Abstract
Context:
Adipose inflammation is a crucial link between obesity and its metabolic complications. Human experimental endotoxemia is a controlled model for the study of inflammatory cardiometabolic responses in vivo.
Objective:
We hypothesized that adipose genes down-regulated during endotoxemia would approximate changes observed with obesity-related inflammation and reveal novel candidates in cardiometabolic disease.
Design, Subjects, and Intervention:
Healthy volunteers (n = 14) underwent a 3 ng/kg endotoxin challenge; adipose biopsies were taken at 0, 4, 12, and 24 h for mRNA microarray. A priority list of highly down-regulated and biologically relevant genes was validated by RT-PCR in an independent sample of adipose from healthy subjects (n = 7) undergoing a subclinical 0.6 ng/kg endotoxemia protocol. Expression of validated genes was screened in adipose of lean and severely obese individuals (n = 11 per group), and cellular source was probed in cultured adipocytes and macrophages.
Results:
Endotoxemia (3 ng/kg) suppressed expression of 353 genes (to <67% of baseline; P < 1 × 10−5) of which 68 candidates were prioritized for validation. In low-dose (0.6 ng/kg) endotoxin validation, 22 (32%) of these 68 genes were confirmed. Functional classification revealed that many of these genes are involved in cell development and differentiation. Of validated genes, 59% (13 of 22) were down-regulated more than 1.5-fold in primary human adipocytes after treatment with endotoxin. In human macrophages, 59% (13 of 22) were up-regulated during differentiation to inflammatory M1 macrophages whereas 64% (14 of 22) were down-regulated during transition to homeostatic M2 macrophages. Finally, in obese vs. lean adipose, 91% (20 of 22) tended to have reduced expression (χ2 = 10.72, P < 0.01) with 50% (11 of 22) reaching P < 0.05 (χ2 = 9.28, P < 0.01).
Conclusions:
Exploration of down-regulated mRNA in adipose during human endotoxemia revealed suppression of genes involved in cell development and differentiation. A majority of candidates were also suppressed in endogenous human obesity, suggesting a potential pathophysiological role in human obesity-related adipose inflammation.
Activation of immune pathways and recruitment of inflammatory leukocytes in adipose tissue are crucial links between obesity and its metabolic and cardiovascular complications (1–4). Adipose inflammation can attenuate normal adipocyte differentiation and function, promoting local and systemic insulin resistance and dyslipidemia. Despite experimental evidence in rodent models, most evidence supporting these concepts in humans derives from observational and correlative studies (5–7). Indeed, validated adipose genes that mediate complications of human adiposity remain limited.
Experimental human endotoxemia can provide unique insights into the relationship of inflammation to metabolic disturbance in man (8). We and others have shown that endotoxemia induces acute metabolic, lipoprotein, and oxidant responses that resemble the chronic changes in insulin resistance and metabolic syndrome (8–11). Notably, endotoxemia induces adipose inflammation (12) with activation of several adipose inflammatory cascades, including cytokines, chemokines, and suppressor of cytokine signaling (SOCS) molecules (11). Activation of these adipose pathways is known to attenuate insulin signaling and contribute to obesity and type 2 diabetes (13). By applying microarray mRNA profiling to human adipose tissue during endotoxemia, we have identified many novel genes modulated by inflammation (12). Validation and exploration of a subset of up-regulated genes encoding secreted proteins have revealed several candidates that may serve as potential biomarkers of and therapeutic targets for obesity-related diseases (12). Indeed, using this strategy, we recently identified fractalkine (CX3CL1) as a novel adipochemokine activated in human metabolic disease (14).
In the current manuscript, we focus on adipose genes that are repressed during endotoxemia. Such mRNA profiling might reveal gene pathways and proteins that are suppressed by adipose inflammation and whose loss of expression and function may contribute to insulin resistance, type 2 diabetes, and atherosclerosis. For example, loss of expression and function of adiponectin in obesity is thought to play a critical role in the development of metabolic and cardiovascular complications. After identification of such genes, we validated our findings in vivo through independent experiments of low-grade human inflammation and identified in vitro the likely human adipose cellular origin of suppressed gene signals. Finally, we compare adipose expression of validated genes in fat tissue of lean and morbidly obese individuals to identify those that are also suppressed in severe obesity and therefore of most potential relevance to clinical disease.
Subjects and Methods
Clinical studies
Each clinical study was performed with approval of the University of Pennsylvania Institutional Review Board after written informed consent was obtained from all research participants.
Endotoxemia protocols
As previously described (11, 12, 15), healthy volunteers, age 18–40 yr, with body mass index (BMI) of 18–30 kg/m2 were recruited. Exclusions included inflammatory disease, pregnancy, or medication, substance, or supplement use. Serial whole blood samples were collected before and 2, 4, 6, 8, 12, 16, and 24 h after iv bolus of either 3 ng/kg (moderate dose) or 0.6 ng/kg (low dose) U.S. standard reference endotoxin [lipopolysaccharide (LPS), lot no. CC-RE-LOT-1 + 2; Clinical Center, Pharmacy Department, National Institutes of Health, Bethesda, MD). Subcutaneous adipose samples were collected by core needle aspiration through a 4-mm gluteal incision from distinct sites 30 min before and 4, 12, and 24 h after LPS and stored at −80 C. A subset of subjects from the moderate-dose endotoxin study (n = 14) was used for adipose tissue microarray, whereas an independent sample from the low-dose endotoxemia protocol (n = 7) was used for real-time PCR validation of microarray results. Baseline demographic and metabolic parameters for both groups have been published previously (15, 16).
Studies of lean and obese adipose tissue
Adipose from lean (n = 11; BMI mean = 24.3; 95% confidence interval = 22.5–26.1) subjects was obtained as described above and at bariatric surgery for severely obese patients (n = 13; BMI mean = 48.3; 95% confidence interval = 43.0–53.7). Samples were stored at −80 C. In a subset of lean subjects (n = 8), a portion of the adipose was minced and digested immediately with type I collagenase (Sigma-Aldrich, St. Louis, MO) for 1 h and centrifuged, and mature adipocytes and stromal vascular fraction were separated.
Adipose microarray and analysis
Arrays
As described previously (12), adipose tissue mRNA was extracted using RNeasy Lipid Tissue Mini kit (QIAGEN Inc., Valencia, CA) and underwent quality control before cDNA synthesis (100 ng), cRNA synthesis, and hybridization to Affymetrix GeneChip Human Genome U133Plus 2.0 arrays (Santa Clara, CA) at the Penn Microarray Facility (https://somapps.med.upenn.edu/pbr/portal/microarr/). Affymetrix Microarray Suite version 5.0 was used to quantitate expression levels. Array signal intensities passed a 2-stage quality control process including affyQCReport package. All arrays had similar background values and distributions of intensity.
Adipose microarray statistical and informatics methods
Arrays were normalized, adjusted for background (17), and filtered to remove probe sets with little evidence of expression, as previously described (12). For the remaining 13,646 genes, mixed-effects models were fit, with the goal of identifying genes with highly significant reductions in expression between baseline and each time point (4, 12, and 24 h). Genes were selected for further study if the permutation-based P value was below 1 × 10−5 for the null hypothesis test of a fold change of unity at any time point (18). In addition, we required a minimum cutoff of at least 33% reduction in intensities per gene. Analyses were performed using Bioconductor (www.bioconductor.org) run under R 2.6 (R Foundation for Statistical Computing, Vienna, Austria).
Prioritization for validation of down-regulated genes
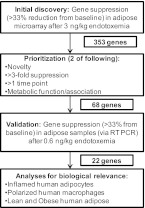
For initial prioritization, we focused on the following attributes: 1) novelty (genes with unknown function); 2) degree of down-regulation (>3-fold down at any time point); 3) sustained down-regulation (>1.5-fold suppression at two or more time points); 4) evidence for functional roles or association with inflammation, insulin signaling, or adipose biology; and 5) known transcription factors. Genes that did not meet at least two of the criteria were not studied further. These 68 genes were then validated in the low-dose endotoxemia study, and the 22 that demonstrated suppressed expression with 0.6 ng/kg endotoxemia were studied further. The process for gene selection is depicted in Fig. 1.
Fig. 1.
Flow chart of gene selection process. Genes were identified as down-regulated if expression at any time point after 3 ng/kg endotoxin was suppressed more than 33% from baseline, yielding 353 genes. These were then prioritized for further study if they met two of the following requirements: 1) novelty (genes with unknown function); 2) degree of down-regulation (>3-fold down at any time point); 3) sustained down-regulation (>1.5-fold suppression at two or more time points); 4) evidence for functional roles or association with inflammation, insulin signaling, or adipose biology; and 5) known transcription factors. Genes that did not meet at least two of the criteria were not studied further. This resulted in 68 genes for validation, of which 22 showed expression suppressed more than 33% from baseline after 0.6 ng/kg endotoxin. These 22 genes were then examined for cellular source and expression in human obesity.
Functional classification
Prioritized genes were uploaded to DAVID (Database for Annotation, Visualization, and Integrated Discovery) Bioinformatics Resource, and the Gene Functional Classification tool was used to generate clusters of related and overrepresented genes (19, 20). The enrichment score (geometric mean of −log P values) is presented.
Cell studies
Primary human adipocyte studies
Fresh human adipose tissue was obtained from abdominal surgery specimens for isolation of adipocyte and stromal vascular fractions as well as culture of human adipocytes (12). Briefly, the specimen was digested with collagenase (Roche Applied Science, Indianapolis, IN), filtered, and centrifuged, and the cellular layer was resuspended in oxidation-fermentation medium [DMEM/F12 plus penicillin/streptomycin, biotin (4 mg/liter), and pantothenate (8 mg/liter)] with 20% fetal bovine serum and plated at 30,000 cells/cm2. Once confluent, cells were differentiated in serum-free differentiation medium [oxidation-fermentation medium, insulin (20 nm), hydrocortisone (1 μm), dexamethasone (250 nm), human transferrin (10 mg/liter), T3 (0.2 nm), isobutylmethylxanthine (500 μm), and peroxisome proliferator-activated receptor-γ agonist GW347845 (2 μm) (gift from GlaxoSmithKline, King of Prussia, PA)] until 80% mature (7–10 d). Cells were washed and treated with or without LPS 100 ng/ml in DMEM/F12 with 0.1% fetal bovine serum (FBS) plus penicillin/streptomycin for 2, 4, 12, and 24 h for three independent experiments, in triplicate. Unless noted, reagents were purchased from Sigma-Aldrich.
Primary human macrophage studies
Human monocytes (peripheral blood mononuclear cells, >95% expression of cluster of differentiation 14 (CD14) and human leukocyte antigen DR (HLA-DR), were isolated from donor blood after apheresis and elutriation in the Penn Center for AIDS Research. As described (21), cells were plated at 3 × 105 cells/cm2 and cultured in RPMI with 20% FBS and penicillin/streptomycin and then supplemented with 100 ng/ml macrophage colony stimulating factor (MCSF) (Sigma Chemical Co., St Louis, MO) to promote differentiation to macrophages over 7 d. In three independent experiments, each in triplicate, macrophages were washed and treated for 16 h with RPMI plus 5% FBS alone or 100 ng/ml LPS and 20 ng/ml interferon-γ (R&D Systems, Minneapolis, MN) for differentiation to the inflammatory (M1) phenotype for differentiation to the M1 phenotype or 20 ng/ml recombinant human IL-4 (R&D Systems) for differentiation to the M2 phenotype (22).
General laboratory methods
RNA extraction, cDNA synthesis, and quantitative PCR
RNA was isolated from adipose tissue, adipocytes, and macrophages using Trizol reagent (Invitrogen, Carlsbad, CA) and was reverse transcribed (500 ng) to cDNA (High Capacity cDNA Archive Kit; Applied BioSystems (ABI), Foster City, CA). For validation, expression of genes was determined by real-time PCR (ABI7900 system) using TaqMan Universal PCR MasterMix, primers and probes. To control for between-sample differences, mRNA levels were normalized to β-actin for each sample by subtracting the cycle threshold (Ct) for β-actin from the Ct for gene of interest, producing a ΔCt value. The ΔCt for each posttreatment sample was compared with the mean ΔCt for all pretreatment samples using the relative quantitation 2−ΔΔCt method to determine fold change from baseline (23).
Statistical analysis of quantitative PCR data
For validation study adipose tissue, fold changes at each time point were estimated using the method of generalized estimating equations to account for within-subject correlations. For adipocytes and macrophages, analysis of mRNA fold change used geometric means of the independent replicated samples. For the time point with peak fold change, the mean and 95% confidence intervals are reported for all studies. Analyses were performed using Stata version 10 (StataCorp LP, College Station, TX).
Results
Clinical response to endotoxin
Demographic and baseline metabolic characteristics for subjects in both endotoxin studies are shown in Supplemental Table 1 (published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org). As reported (12, 15), there was a transient clinical and biochemical inflammatory response to the moderate 3-ng/kg LPS endotoxin dose, whereas the lower 0.6-ng/kg LPS dose produced a subclinical response with modest increases in plasma cytokines but no significant symptoms or fever.
Endotoxin reduced expression of many adipose genes
Of the 776 unique genes with differential expression at any time point after 3 ng/kg endotoxin administration (12), approximately half (353 genes) showed reduced expression, with the majority of these (73%) suppressed at the 4-h time point. A total of 110 genes were down-regulated more than 3-fold from baseline.
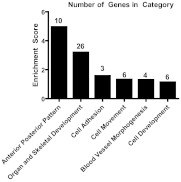
Prioritization as outlined in Subjects and Methods identified 68 of the most highly repressed, biologically relevant, and novel down-regulated genes for further study. Figure 2 depicts the functional classification of these genes. Remarkably, these LPS-repressed genes were almost all exclusively linked to developmental functions and pathways, including organogenesis, vascularization, and anterior-posterior patterning. Suppression of expression for this set of genes was examined by RT-PCR in the validation study samples, an independent sample of human adipose tissue obtained after lower-dose (0.6 ng/kg) endotoxin. In this study, 22 of the 68 genes (32%) were found to be down-regulated more than 1.5-fold (>33% reduction in expression) from baseline in this milder, subclinical model of induced inflammation. Supplemental Table 2 describes mRNA fold changes of these 22 genes at peak suppression in the low-dose validation study, with the microarray peak suppression during moderate-dose LPS presented for comparison.
Fig. 2.
DAVID (Database for Annotation, Visualization, and Integrated Discovery) functional categories with enrichment scores. Functional classification is of all prioritized down-regulated genes (n = 68). Numbers of genes in each category are listed above each bar. Nine genes (many with unknown functions) did not classify into any known categories.
A subset of genes repressed in adipose tissue by inflammation are also suppressed by inflammation in cultured adipocytes and M2 macrophages
The cellular source of validated adipose down-regulated genes was probed in primary human adipocytes after endotoxin treatment and primary human macrophages after polarization to M1 (classically activated, inflammatory) or M2 (alternatively activated, antiinflammatory) macrophages. Of the 22 validated genes, 59% (13 of 22) were present and down-regulated more than 1.5-fold in primary human adipocytes after treatment with endotoxin (P < 0.05 vs. untreated adipocytes). In fact, of the 18 genes detectable in adipocytes, all 18 trended toward decreased expression. Macrophages displayed a markedly different pattern with a tendency toward up-regulation in M1 macrophages and down-regulation in M2 macrophages. Of the 22 genes studied, 13 (59%) showed increased expression (fold change >1) upon polarization to M1 macrophages, with 10 genes (45%) induced more than 1.5-fold (P < 0.05 vs. resting macrophages). Polarization to the M2 phenotype resulted in reduced expression (fold change <1) in 14 of 22 genes (64%), with nine of these (41%) suppressed more than 1.5-fold (P < 0.05 vs. resting macrophages). Nine of 22 genes were suppressed in M2 macrophages but up-regulated in the M1 subtype. Two genes (LRMP and FLJ14213) were down-regulated in both M1 and M2 macrophages, whereas two (SSTR1 and KIAA0644) were up-regulated in both phenotypes. Comparing adipocytes and macrophages, 12 of 22 genes were suppressed in both M2 macrophages and adipocytes, but only six of 22 were suppressed in M1 macrophages and adipocytes. The maximal fold changes in adipocytes and the fold change in M1 and M2 macrophages are shown in Table 1.
Table 1.
Expression pattern of validated adipose genes (n = 22) by endotoxin in human adipocytes and during polarization to M1 and M2 macrophages
| Adipocytes with endotoxin | M1 macrophage | M2 macrophage | |
|---|---|---|---|
| ALS2CR13 | 0.58 (2 h)a | 1.29 | 0.91 |
| CARD10 | 0.86 (24 h) | 1.90 | 0.43a |
| CRHBP | 0.58 (2 h)a | 0.25a | 0.70 |
| HOXA9 | 0.65 (4 h)a | 2.71a | 1.38 |
| ISLR2 | 0.33 (12 h)a | 2.30a | 0.22a |
| NR1D1 | 0.26 (4 h)a | 2.69a | 0.14a |
| NR1D2 | 0.89 (4 h) | 4.76a | 1.33 |
| OTOA1 | 0.38 (2 h)a | 1.94a | 0.97 |
| SSTR1 | 0.15 (12 h)a | 3.88a | 11.7a |
| THSD1 | 0.42 (2 h)a | 4.29a | 0.23a |
| C1ORF51 | 0.61 (4 h)a | 2.10a | 0.89 |
| CETP | ND | 1.58 | 0.52a |
| FLJ14213 | 0.54 (4 h)a | 0.57a | 0.58a |
| GNAZ | 0.75 (12 h) | ND | ND |
| KIAA0644 | 0.6 (12 h)a | 39.8a | 19.2a |
| KLH14 | ND | 3.81a | 0.57a |
| LHX6 | ND | ND | ND |
| LRMP | 0.33 (4 h)a | 0.42a | 0.31a |
| NOG | 0.44 (4 h)a | 0.79 | 1.31 |
| OR51E1 | ND | ND | ND |
| PER3 | 0.83 (24 h) | 0.81 | 0.44a |
| SERTAD4 | 0.74 (2 h) | 0.66a | 0.83 |
Maximal fold changes (time point) after endotoxin treatment in adipocytes and fold changes in M1 or M2 compared with unpolarized resting macrophages are presented. Values shown represent mean values of three experiments in triplicate. ND, Not detectable; Ct higher than 35.
P < 0.05 from unstimulated adipocytes or unpolarized macrophages.
Adipose tissue genes repressed by induced inflammation are also suppressed in human obesity
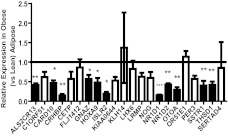
Of the 22 inflammation-suppressed genes validated in the low-dose endotoxemia study, the expression of 20 tended to be reduced in obese vs. lean adipose (20 observed vs. 11 expected under null association, χ2 = 10.72, P < 0.01). Furthermore, 11 (50%) had reduced levels of expression, reaching at least a nominal statistical threshold (P < 0.05) in adipose tissue of obese vs. lean (11 observed vs. approximately one expected by chance under the null of no association, χ2 = 9.28, P < 0.01), all of which were down-regulated by at least 2-fold. This subset of genes encode proteins with a variety of functions including transcriptional regulation (HOXA9), endocrine axes (CRHBP and SSTR1), molecular clock (NR1D1 and NR1D2), cell adhesion (THSD1), and immune regulation (CARD10 and ISLR2), as well as those with no known relevant function (ALS2CR13 and OTOA1). Figure 3 depicts the relative expression level of all 22 genes in obese compared with lean adipose tissue.
Fig. 3.
Expression of validated genes in obese adipose tissue. Values shown are mean ± sd of mRNA expression levels of 22 validated genes in obese tissue, when expression in lean tissue is set to 1. Twenty of 22 genes tended to have lower expression in obese vs. lean tissues (observed vs. expected χ2 = 10.72, P < 0.01). Shaded bars represent the 11 genes (50%) that were reduced at a P value <0.05 threshold (observed vs. expected χ2 = 9.28, P < 0.01) in obese vs. lean adipose. *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Discussion
Obesity-related disease, including type 2 diabetes, presents a major medical challenge; elucidating the underlying biologic mechanisms in humans will expand available preventive and therapeutic options. A crucial link between obesity and its metabolic complications is adipose tissue inflammation. Determination of mechanistic factors in inflammation-induced metabolic perturbations may lead to novel treatments selectively targeting involved adipose inflammatory pathways. Although such genes and pathways have been explored extensively in animal models, validated candidates that mediate, or serve as biomarkers for, complications of human adiposity remain limited. Here, we explored genes down-regulated in adipose tissue during evoked inflammation in human and identified suppression of novel adipose genes involved in cell development and differentiation. Remarkably, the majority of these were also suppressed in severe endogenous human obesity, suggesting their involvement in clinical obesity-related adipose inflammation and disease.
The use of the human endotoxemia model has been validated by our group and others as a reproducible probe to evoke insulin resistance and systemic and adipose-tissue-specific inflammatory changes similar to those observed in obesity (8, 11, 15). Thus, we chose this in vivo model to pursue an unbiased approach to identify novel adipose tissue inflammatory genes. Adipose genes modulated by inflammation may represent mediators in the link between inflammation and obesity-related metabolic dysregulation. Many adipose genes and proteins regulated during endotoxemia are known to modulate metabolic pathways, cell differentiation, or cell-cell interactions, whereas others have unknown function in adipose. Indeed, our previous focus on endotoxemia-up-regulated and secreted genes and proteins led to our identification of fractalkine (CX3CL1) as a novel inflammatory adipochemokine in humans (12, 14).
Functional classification of validated down-regulated genes during endotoxemia reveals many genes, including transcription factors, with developmental functions suggesting regression of adipose tissue to a less differentiated state. This is particularly relevant in adipose biology because decreased activity of several developmental genes (e.g. BMP and HOX genes) has been shown in vitro and in vivo in animal models to prevent adipose differentiation (24, 25). The subset of genes repressed in obese adipose tissue encode proteins with a wide variety of functions: transcriptional regulation (HOXA9), endocrine axes (CRHBP and SSTR1), molecular clock (NR1D1 and NR1D2), cell adhesion (THSD1), and immune regulation (CARD10 and ISLR2) as well as those with no known relevant function (ALS2CR13 and OTOA1). Of note, the majority of these genes have not yet been implicated in obesity, adipose inflammation, or metabolic diseases. Thus, they may represent novel functional mediators of obesity-induced adipose inflammation in human cardiometabolic disease.
Previous work on several of these suppressed genes, or those in related pathways, suggests that they might be involved in adipose biology and/or inflammatory response. Multiple human and animal studies have shown alteration in adipose tissue molecular clock gene expression with obesity, high-fat feeding, or metabolic syndrome (26–29), although Otway et al. (30) has recently suggested that there is no change in the rhythmic expression of these genes in human sc adipose tissue with obesity or type 2 diabetes. Expression of somatostatin receptors, including SSTR1, is regulated in human adipocytes with inflammatory stimuli. Protein levels of the ligand, somatostatin, are also increased in adipose tissue with cytokine or endotoxin treatment, supporting a role of this system in adipose inflammation in obesity (31). CARD10 has been implicated directly in nuclear factor-κB activation by various cytokines and chemokines that themselves are associated with cardiometabolic disease (32, 33), suggesting that this gene product may have a unifying role in mediating inflammatory signaling.
In contrast to our previous findings in which genes up-regulated by endotoxin in adipose were induced in inflamed cultured adipocytes and inflammatory M1 macrophages (12), here we reveal a more complex pattern of cellular interaction and macrophage gene regulation. The majority of our validated down-regulated genes were suppressed in inflamed cultured adipocytes, suggesting that adipocytes are the major adipose origin of the observed in vivo changes. In macrophages, many of the validated down-regulated genes also tended to be suppressed in the M2 phenotype, suggesting the loss of homeostatic antiinflammatory macrophage functions similar to that reported in human and animal models of obesity (22, 34). In contrast, the majority of down-regulated genes were actually induced during differentiation to M1 macrophages, suggesting cell-specific regulation and activation of the M1 inflammatory subtype in inflamed human adipose. It remains to be determined in future studies whether coordinated but cell-specific and differential responses in adipocytes and M2 macrophages (often considered more homeostatic) vs. M1 macrophages (often considered more inflammatory and pathophysiological in chronic settings) may be critical for normal physiological vs. pathophysiological responses in adipose to inflammatory stress and energy excess.
Our study has several limitations. Gene expression was compared in different sc adipose depots; the lean samples were obtained from gluteal adipose biopsies, whereas the obese samples were obtained from abdominal adipose during bariatric surgery. We recognize that differences in expression may in some cases be driven by depot and not necessarily by obesity. However, recent studies examining gene expression in these two depots suggest significant overlap in expression between the two sites. Furthermore, genes that do show differential expression by depot are likely to be associated with metabolic phenotype, underscoring biological relevance in obesity-related metabolic disease (35). Our obese subjects were morbidly obese and likely had comorbid medical conditions. Thus, the relevance of our findings is limited to this population, and additional studies in early obesity or less extreme phenotypes are needed. Additionally, we acknowledge that our acute evoked inflammatory model does not reproduce the chronic low-grade changes in diet- and obesity-related adipose inflammation. However, several lines of evidence support its utility in modeling the chronic low-grade activation of innate immunity observed in cardiometabolic disorders. First, the transient inflammatory and metabolic responses to endotoxemia resemble those observed in insulin resistance and metabolic syndrome (8, 9). Second, rodent models have proven that adipose inflammatory pathways play important roles in diet- and obesity-related metabolic disturbance (36–38). Finally, mRNA microarray and secretome profiling of human adipose tissue and adipocytes identify inflammatory pathways in obesity similar to those classically activated by endotoxin (1, 39, 40).
For our top validated candidates, mechanistic studies in animal and human in vitro models are required to determine functional role in pathophysiology of obesity and high-fat diet-induced adipose inflammation and metabolic disturbance. Human studies examining association of genetic variation or expression changes in these genes with specific metabolic phenotypes are needed to support relevance to human disease. Ultimately, human clinical studies examining the effects of therapeutics that target specific genes and proteins will be needed to translate our findings into clinical practice in the treatment of obesity and related disease.
In summary, exploration of gene expression changes in adipose tissue during human endotoxemia identified many genes with reduced expression that have potential roles in obesity and related metabolic dysfunction. The cellular origins of the suppressed adipose genes include adipocytes and homeostatic antiinflammatory macrophages. Remarkably, the vast majority of the genes down-regulated by evoked human inflammation were also suppressed in severe endogenous human obesity, suggesting a biological role in the pathophysiology of obesity-related adipose inflammation in clinical disease.
Acknowledgments
This work was supported by a Clinical and Translational Science Award (UL1RR024134) from the National Center for Research Resources and a Diabetes and Endocrine Research Center Award (P20-DK 019525), both to University of Pennsylvania, and by R01 HL-073278 and a P50 HL-083799-SCCOR Project Award (to M.P.R.). M.P.R. is also supported by R01-DK-090505, R01-DK071224, U01-HL108636, and K24-HL107643 from the National Institutes of Health (NIH). N.N.M. is supported by 5K23HL097151 from NIH. R.S. is supported by the University of Pennsylvania Clinical and Translational Science Award K12 KL1RR024132 from NIH.
R.S. researched data, contributed to discussion, and wrote the manuscript. C.C.H. researched data and contributed to discussion. R.S. researched data. N.N.M. researched data and edited the manuscript. M.E.P. researched data and reviewed and edited the manuscript. M.P.R. researched data, contributed to discussion, and reviewed and edited the manuscript.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- BMI
- Body mass index
- Ct
- cycle threshold
- LPS
- lipopolysaccharide.
References
- 1. Alvarez-Llamas G, Szalowska E, de Vries MP, Weening D, Landman K, Hoek A, Wolffenbuttel BH, Roelofsen H, Vonk RJ. 2007. Characterization of the human visceral adipose tissue secretome. Mol Cell Proteomics 6:589–600 [DOI] [PubMed] [Google Scholar]
- 2. Huber J, Kiefer FW, Zeyda M, Ludvik B, Silberhumer GR, Prager G, Zlabinger GJ, Stulnig TM. 2008. CC chemokine and CC chemokine receptor profiles in visceral and subcutaneous adipose tissue are altered in human obesity. J Clin Endocrinol Metab 93:3215–3221 [DOI] [PubMed] [Google Scholar]
- 3. Kim F, Pham M, Luttrell I, Bannerman DD, Tupper J, Thaler J, Hawn TR, Raines EW, Schwartz MW. 2007. Toll-like receptor-4 mediates vascular inflammation and insulin resistance in diet-induced obesity. Circ Res 100:1589–1596 [DOI] [PubMed] [Google Scholar]
- 4. Nguyen MT, Favelyukis S, Nguyen AK, Reichart D, Scott PA, Jenn A, Liu-Bryan R, Glass CK, Neels JG, Olefsky JM. 2007. A subpopulation of macrophages infiltrates hypertrophic adipose tissue and is activated by free fatty acids via Toll-like receptors 2 and 4 and JNK-dependent pathways. J Biol Chem 282:35279–35292 [DOI] [PubMed] [Google Scholar]
- 5. Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. 1999. Elevated C-reactive protein levels in overweight and obese adults. JAMA 282:2131–2135 [DOI] [PubMed] [Google Scholar]
- 6. Poitou C, Viguerie N, Cancello R, De Matteis R, Cinti S, Stich V, Coussieu C, Gauthier E, Courtine M, Zucker JD, Barsh GS, Saris W, Bruneval P, Basdevant A, Langin D, Clément K. 2005. Serum amyloid A: production by human white adipocyte and regulation by obesity and nutrition. Diabetologia 48:519–528 [DOI] [PubMed] [Google Scholar]
- 7. Olszanecka-Glinianowicz M, Zahorska-Markiewicz B, Janowska J, Zurakowski A. 2004. Serum concentrations of nitric oxide, tumor necrosis factor (TNF)-α and TNF soluble receptors in women with overweight and obesity. Metabolism 53:1268–1273 [DOI] [PubMed] [Google Scholar]
- 8. Agwunobi AO, Reid C, Maycock P, Little RA, Carlson GL. 2000. Insulin resistance and substrate utilization in human endotoxemia. J Clin Endocrinol Metab 85:3770–3778 [DOI] [PubMed] [Google Scholar]
- 9. Hudgins LC, Parker TS, Levine DM, Gordon BR, Saal SD, Jiang XC, Seidman CE, Tremaroli JD, Lai J, Rubin AL. 2003. A single intravenous dose of endotoxin rapidly alters serum lipoproteins and lipid transfer proteins in normal volunteers. J Lipid Res 44:1489–1498 [DOI] [PubMed] [Google Scholar]
- 10. Suffredini AF, Fromm RE, Parker MM, Brenner M, Kovacs JA, Wesley RA, Parrillo JE. 1989. The cardiovascular response of normal humans to the administration of endotoxin. N Engl J Med 321:280–287 [DOI] [PubMed] [Google Scholar]
- 11. Anderson PD, Mehta NN, Wolfe ML, Hinkle CC, Pruscino L, Comiskey LL, Tabita-Martinez J, Sellers KF, Rickels MR, Ahima RS, Reilly MP. 2007. Innate immunity modulates adipokines in humans. J Clin Endocrinol Metab 92:2272–2279 [DOI] [PubMed] [Google Scholar]
- 12. Shah R, Lu Y, Hinkle CC, McGillicuddy FC, Kim R, Hannenhalli S, Cappola TP, Heffron S, Wang X, Mehta NN, Putt M, Reilly MP. 2009. Gene profiling of human adipose tissue during evoked inflammation in vivo. Diabetes 58:2211–2219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. 2001. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-α in the adipose tissue of obese mice. J Biol Chem 276:47944–47949 [DOI] [PubMed] [Google Scholar]
- 14. Shah R, Hinkle CC, Ferguson JF, Mehta NN, Li M, Qu L, Lu Y, Putt ME, Ahima RS, Reilly MP. 2011. Fractalkine is a novel human adipochemokine associated with type 2 diabetes. Diabetes 60:1512–1518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mehta NN, McGillicuddy FC, Anderson PD, Hinkle CC, Shah R, Pruscino L, Tabita-Martinez J, Sellers KF, Rickels MR, Reilly MP. 2010. Experimental endotoxemia induces adipose inflammation and insulin resistance in humans. Diabetes 59:172–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Shah A, Mehta N, Reilly MP. 2008. Adipose inflammation, insulin resistance, and cardiovascular disease. JPEN J Parenter Enteral Nutr 32:638–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bolstad BM, Irizarry RA, Astrand M, Speed TP. 2003. A comparison of normalization methods for high density oligonucleotide array data based on variance and bias. Bioinformatics 19:185–193 [DOI] [PubMed] [Google Scholar]
- 18. Cui X, Hwang JT, Qiu J, Blades NJ, Churchill GA. 2005. Improved statistical tests for differential gene expression by shrinking variance components estimates. Biostatistics 6:59–75 [DOI] [PubMed] [Google Scholar]
- 19. Dennis G, Jr, Sherman BT, Hosack DA, Yang J, Gao W, Lane HC, Lempicki RA. 2003. DAVID: Database for Annotation, Visualization, and Integrated Discovery. Genome Biol 4:P3. [PubMed] [Google Scholar]
- 20. Huang da W, Sherman BT, Lempicki RA. 2009. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc 4:44–57 [DOI] [PubMed] [Google Scholar]
- 21. Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. 2004. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med 1:e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Martinez FO, Sica A, Mantovani A, Locati M. 2008. Macrophage activation and polarization. Front Biosci 13:453–461 [DOI] [PubMed] [Google Scholar]
- 23. Schmittgen TD, Livak KJ. 2008. Analyzing real-time PCR data by the comparative C(T) method. Nat Protoc 3:1101–1108 [DOI] [PubMed] [Google Scholar]
- 24. Cantile M, Procino A, D'Armiento M, Cindolo L, Cillo C. 2003. HOX gene network is involved in the transcriptional regulation of in vivo human adipogenesis. J Cell Physiol 194:225–236 [DOI] [PubMed] [Google Scholar]
- 25. Maekawa T, Jin W, Ishii S. 2010. The role of ATF-2 family transcription factors in adipocyte differentiation: antiobesity effects of p38 inhibitors. Mol Cell Biol 30:613–625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ando H, Kumazaki M, Motosugi Y, Ushijima K, Maekawa T, Ishikawa E, Fujimura A. 2011. Impairment of peripheral circadian clocks precedes metabolic abnormalities in ob/ob mice. Endocrinology 152:1347–1354 [DOI] [PubMed] [Google Scholar]
- 27. Gómez-Santos C, Gómez-Abellán P, Madrid JA, Hernández-Morante JJ, Lujan JA, Ordovas JM, Garaulet M. 2009. Circadian rhythm of clock genes in human adipose explants. Obesity 17:1481–1485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kohsaka A, Laposky AD, Ramsey KM, Estrada C, Joshu C, Kobayashi Y, Turek FW, Bass J. 2007. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab 6:414–421 [DOI] [PubMed] [Google Scholar]
- 29. Gómez-Abellán P, Hernández-Morante JJ, Lujan JA, Madrid JA, Garaulet M. 2008. Clock genes are implicated in the human metabolic syndrome. Int J Obes (Lond) 32:121–128 [DOI] [PubMed] [Google Scholar]
- 30. Otway DT, Mantele S, Bretschneider S, Wright J, Trayhurn P, Skene DJ, Robertson MD, Johnston JD. 2011. Rhythmic diurnal gene expression in human adipose tissue from individuals who are lean, overweight, and have type 2 diabetes. Diabetes 60:1577–1581 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Seboek D, Linscheid P, Zulewski H, Langer I, Christ-Crain M, Keller U, Müller B. 2004. Somatostatin is expressed and secreted by human adipose tissue upon infection and inflammation. J Clin Endocrinol Metab 89:4833–4839 [DOI] [PubMed] [Google Scholar]
- 32. Rehman AO, Wang CY. 2009. CXCL12/SDF-1 α activates NF-κB and promotes oral cancer invasion through the Carma3/Bcl10/Malt1 complex. Int J Oral Sci 1:105–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Martin D, Galisteo R, Gutkind JS. 2009. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFκB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem 284:6038–6042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lumeng CN, Bodzin JL, Saltiel AR. 2007. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 117:175–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Min JL, Nicholson G, Halgrimsdottir I, Almstrup K, Petri A, Barrett A, Travers M, Rayner NW, Mägi R, Pettersson FH, Broxholme J, Neville MJ, Wills QF, Cheeseman J, Allen M, Holmes CC, Spector TD, Fleckner J, McCarthy MI, Karpe F, Lindgren CM, Zondervan KT. 2012. Coexpression network analysis in abdominal and gluteal adipose tissue reveals regulatory genetic loci for metabolic syndrome and related phenotypes. PLoS Genet 8:e1002505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hotamisligil GS. 2003. Inflammatory pathways and insulin action. Int J Obes Relat Metab Disord 27(Suppl 3):S53–S55 [DOI] [PubMed] [Google Scholar]
- 37. McCowen KC, Ling PR, Ciccarone A, Mao Y, Chow JC, Bistrian BR, Smith RJ. 2001. Sustained endotoxemia leads to marked down-regulation of early steps in the insulin-signaling cascade. Crit Care Med 29:839–846 [DOI] [PubMed] [Google Scholar]
- 38. Shoelson SE, Lee J, Goldfine AB. 2006. Inflammation and insulin resistance. J Clin Invest 116:1793–1801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gómez-Ambrosi J, Catalán V, Diez-Caballero A, Martinez-Cruz LA, Gil MJ, García-Foncillas J, Cienfuegos JA, Salvador J, Mato JM, Frühbeck G. 2004. Gene expression profile of omental adipose tissue in human obesity. FASEB J 18:215–217 [DOI] [PubMed] [Google Scholar]
- 40. Nair S, Lee YH, Rousseau E, Cam M, Tataranni PA, Baier LJ, Bogardus C, Permana PA. 2005. Increased expression of inflammation-related genes in cultured preadipocytes/stromal vascular cells from obese compared with nonobese Pima Indians. Diabetologia 48:1784–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]