic | Overall | Vitamin D | Placebo | P value |
|---|---|---|---|---|
| n | 203 | 102 | 101 | |
| Age (yr) | ||||
| Mean (sd) | 20.9 (2.0) | 20.9 (2.1) | 20.9 (1.9) | 0.803 |
| Median (range) | 21 (18–24) | 21 (18–24) | 21 (18–24) | |
| Sex | ||||
| Male | 127 (63%) | 63 (62%) | 64 (63%) | 0.813 |
| Female | 76 (37%) | 39 (38%) | 37 (37%) | |
| Race | ||||
| African-American | 106 (52%) | 52 (51%) | 54 (54%) | 0.890 |
| Caucasian | 45 (22%) | 24 (24%) | 21 (21%) | |
| Other or mixed | 52 (26%) | 26 (26%) | 26 (26%) | |
| Ethnicity | ||||
| Hispanic | 64 (32%) | 31 (31%) | 33 (33%) | 0.726 |
| BMI (kg/m2) | ||||
| Mean (sd) | 25.6 (7.0) | 26.0 (7.4) | 25.2 (6.6) | 0.628 |
| Median (range) | 23.7 (11.3–56.2) | 23.8 (16.1–56.2) | 23.6 (11.3–56.2) | |
| Lifestyle | ||||
| Exercise regularly | 116 (57%) | 61 (60%) | 55 (54%) | 0.441 |
| Trying to lose weight | 62 (31%) | 34 (33%) | 28 (28%) | 0.385 |
| Use multivitamins | 43 (21%) | 23 (23%) | 20 (20%) | 0.632 |
| Season enrolled | ||||
| Winter | 44 (22%) | 23 (23%) | 21 (21%) | 0.978 |
| Spring | 62 (31%) | 31 (30%) | 31 (31%) | |
| Summer | 53 (26%) | 27 (26%) | 26 (26%) | |
| Fall | 44 (22%) | 21 (21%) | 23 (23%) | |
| Geographic latitude | ||||
| ≤35° N | 77 (38%) | 40 (29%) | 37 (37%) | 0.415 |
| >35–40° N | 40 (20%) | 23 (23%) | 17 (17%) | |
| >40° N | 86 (42%) | 39 (38%) | 47 (47%) | |
| HIV duration (yr) | ||||
| Mean (sd) | 6.6 (6.4) | 6.7 (6.5) | 6.5 (6.3) | 0.815 |
| Median (range) | 4 (<1–22) | 4 (<1–22) | 3 (<1–21) | |
| CDC disease stage | ||||
| A | 119 (59%) | 60 (59%) | 59 (58%) | 0.974 |
| B | 35 (17%) | 17 (17%) | 18 (18%) | |
| C | 49 (24%) | 25 (25%) | 24 (24%) | |
| CD4 cell count current (cells/μl) | ||||
| Mean (sd) | 587 (246) | 562 (226) | 612 (263) | 0.210 |
| Median (range) | 543 (72–1488) | 533 (191–1488) | 559 (72–1372) | |
| Viral load, current (below quantitation limit) | 139 (68%) | 70 (69%) | 68 (68%) | 0.962 |
| Maximum ART exposure (months) | ||||
| <6 | 30 (15%) | 14 (14%) | 16 (16%) | 0.661 |
| 6 to ≤24 | 62 (31%) | 29 (28%) | 33 (33%) | |
| >24 | 111 (55%) | 59 (58%) | 52 (52%) | |
| Antiretroviralsa | ||||
| PI without NNRTI | 112 (55%) | 59 (58%) | 53 (53%) | 0.576c |
| NNRTI without PI | 76 (37%) | 37 (36%) | 39 (39%) | |
| PI + NNRTI | 12 (6%) | 4 (4%) | 8 (8%) | |
| Other | 3 (2%) | 2 (2%) | 1 (1%) | |
| Tenofovir in ARTb | 118 (58%) | 59 (58%) | 59 (58%) | 1.00 |
| Efavirenz in ARTb | 84 (41%) | 40 (39%) | 44 (44%) | 0.529 |
| UCa/UCr ratio | ||||
| Mean (sd) | 0.06 (0.04) | 0.06 (0.05) | 0.06 (0.04) | 0.790 |
| Median (range) | 0.05 (0–0.26) | 0.05 (0–0.26) | 0.05 (0–0.20) | |
| Serum Calcium (mg/dl) | ||||
| Mean (sd) | 9.4 (0.4) | 9.5 (0.4) | 9.4 (0.4) | 0.201 |
| Median (range) | 9.5 (8.2–10.5) | 9.5 (8.6–10.5) | 9.4 (8.2–10.1) | |
| Total calcium intake (mg/d) | ||||
| Mean (sd) | 796 (523) | 847 ((544) | 746 (498) | 0.111 |
| Median (range) | 619 (63–2832) | 702 (78–2832) | 596 (63–2571) | |
| Total vitamin D intake (IU/d) | ||||
| Mean (sd) | 211 (199) | 227 (206) | 196 (190) | 0.156 |
| Median (range) | 131 (3–815) | 151 (14–815) | 114 (3–750) | |
| Vitamin D intake category, <600 IU/d | 191 (95%) | 94 (93%) | 97 (96%) | 0.352 |
| Serum 25-OHD (ng/ml) | ||||
| Mean (sd) | 21.2 (12.3) | 21.4 (13.1) | 20.9 (11.5) | 0.941 |
| Median (range) | 18.5 (2.2–100.1) | 18.9 (4.5–100.1) | 18.0 (2.2–65.5) | |
| Serum 25-OHD category | ||||
| Deficient (<12 ng/ml) | 42 (21%) | 22 (22%) | 20 (20%) | 0.989c |
| Insufficient (12–<20) | 68 (34%) | 34 (33%) | 34 (34%) | |
| Sufficient (≥20–50) | 87 (43%) | 43 (42%) | 44 (44%) | |
| Excess (>50) | 6 (3%) | 3 (3%) | 3 (3%) | |
| Serum 1,25-(OH)2D (pmol/liter) | ||||
| Mean (sd) | 111.5 (47.7) | 111.2 (48.2) | 111.8 (47.5) | 0.872 |
| Median (range) | 104.6 (6.0–278.4) | 102.7 (6.0–278.4) | 108.1 (25.8–245.9) |
The data describe all participants with baseline data (n = 203) There were no clinically important statistically significant differences between participants in the fully evaluable subset (n = 169) compared with the not-fully-evaluable subset (n = 34) except in BMI [25.2 (sd 6.9) vs. 27.8 (7.2) kg/m2, respectively]. Although supplemental vitamin D intake was higher in the evaluable subset compared with the not-fully-evaluable subset [376 (96) vs. 267 (200) IU, respectively], only 34 evaluable and nine not-fully evaluable group members took supplements. Continuous-data P values were calculated by Wilcoxon rank sum test and categorical-data P values by Pearson χ2 test unless indicated otherwise. CDC, Centers for Disease Control and Prevention; NRTI, nucleoside analog reverse transcriptase inhibitor; NNRTI, non-nucleoside analog reverse transcriptase inhibitor; PI, protease inhibitor.
Antiretroviral classes of the current regimen in addition to NRTI.
Note that tenofovir and efavirenz use were not mutually exclusive.
Fisher's exact test.
Effects of vitamin D supplementation
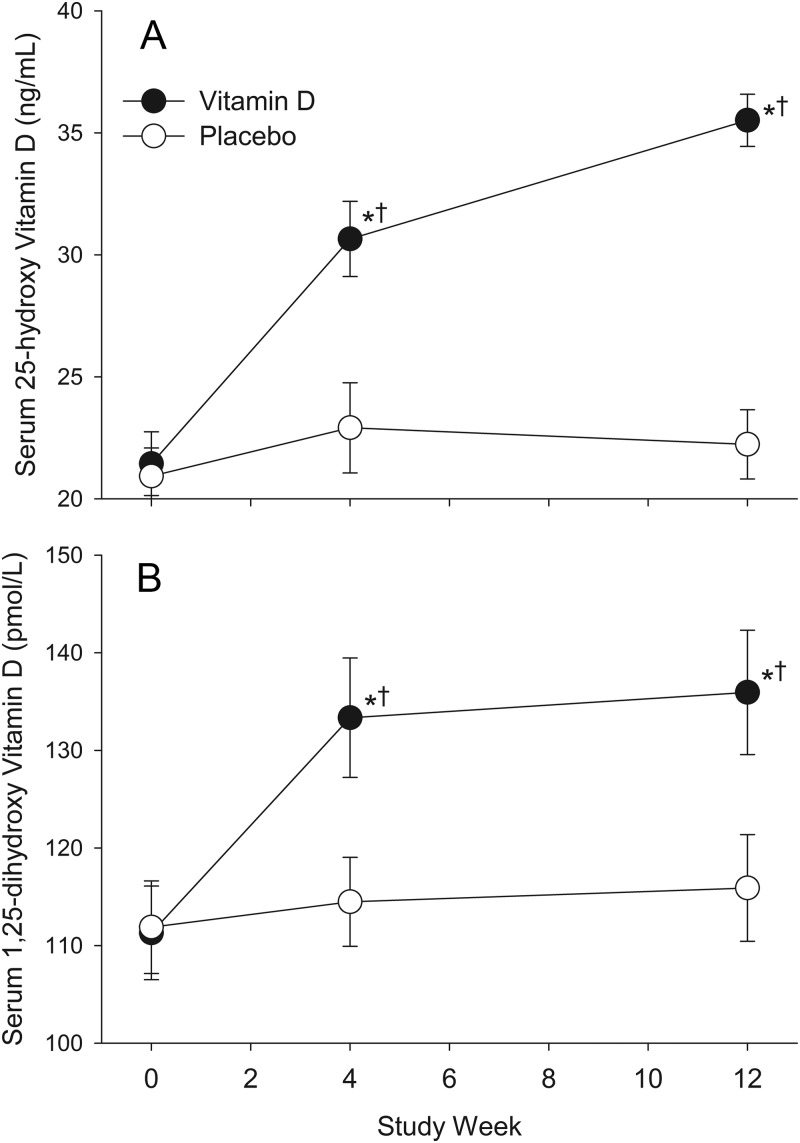
In the evaluable population, a single dose of 50,000 IU vitamin D3 raised the 25-OHD serum concentration from a mean of 21.9 (13.3) ng/ml at baseline to 30.8 (15.1) ng/ml at wk 4 (P < 0.001 compared with baseline), and additional 50,000 IU doses at wk 4 and 8 resulted in a mean 25-OHD serum concentration of 35.9 (19.1) ng/ml at wk 12 (P < 0.001 compared with baseline; Fig. 1). Concentrations of 1,25-OHD increased from 111.3 (48.2) pmol/liter at baseline to 135.9 (60.1) pmol/liter at wk 12 in the vitamin D treatment group (P < 0.001). Neither 25-OHD nor 1,25-(OH)2D concentrations changed from baseline to wk 12 in the placebo group (Fig. 1).
Fig. 1.
Mean (±se) serum concentration of 25-OHD (A) and 1,25-(OH)2D (B) by treatment group and study week. Vitamin D (50,000 IU) or placebo treatments were administered at baseline and 4 and 8 wk. *, P < 0.001, significant differences from baseline within treatment group; †, P < 0.001, significant differences from the placebo group at the same time point.
Among those with fully evaluable data, at wk 12, 84 of 88 (95%) in the group randomized to vitamin D had 25-OHD concentration of 20 ng/ml or higher; this included 76 with 25-OHD concentration in the sufficient range (20–50 ng/ml), and eight in the excess range (>50 ng/ml) (Table 2). Of the 46 participants with baseline 25-OHD below 20 ng/ml, only three (7%) remained in the insufficient range at wk 12 after three doses of vitamin D. In contrast, in the group randomized to placebo, 25-OHD concentration below 20 ng/ml was observed in 42 of 81 (52%) at baseline and 41 of 81 (51%) at wk 12 (P < 0.001 compared with the vitamin D treatment group).
Table 2.
Effect of vitamin D and placebo treatment on vitamin D status at 4 and 12 wk in evaluable subjects categorized by vitamin D status at baseline
| Baseline vitamin D serum concentration category | na | Vitamin D serum concentration category at study wk 4 |
Vitamin D serum concentration category at study wk 12 |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Deficient | Insufficient | Sufficient | Excess | Totalb | Deficient | Insufficient | Sufficient | Excess | Totalc | ||
| Vitamin D | |||||||||||
| Deficient (<12 ng/ml) | 18 (20%) | 2 (11%)c | 10 (56%) | 6 (33%) | 0 | 18 | 0 | 3 (17%) | 15 (83%) | 0 | 18 |
| Insufficient (12–<20 ng/ml) | 28 (32%) | 0 | 5 (18%) | 22 (79%) | 1 (4%) | 28 | 0 | 0 | 27 (96%) | 1 (4%) | 28 |
| Sufficient (20–50 ng/ml) | 40 (45%) | 0 | 0 | 36 (90%) | 4 (10%) | 40 | 0 | 1 (3%) | 34 (85%) | 5 (13%) | 40 |
| Excess (>50 ng/ml) | 2 (2%) | 0 | 0 | 0 | 2 (100%) | 2 | 0 | 0 | 0 | 2 (100%) | 2 |
| Total | 88 | 2 (2%) | 15 (17%) | 64 (73%) | 7 (8%) | 88 | 0 (0%) | 4 (5%) | 76 (86%) | 8 (9%) | 88 |
| Placebo | |||||||||||
| Deficient (<12 ng/ml) | 14 (17%) | 11 (79%) | 2 (14%) | 0 | 0 | 13 | 7 (50%) | 4 (29%) | 3 (21%) | 0 | 14 |
| Insufficient (12–<20 ng/ml) | 28 (35%) | 2 (7%) | 20 (71%) | 6 (21%) | 0 | 28 | 3 (11%) | 17 (61%) | 8 (29%) | 0 | 28 |
| Sufficient (20–50 ng/ml) | 36 (44%) | 0 | 1 (3%) | 34 (94%) | 1 (3%) | 36 | 1 (3%) | 9 (25%) | 26 (72%) | 0 | 36 |
| Excess (>50 ng/ml) | 3 (4%) | 0 | 0 | 0 | 3 (100%) | 3 | 0 | 0 | 0 | 3 (100%) | 3 |
| Total | 81 | 13 (16%) | 23 (29%) | 40 (50%) | 4 (5%) | 80 | 11 (14%) | 30 (37%) | 37 (46%) | 3 (4%) | 81 |
Vitamin D status was based on classification of 25-OHD serum concentration in accordance with IOM 2010 criteria (20).
Percentage within column at baseline.
Distribution of participants by vitamin D status category was statistically significantly different between randomized treatment groups at wk 4 and 12 (P ≤ 0.001, Fisher's exact test for treatment group differences) but not at study baseline (P = 0.882.
Percentage within row (baseline status category) at each visit.
Univariate analysis showed that participants with the lowest baseline vitamin D (25-OHD <12 ng/ml) had a greater increase in 25-OHD serum concentration in response to vitamin D supplementation from wk 4 and 12 compared with those with higher baseline vitamin D (P = 0.019; Table 3). However, baseline 25-OHD concentration was not statistically significantly associated with response to vitamin D supplementation in multivariable models that adjusted for season of the year at study baseline, latitude of residence, race, body weight, tenofovir, and efavirenz (Table 4).
Table 3.
Effect of vitamin D and placebo treatment on serum 25-OHD concentration in evaluable subjects categorized by vitamin D status at baseline
| Category of baseline 25-OHD serum concentration | na | 25-OHD concentration (ng/ml), mean (sd; maximal value)b |
Change in 25-OHD, mean (sd) |
|||
|---|---|---|---|---|---|---|
| Baseline | wk 4 | wk 12 | Baseline to wk 4 | wk 4 to wk 12 | ||
| Vitamin D treatment group | ||||||
| All vitamin D | 88 | 21.9 (13.3; 100) | 30.8 (15.1; 104) | 35.9 (19.1; 144) | 9.0 (8.6)c | 5.1 (8.3)c |
| Deficient (<12 ng/ml) | 18 | 9.3 (2.2; 12) | 19.3 (5.3; 31) | 27.1 (6.7; 42) | 10.0 (5.3)c | 7.7 (4.9)c |
| Insufficient (12–<20 ng/ml) | 28 | 15.9 (2.3; 20) | 26.5 (9.1; 64) | 31.9 (14.2; 97) | 10.6 (9.3)c | 5.4 (7.3)c |
| Sufficient (20–50 ng/ml) | 40 | 28.9 (7.3; 46) | 36.2 (12.4; 94) | 40.0 (20.1; 144) | 7.3 (9.3)c | 3.8 (10.0)d |
| Excess (>50 ng/ml) | 2 | 78.3 (30.7; 100) | 88.2 (22.3; 104) | 91.6 (31.0; 114) | 9.9 (8.5) | 3.4 (8.8) |
| 0.065e | 0.019e | |||||
| Placebo treatment group | ||||||
| All placebo | 80 | 21.8 (12.0; 65) | 23.6 (17.7; 144) | 22.2 (12.6; 80) | 1.6 (9.4) | −1.2 (9.5) |
| Deficient (<12 ng/ml) | 13 | 8.0 (2.3; 11) | 9.5 (3.4; 17) | 13.5 (8.2; 30) | 1.7 (3.6) | 4.6 (6.4)d |
| Insufficient (12–<20 ng/ml) | 28 | 15.9 (2.0; 20) | 16.6 (3.4; 23) | 17.3 (5.4; 28) | 0.7 (3.1) | 0.8 (4.9) |
| Sufficient (20–50 ng/ml) | 36 | 28.7 (6.7; 46) | 28.8 (8.0; 58) | 25.8 (8.9; 48) | 0.1 (3.4) | −3.0 (6.1)c |
| Excess (>50 ng/ml) | 3 | 60.0 (5.6; 65) | 87.9 (49.4; 144) | 66.2 (12.0; 80) | 27.9 (44.4) | −21.7 (37.4) |
| 0.302e | 0.001e | |||||
Number with values for 25-OHD at both baseline and wk 4.
Mean (sd; maximal 25-OHD in participants in each category).
P value <0.001 for change in 25-OHD concentration between visits by Wilcoxon signed rank test.
P value >0.01 and <0.05 for change in 25-OHD concentration between visits by Wilcoxon signed rank test.
P value for differences of change in 25-OHD serum concentrations from baseline to wk 4, or wk 4 to wk 12, by category of baseline vitamin D serum concentration by Kruskal-Wallis test.
Table 4.
Effect of baseline serum 25-OHD on change in serum 25-OHD by wk 12 in subjects receiving vitamin D supplements, with and without adjustment for antiretroviral therapy and other covariates
| Variables included | Coefficienta | P valueb |
|---|---|---|
| Model 1 | ||
| Baseline 25-OHD serum concentration (ng/ml) | −0.11 | 0.004 |
| Model 2b | ||
| Baseline 25-OHD serum concentration (ng/ml) | 0.05 | 0.141 |
| Season (referent is winter) | <0.001 | |
| Spring | 8.70 | |
| Summer | −6.60 | |
| Fall | −2.67 | |
| Latitude (referent is ≥40° N) | 0.030 | |
| >35–40° N | −7.29 | |
| ≤35° N | −8.60 | |
| Race (African-American vs. all others) | 9.85 | <0.001 |
| Body weight (kg) | −0.15 | 0.016 |
| Tenofovir included in cART | 1.41 | 0.156 |
| Efavirenz included in cART | 2.06 | 0.776 |
Outcome is the change from baseline to wk 12.
Coefficient from generalized linear model on unranked data.
P value from generalized linear model based on ranked data, by likelihood ratio χ2 test.
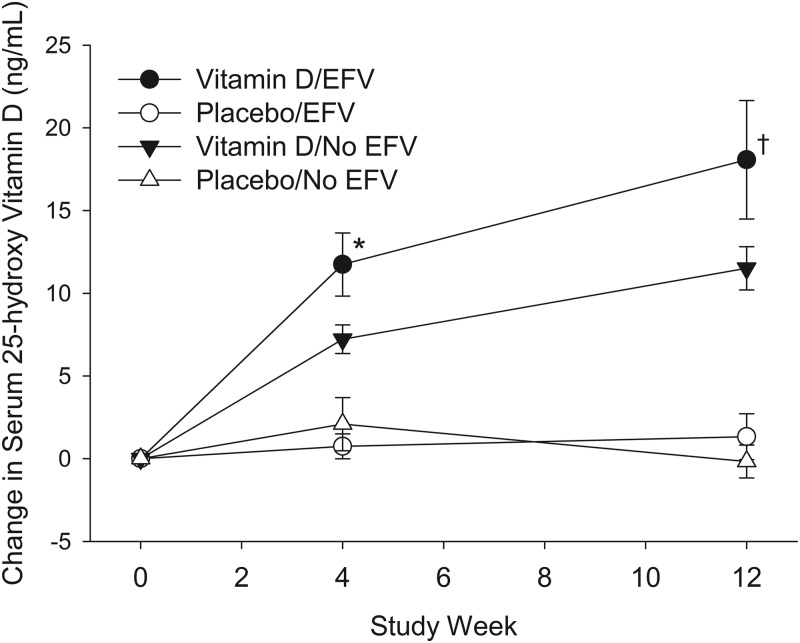
Use of cART containing efavirenz (n = 64), compared with use of cART not containing efavirenz (n = 105), was associated with lower baseline 25-OHD concentration, with mean 25-OHD serum concentrations of 19.5 (14.4) and 23.3 (11.3) ng/ml, respectively (P = 0.002). The presence of efavirenz in the cART regimen was not associated with the response to vitamin D treatment at wk 4 or 12 (Fig. 2 and Table 4). The use of tenofovir was not associated with either baseline levels of 25-OHD (data not shown) or the response to vitamin D supplementation (Table 4). Sixty-three participants were taking cART that included both efavirenz and tenofovir.
Fig. 2.
Mean (±se) change in serum concentration of 25-OHD from baseline (wk 0) by treatment group (placebo and vitamin D) and efavirenz (EFV) use. Vitamin D (50,000 IU) or placebo treatments were administered at baseline and 4 and 8 wk. Differences between those receiving and not receiving efavirenz were seen for subjects in the vitamin D group (*, P = 0.026; †, P = 0.059) but not the placebo group (P > 0.10).
Safety
There were no differences in the estimated glomerular filtration rate, UCa/UCr, or SCa from baseline to wk 12 in the vitamin D or placebo groups (data not shown). One participant randomized to the vitamin D group had a baseline elevation in UCa/UCr over 0.20 mg/mg that persisted throughout the study, associated with 25-OHD levels of 5.73 and 13.63 ng/ml at baseline and wk 12, respectively. Five participants (two in the vitamin D group and three in placebo group) had 25-OHD levels above 50 ng/ml at baseline that persisted through the study; six participants (all in the vitamin D treatment group) developed 25-OHD above 50 ng/ml during the study (Table 2). One participant in the vitamin D group, whose 25-OHD concentration at wk 12 was 33.8 ng/ml, was treated for renal stones 2 months after the last study dose. Fourteen participants (nine in the vitamin D group and five in the placebo group) had a single UCa/UCr measurement over 0.20 mg/mg that reverted to normal when repeated. There were no increases in SCa, and there were no clinical toxicities related to treatment.
Discussion
This study, which used directly observed monthly dosing of vitamin D3 50,000 IU, demonstrated a rapid increase in 25-OHD serum concentration to a mean level of 35.5 (19.1) ng/ml in adolescents and young adults with HIV infection. This regimen reduced the proportion of participants with vitamin D concentrations in the deficient/insufficient range from 52% at baseline to 5% after three doses among evaluable participants. We enrolled participants without regard to baseline serum vitamin D concentration, used a uniform, directly observed dose for treatment, and measured serum concentrations of 25-OHD 4 wk after the first and third doses. This allowed us to measure the change in serum 25-OHD as it varied by baseline concentration and total administered dose. There were no clinical or biochemical toxicities related to supplementation, even in those with 25-OHD over 50 ng/ml at study baseline. As reported elsewhere, this regimen decreased PTH concentrations in participants treated with tenofovir-containing cART but not in those treated with cART not containing tenofovir (29).
Recommendations for correction of vitamin D deficiency using vitamin D2 or vitamin D3 include daily doses of 2000 IU (3) to 5000 IU (5), weekly doses of 50,000 IU for 5 wk (4) to 12 wk (7, 12), and single doses of 100,000 IU (9), 150,000 IU (6), 300,000 IU (6), 500,000 IU (10), or 600,000 IU (6, 11). A single dose of 500,000 IU was associated with an increased risk of falls and fractures in older women (10) and is not currently recommended (8), but vitamin D2 50,000 IU weekly for 5 (13) to 8 (14) weeks is commonly used to correct vitamin D deficiency. Results from our study suggest that for many patients, a single monthly 50,000-IU dose of vitamin D3 might be sufficient to reach and maintain a 25-OHD concentration between 20 and 50 ng/ml, at least while such dosing is continued.
This study used vitamin D3 (cholecalciferol), which is the form of vitamin D that is produced by human metabolism. It can be found naturally in cod liver oil or fish (e.g. salmon) and is the supplement preferred by some experts (16, 35, 36). Compared with vitamin D2 (ergocalciferol, of fungal and plant origin), vitamin D3 is more effective at increasing 25-OHD serum concentrations to higher levels (12, 18) and maintaining the effect for a longer duration (17), which may be especially important when dosing at weekly or monthly intervals (15, 35). Vitamin D3 may have greater potency at reducing serum PTH concentrations than vitamin D2 (37). However, when smaller daily doses of vitamin D supplementation are given, D2 and D3 may be equally effective at maintaining serum 25-OHD concentrations (38, 39). Doses based on findings in this study are applicable to supplementation with vitamin D3, and not D2.
We used a dosing frequency of every 4 wk, with follow-up measurements 4 or 8 wk after the administered dose. Serum 25-OHD concentrations may peak from a few days to 2 wk after an administered dose (15, 39), so our measurements presumably do not represent peak concentrations. Lower doses of vitamin D3 given more frequently (e.g. daily or weekly) may have a lower peak (8, 12, 39), but the monthly dose used here was well tolerated, and once-monthly dosing in adolescents with HIV infection minimizes pill burden and potential interference with adherence to ART and allows for directly observed therapy. In a study of children and youth (ages 6–16 yr) with HIV infection, a higher vitamin D dose (100,000 IU vitamin D3) administered less frequently (every 2 months) resulted in serum concentrations of 25-OHD below 20 ng/ml in two (6.7%) of 29 treated participants after 12 months of therapy, but 75% had at least one monthly serum 25-OHD below 30 ng/ml (2).
In univariate analysis, we found the inverse relationship between baseline 25-OHD concentration and the increase in 25-OHD concentration that has been seen in other studies (5, 12, 18, 28). However, baseline 25-OHD serum concentration was not associated with the magnitude of change in response to supplementation in multivariable models that included latitude of residence, season of the year at the baseline visit, body weight, race, tenofovir, and efavirenz.
A particular strength of this study is the use of directly observed dosing, which minimized variability that may arise from poor adherence to study medication. In clinical practice, HIV-infected patients can obtain a single vitamin D capsule at the same time they fill their antiretroviral prescriptions monthly, a convenience that may enhance adherence outside of the setting of a clinical trial, even in the absence of directly observed therapy. In the United States, vitamin D3 is not available by prescription as a 50,000-IU capsule, so government insurance will not cover its cost. Because nonprescription sources of this formulation must be used, patients may incur an extra out-of-pocket expense, limiting usefulness in some practices. The vitamin D3 capsules used for this study are readily available commercially.
Use of the antiretroviral efavirenz has been associated with lower 25-OHD levels in cross-sectional studies (24) and decreases in 25-OHD serum concentrations with initiation of ART (23). In addition, removal of efavirenz from an ART regimen resulted in increases in 25-OHD concentrations (25). Efavirenz was used by 41% of all participants at baseline, and whereas those on efavirenz had lower baseline 25-OHD concentrations, use of this antiretroviral did not diminish the response to vitamin D administration.
Young persons have more rapid clearance of 25-OHD after a single large dose compared with older persons (9), suggesting another caution for the generalizability of this study. Applying this dosing algorithm to older persons may lead to higher serum concentrations. This study, in participants with HIV infection, might not be directly applicable to those without HIV, but our study population was in general good health, on stable antiretroviral therapy, and had no evidence of malabsorption.
Once vitamin D deficiency is corrected, maintenance doses of 600 IU vitamin D daily are currently recommended by the IOM (20) for persons from 1–70 yr of age, although others recommend higher daily (14) or monthly (13) doses, and vitamin D3 100,000 IU every 2 months has been used for chronic supplementation in children and youth with HIV infection (2). The IOM report states that the safe upper limit of the daily dose is 4000 IU/d for persons older than 9 yr of age (20). The current study did not evaluate dosing required for maintenance of steady-state serum concentrations of 25-OHD. A study of vitamin D3 50,000 IU monthly in older adults showed safety of this regimen (i.e. no increased risk of hypercalcemia or hypercalciuria was observed) over 12 months of study (39).
These data offer a practical approach to targeting vitamin D replacement in young adults with HIV infection and with vitamin D insufficiency/deficiency, using a youth-friendly dosing regimen that is safe, does not increase the daily pill burden, and reaches appropriate target serum 25-OHD concentrations in the presence or absence of efavirenz in the antiretroviral regimen.
Acknowledgments
We thank the ATN Community Advisory Board and the youth who participated in the study.
The following ATN sites participated in this study: University of South Florida, Tampa (Emmanuel, Lujan-Zilberman, Julian), Children's Hospital of Los Angeles (Belzer, Flores, Tucker), University of Southern California at Los Angeles (Kovacs, Homans, Lozano), Children's National Medical Center (D'Angelo, Hagler, Trexler), Children's Hospital of Philadelphia (Douglas, Tanney, DiBenedetto), John H. Stroger Jr. Hospital of Cook County and the Ruth M. Rothstein CORE Center (Martinez, Bojan, Jackson), University of Puerto Rico (Febo, Ayala-Flores, Fuentes-Gomez), Montefiore Medical Center (Futterman, Enriquez-Bruce, Campos), Mount Sinai Medical Center (Steever, Geiger), University of California-San Francisco (Moscicki, Auerswald, Irish), Tulane University Health Sciences Center (Abdalian, Kozina, Baker), University of Maryland (Peralta, Gorle), University of Miami School of Medicine (Friedman, Maturo, Major-Wilson), Children's Diagnostic and Treatment Center (Puga, Leonard, Inman), St. Jude's Children's Research Hospital (Flynn, Dillard), and Children's Memorial (Garofalo, Brennan, Flanagan).
The following International Maternal Pediatric Adolescent AIDS Clinical Trials (IMPAACT) sites participated in the study: Children's Memorial-Chicago (Yogev, Sanders), Duke University Medical Center (Cunningham, Patil, Wilson), New York University School of Medicine (Borkowsky, Deygoo), University of Medicine and Dentistry of New Jersey (Dieudonne, Bettica, Monti), Bronx-Lebanon Hospital (Purswani, Vachon, Chittalae), Baylor College of Medicine (Shearer, Cooper, McMullen-Jackson), Boston Medical Center (Cooper, McLaud, Tucker), University of Colorado/The Children's Hospital of Denver (McFarland, Chambers, Katai), San Juan Hospital (Acevedo, Gonzalez, Angeli), Seattle Children's Hospital (Frenkel, Venema-Weiss, Bowen), University of California at San Diego (Spector, Viani, Manning), State University of New York-Stony Brook (Nachman, Puccio, Ferraro), Howard University (Rana,Yu), Miller Children's Hospital (Chen, Michalik, Jackson-Alvarez), Metropolitan Hospital-New York (Bamji, Paul, Riley), Jacobi Medical Center (Wiznia, Kassen, Burey), Harbor UCLA Medical Center (Keller, Hayes, Gonzalez), Children's Hospital of Los Angeles (Rodier, Rockwood), University of Miami (Scott, Falk, Florenz), and Columbia University (LaRussa, Higgins).
This work was supported by the ATN from the National Institutes of Health (NIH) (U01 HD 040533 and U01 HD 040474) through the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD) (to B.G.K.), with supplemental funding from the National Institutes on Drug Abuse (N. Borek) and Mental Health (P. Brouwers and S. Allison). The protocol was co-endorsed by the IMPAACT Group. Support for the IMPAACT Group was provided by the National Institute of Allergy and Infectious Diseases, the NICHD, and the National Institute of Mental Health (U01 A1068632). The study was scientifically reviewed by the ATN′s Therapeutic Leadership Group. Network, scientific, and logistical support was provided by the ATN Coordinating Center (C.W. and C. Partlow) at The University of Alabama at Birmingham. Network operations and analytic support was provided by the ATN Data and Operations Center at Westat, Inc. (J. Korelitz and B. Driver).
Vitamin D and placebo were supplied by Bio-Tech Pharmacal, Fayetteville, AR. Laboratory assays were performed by Xiaowen Jiang, Melissa Zerofsky, Brian Piccolo, and Gertrud Schuster at the U.S. Department of Agriculture Agricultural Research Service, Western Human Nutrition Research Center, Davis, CA.
Eight of the participating sites used their General Clinical Research Center/Pediatric Clinical Research Center for the study; the centers were supported by grants from the General Clinical Research Center Program of the National Center for Research Resources, NIH, Department of Health and Human Services as follows: Children's National Medical Center, M01RR020359; University of Pennsylvania/Children's Hospital of Philadelphia, NCRRUL1-RR-024134; University of California at San Francisco, UL1 RR024131; Seattle Children's Hospital, UL1-RR025014; Texas Children's Hospital and Baylor College of Medicine, M01-RR00188; Boston University Medical Center, UL1-RR02517; and SUNY Stony Brook, M01-RR10710. The Tulane University Health Sciences Center used its Clinical and Translational Research Center for the study; the center was supported in whole or in part by funds provided through the Louisiana Board of Regents RC/EEP (RC/EEP-06).
This work was presented in part at the 18th Conference on Retroviruses and Opportunistic Infections (CROI), Boston, MA, February, 2011, and the 13th International Workshop on Comorbidities and Adverse Drug Reactions in HIV in Rome, 2011.
The clinical trials registration number for this study is NCT00490412
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- ATN
- Adolescent Medicine Trials Network for HIV/AIDS Intervention
- cART
- combination antiretroviral therapy
- IOM
- Institute of Medicine
- 25-OHD
- 25-hydroxyvitamin D
- 1,25-(OH)2D
- 1,25-dihydroxyvitamin D
- SCa
- serum calcium
- UCa
- urine calcium
- UCr
- urine creatinine.
References
- 1. Stephensen CB, Marquis GS, Kruzich LA, Douglas SD, Aldrovandi GM, Wilson CM. 2006. Vitamin D status in adolescents and young adults with HIV infection. Am J Clin Nutr 83:1135–1141 [DOI] [PubMed] [Google Scholar]
- 2. Arpadi SM, McMahon D, Abrams EJ, Bamji M, Purswani M, Engelson ES, Horlick M, Shane E. 2009. Effect of bimonthly supplementation with oral cholecalciferol on serum 25-hydroxyvitamin D concentrations in HIV-infected children and adolescents. Pediatrics [Erratum (2009) 123:1437] 123:e121–e126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE, Gordon CM, Williams AL, Feldman HA, May J, Sinclair L, Vasquez A, Cox JE. 2008. Treatment of hypovitaminosis D in infants and toddlers. J Clin Endocrinol Metab 93:2716–2721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Adams JS, Kantorovich V, Wu C, Javanbakht M, Hollis BW. 1999. Resolution of vitamin D insufficiency in osteopenic patients results in rapid recovery of bone mineral density. J Clin Endocrinol Metab 84:2729–2730 [DOI] [PubMed] [Google Scholar]
- 5. Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK, Aloia JF, Patel M, Dimaano R, Li-Ng M, Talwar SA, Mikhail M, Pollack S, Yeh JK. 2008. Vitamin D intake to attain a desired serum 25-hydroxyvitamin D concentration. Am J Clin Nutr 87:1952–1958 [DOI] [PubMed] [Google Scholar]
- 6. Cesur Y, Caksen H, Gundem A, Kirimi E, Odabas D. 2003. Comparison of low and high dose of vitamin D treatment in nutritional vitamin D deficiency rickets. J Pediatr Endocrinol 16:1105–1109 [DOI] [PubMed] [Google Scholar]
- 7. Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V, Chandra P, Binongo JNG, Ziegler TR, Schlanger LE, Wang W, Someren JT, Tangpricha V. 2008. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract 14:10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Dawson-Hughes B, Harris SS. 2010. High-dose vitamin D supplementation: too much of a good thing? JAMA 303:1861–1862 [DOI] [PubMed] [Google Scholar]
- 9. Ilahi M, Armas LA, Heaney RP. 2008. Pharmacokinetics of a single, large dose of cholecalciferol. Am J Clin Nutr 87:688–691 [DOI] [PubMed] [Google Scholar]
- 10. Sanders KM, Stuart AL, Williamson EJ, Simpson JA, Kotowicz MA, Young D, Nicholson GC. 2010. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA [Erratum (2010) 303:2357] 303:1815–1822 [DOI] [PubMed] [Google Scholar]
- 11. Shah BR, Finberg L. 1994. Single-day therapy for nutritional vitamin D-deficiency rickets: a preferred method. J Pediatr 125:487–490 [DOI] [PubMed] [Google Scholar]
- 12. Khazai NB, Judd SE, Jeng L, Wolfenden LL, Stecenko A, Ziegler TR, Tangpricha V. 2009. Treatment and prevention of vitamin D insufficiency in cystic fibrosis patients: comparative efficacy of ergocalciferol, cholecalciferol, and UV light. J Clin Endocrinol Metab 94:2037–2043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adams JS, Hewison M. 2010. Update in vitamin D. J Clin Endocrinol Metab 95:471–478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Holick MF. 2007. Vitamin D deficiency. N Engl J Med 357:266–281 [DOI] [PubMed] [Google Scholar]
- 15. Armas LA, Hollis BW, Heaney RP. 2004. Vitamin D2 is much less effective than vitamin D3 in humans. J Clin Endocrinol Metab 89:5387–5391 [DOI] [PubMed] [Google Scholar]
- 16. Heaney RP, Recker RR, Grote J, Horst RL, Armas LA. 2011. Vitamin D3 is more potent than vitamin D2 in humans. J Clin Endocrinol Metab 96:E447–E452 [DOI] [PubMed] [Google Scholar]
- 17. Thacher TD, Fischer PR, Obadofin MO, Levine MA, Singh RJ, Pettifor JM. 2010. Comparison of metabolism of vitamins D2 and D3 in children with nutritional rickets. J Bone Miner Res 25:1988–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trang HM, Cole DE, Rubin LA, Pierratos A, Siu S, Vieth R. 1998. Evidence that vitamin D3 increases serum 25-hydroxyvitamin D more efficiently than does vitamin D2. Am J Clin Nutr 68:854–858 [DOI] [PubMed] [Google Scholar]
- 19. Institute of Medicine 2011. Dietary reference intakes for calcium and vitamin D. Washington, DC: National Academies Press; [PubMed] [Google Scholar]
- 20. Ross AC, Manson JE, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Mayne ST, Rosen CJ, Shapses SA. 2011. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: what clinicians need to know. J Clin Endocrinol Metab 96:53–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Rosen CJ, Abrams SA, Aloia JF, Brannon PM, Clinton SK, Durazo-Arvizu RA, Gallagher JC, Gallo RL, Jones G, Kovacs CS, Manson JE, Mayne ST, Ross AC, Shapses SA, Taylor CL. 2012. IOM committee members respond to Endocrine Society vitamin D guideline. J Clin Endocrinol Metab 97:1146–1152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Holick MF, Binkley NC, Bischoff-Ferrari HA, Gordon CM, Hanley DA, Heaney RP, Murad MH, Weaver CM. 2011. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:1911–1930 [DOI] [PubMed] [Google Scholar]
- 23. Brown TT, McComsey GA. 2010. Association between initiation of antiretroviral therapy with efavirenz and decreases in 25-hydroxyvitamin D. Antivir Ther 15:425–429 [DOI] [PubMed] [Google Scholar]
- 24. Welz T, Childs K, Ibrahim F, Poulton M, Taylor CB, Moniz CF, Post FA. 2010. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. Aids 24:1923–1928 [DOI] [PubMed] [Google Scholar]
- 25. Fox J, Peters B, Prakash M, Arribas J, Hill A, Moecklinghoff C. 2011. Improvement in vitamin D deficiency following antiretroviral regime change: results from the MONET trial. AIDS Res Hum Retrovir 27:29–34 [DOI] [PubMed] [Google Scholar]
- 26. Murphy DA, Belzer M, Durako SJ, Sarr M, Wilson CM, Muenz LR, Adolescent Medicine HIVARN 2005. Longitudinal antiretroviral adherence among adolescents infected with human immunodeficiency virus. Arch Pediatr Adolesc Med 159:764–770 [DOI] [PubMed] [Google Scholar]
- 27. Chesney MA. 2000. Factors affecting adherence to antiretroviral therapy. Clin Infect Dis 30:S171–S176 [DOI] [PubMed] [Google Scholar]
- 28. Talwar SA, Aloia JF, Pollack S, Yeh JK. 2007. Dose response to vitamin D supplementation among postmenopausal African American women. Am J Clin Nutr 86:1657–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Havens PL, Stephensen CB, Hazra R, Flynn PM, Wilson CM, Rutledge B, Bethel J, Pan CG, Woodhouse LR, Van Loan MD, Liu N, Lujan-Zilbermann J, Baker A, Kapogiannis BG, Mulligan K; Adolescent Medicine Trials Network for HIV/AIDS Interventions 063 Study Team 2012. Vitamin D3 decreases parathyroid hormone in HIV-infected youth being treated with tenofovir: a randomized, placebo-controlled trial. Clin Infect Dis 54:1013–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gallant JE, Staszewski S, Pozniak AL, DeJesus E, Suleiman JM, Miller MD, Coakley DF, Lu B, Toole JJ, Cheng AK, Study G. 2004. Efficacy and safety of tenofovir DF vs stavudine in combination therapy in antiretroviral-naive patients: a 3-year randomized trial. JAMA 292:191–201 [DOI] [PubMed] [Google Scholar]
- 31. Stellbrink HJ, Orkin C, Arribas JR, Compston J, Gerstoft J, Van Wijngaerden E, Lazzarin A, Rizzardini G, Sprenger HG, Lambert J, Sture G, Leather D, Hughes S, Zucchi P, Pearce H. 2010. Comparison of changes in bone density and turnover with abacavir-lamivudine versus tenofovir-emtricitabine in HIV-infected adults: 48-week results from the ASSERT study. Clin Infect Dis 51:963–972 [DOI] [PubMed] [Google Scholar]
- 32. McComsey GA, Kitch D, Daar ES, Tierney C, Jahed NC, Tebas P, Myers L, Melbourne K, Ha B, Sax PE. 2011. Bone mineral density and fractures in antiretroviral-naive persons randomized to receive abacavir-lamivudine or tenofovir disoproxil fumarate-emtricitabine along with efavirenz or atazanavir-ritonavir: AIDS Clinical Trials Group A5224s, a substudy of ACTG A5202. J Infect Dis 203:1791–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, Hendriksen S, Kusek JW, Van Lente F, Chronic Kidney Disease Epidemiology Collaboration 2006. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med [Erratum (2008) 149:519] 145:247–254 [DOI] [PubMed] [Google Scholar]
- 34. Cummings SR, Block G, McHenry K, Baron RB. 1987. Evaluation of two food frequency methods of measuring dietary calcium intake. Am J Epidemiol 126:796–802 [DOI] [PubMed] [Google Scholar]
- 35. Houghton LA, Vieth R. 2006. The case against ergocalciferol (vitamin D2) as a vitamin supplement. Am J Clin Nutr 84:694–697 [DOI] [PubMed] [Google Scholar]
- 36. Kennel KA, Drake MT, Hurley DL. 2010. Vitamin D deficiency in adults: when to test and how to treat. Mayo Clin Proc 85:752–757; quiz 757–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Romagnoli E, Mascia ML, Cipriani C, Fassino V, Mazzei F, D'Erasmo E, Carnevale V, Scillitani A, Minisola S. 2008. Short and long-term variations in serum calciotropic hormones after a single very large dose of ergocalciferol (vitamin D2) or cholecalciferol (vitamin D3) in the elderly. J Clin Endocrinol Metab 93:3015–3020 [DOI] [PubMed] [Google Scholar]
- 38. Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD, Holick MF, Biancuzzo RM, Chen TC, Klein EK, Young A, Bibuld D, Reitz R, Salameh W, Ameri A, Tannenbaum AD. 2008. Vitamin D2 is as effective as vitamin D3 in maintaining circulating concentrations of 25-hydroxyvitamin D. J Clin Endocrinol Metab 93:677–681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Binkley N, Gemar D, Engelke J, Gangnon R, Ramamurthy R, Krueger D, Drezner MK. 2011. Evaluation of ergocalciferol or cholecalciferol dosing, 1,600 IU daily or 50,000 IU monthly in older adults. J Clin Endocrinol Metab 96:981–988 [DOI] [PMC free article] [PubMed] [Google Scholar]