Abstract
Context:
Microcephalic primordial dwarfism (MPD) is a rare, severe form of human growth failure in which growth restriction is evident in utero and continues into postnatal life. Single causative gene defects have been identified in a number of patients with MPD, and all involve genes fundamental to cellular processes including centrosome functions.
Objective:
The objective of the study was to find the genetic etiology of a novel presentation of MPD.
Design:
The design of the study was whole-exome sequencing performed on two affected sisters in a single family. Molecular and functional studies of a candidate gene were performed using patient-derived primary fibroblasts and a zebrafish morpholino oligonucleotides knockdown model.
Patients:
Two sisters presented with a novel subtype of MPD, including severe intellectual disabilities.
Main Outcome Measures:
NIN, encoding Ninein, a centrosomal protein critically involved in asymmetric cell division, was identified as a candidate gene, and functional impacts in fibroblasts and zebrafish were studied.
Results:
From 34,606 genomic variants, two very rare missense variants in NIN were identified. Both probands were compound heterozygotes. In the zebrafish, ninein knockdown led to specific and novel defects in the specification and morphogenesis of the anterior neuroectoderm, resulting in a deformity of the developing cranium with a small, squared skull highly reminiscent of the human phenotype.
Conclusion:
We identified a novel clinical subtype of MPD in two sisters who have rare variants in NIN. We show, for the first time, that reduction of ninein function in the developing zebrafish leads to specific deficiencies of brain and skull development, offering a developmental basis for the myriad phenotypes in our patients.
Microcephalic primordial dwarfism (MPD) is a rare, severe form of human growth failure in which growth restriction is evident in utero and continues into postnatal life. A number of disease entities share this growth phenotype, including Seckel syndrome (1, 2) (online inheritance in man 210600, 606744, 61376, and 613823), Microcephalic osteodysplastic primordial dwarfism types I and II (MOPDII; online inheritance in man 210720) (3, 4), and Meier-Gorlin syndrome (5, 6). Single causative gene defects have recently been identified in a number of these affected patients (7), and all involve genes fundamental to cellular processes such as genomic replication (ORC1, ORC4, ORC6, CDT1, and CDC6), DNA damage response [ATR (8)], and centrosome functions (PCNT, CENPJ, CEP152, CEP63, and RBBP8) (7). Interestingly, the total loss of the endocrine growth-promoting factor, IGF-I, due to inactivating mutations in the IGF1 gene, produces a growth phenotype similar to MPD (9, 10). Mutations in the IGF-I receptor (IGF1R), which confer resistance to IGF-I growth effects, result in a phenotype that resembles, in the most severe cases, a milder form of MPD.
Centrosomes are the main cytoplasmic microtubule-organizing centers in animal cells and are integral for faithful cell division, cell polarity, and cell cycle control (11). The core structure during the centrosome cycle is composed of two asymmetric microtubular centrioles, a mature mother and a younger daughter centriole, which recruit a matrix of associated pericentriolar material. Mutations in five centrosomal genes identified in subjects with MPD have been shown to disrupt centrosome integrity (PCNT) or centriole biogenesis (CENPJ, CEP152, CEP63, and RBBP8), thereby impacting mitosis and globally altering the efficiency of cell proliferation during prenatal and postnatal growth.
We now report the association of MPD with rare genetic variants in the NIN gene, encoding Ninein, a protein that is targeted to centrosomes during interphase (12–14) and is associated with asymmetric cell division (15–17). The two affected siblings presented clinical MPD features that were not consistent with MOPDII or Seckel syndrome. Both siblings were insensitive to the growth-promoting effects of GH therapy, despite normal levels of serum IGF-I. Analysis of proliferation and spindle organization in patient fibroblasts was unrevealing. Studies of ninein function in the zebrafish, however, indicated essential functions of ninein during early patterning and morphogenesis of the brain leading to cranial phenotypes qualitatively similar to MPD. Our data suggest that elements of the MPD pathophysiology may arise due to specific developmental roles for centrosome regulators apart from their role(s) in cell division.
Subjects and Methods
Case descriptions
The subject cases of this report are female siblings, currently 22 (subject 1) and 18 (subject 2) yr of age. Their parents are of native Indian ancestry from Michoacán, Mexico; to their knowledge, they are not related. Maternal and paternal heights are −2.10 and −1.62 sd, respectively [standards of the Centers for Disease Control and Prevention (18)]; they do not have dysmorphic features, are of normal intellect, and in good health. Maternal head circumference is 54.0 cm (35%) and paternal head circumference is 56.7 cm (75%).
Subject 1
The product of a term gestation, subject 1 has severe prenatal and postnatal growth retardation, microcephaly, and developmental delay (Fig. 1 and Table 1). Diagnostic laboratory evaluation included a normal chromosome analysis and assessment of the GH-IGF-1 axis (Table 1). At age 2 yr 4 months, she was started on a trial of GH treatment, which she received intermittently until age 14 yr. Her growth velocity was subnormal for age when not receiving GH therapy and showed only a small improvement with treatment, which was not sustained. Throughout childhood, her bone age was delayed, on average by 3 yr compared with her chronological age. However, at age 14 yr, her bone age was equivalent to her chronological age and GH treatment was discontinued. Her final adult height is 115 cm (−7.35 sd). Measurement of body proportion showed short limbs, with legs affected more than arms (Table 1). She first developed pubic hair by age 13 yr but had no breast development at that time. At age 18 yr, she had primary amenorrhea and Tanner 1 breast development, with Tanner 5 pubic hair. At that time, serum LH and FSH were in the normal ranges at 4.5 mIU/ml [normal range (NR) 1.5–9.0] and 3.6 mIU/ml (NR 2.0–9.2), respectively, with a modestly low estradiol of 26 pg/ml (NR 30–300). Pelvic ultrasound showed relatively normal ovarian development, with the right ovary measuring 11.6 cc and the left ovary measuring 6.6 cc. Bilateral follicles were present, but her uterus was prepubertal, measuring 3.5 cm in length. She was started on estrogen replacement therapy. Serum thyroid function tests showed borderline central hypothyroidism (Table 1), and with worsening obesity, she was started on a trial of thyroid hormone. Adrenal function was normal on stimulation testing (Table 1).
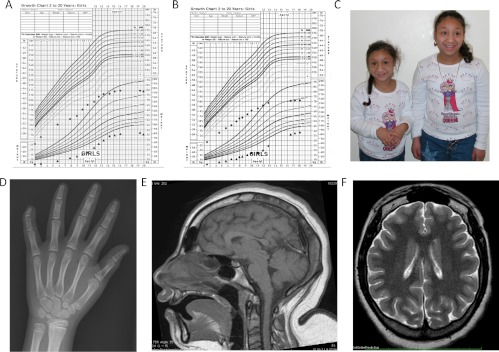
Fig. 1.
Growth curves, MRI, and pictures. A, Subject 1's growth chart. B, Subject 2's growth chart. C, Subjects 1 and 2 at ages 11 and 16 yr. D, Bone age x-ray of subject 2 at age 11 yr demonstrating clinodactyly. E and F, MRI images from subject 2's MRI at age 20 yr show severe microcephaly with the cerebral hemispheres affected more severely than the cerebellum in addition to a slightly immature sulcation pattern.
Table 1.
Growth data, physical examination, and laboratory findings in the cases
| Case 1 | Case 2 | |
|---|---|---|
| Sex | Female | Female |
| Growth characteristics | ||
| Birth weight (g/SDS) | 1590 (−3.6) | 2017 (−3.0) |
| Birth length (cm/SDS) | Unknown | 38.1 (−7.45) |
| Body proportions: upper/lower (cm) | 65/50 = 1.30 | 63.7/43 = 1.48 |
| Adult height (cm/SDS) | 115 (−7.35) | 106.7 (−8.63) |
| Adult weight (kg/SDS) | 35.5 (−4.38) | 38.0 (−3.45) |
| Adult head circumference (cm/SDS) | 44.8 cm (−6.8) | 45.0 cm (−6.7 sd) |
| Puberty | Absent breast development, primary amenorrhea | Absent breast development, primary amenorrhea |
| Paternal height (cm/SDS) | 165.2 (−1.62) | 165.2 (−1.62) |
| Maternal height (cm/SDS) | 149.7 (−2.10) | 149.7 (−2.10) |
| Physical characteristics | ||
| Microcephaly | Severe | Severe |
| Facies | Hypotelorism | Hypotelorism |
| Small ears | Small ears | |
| Slightly large nose | Slightly large nose | |
| Vision | Normal | Normal |
| Hearing | Normal | Normal |
| Fingers | Clinodactyly fifth fingers | Clinodactyly fifth fingers |
| Short fifth middle phalanx | ||
| Abnormal carpal bones | ||
| Extremities | Madelung deformity | Madelung deformity |
| Spine | Lumbar scoliosis, mild | Lumbar scoliosis, mild |
| Hip | Bilateral hip dysplasia | Bilateral hip dysplasia |
| Laboratory findings | ||
| Karyotype | 46XX (19 months)a | ND |
| Bone age | 2- to 3-yr delay during childhood | 2- to 3-yr delay during childhood |
| Free T4 (0.6–1.2 ng/dl) | 0.7 (19 yr) | 0.6 (16 yr) |
| TSH (0.34–5.60 mU/liter) | 3.91 (19 yr) | 1.67 (19 yr) |
| GH peak (ng/ml, >7) | 21 (19 months) | NDa |
| GHBP (534–5785 pmol/liter) | 4191 (16 yr) | 3039 (12 yr) |
| IGF-I basal (ng/ml) | 83 (30–122) (19 months) | 126.2 (117–771) (2 yr) |
| IGF-I on GH (261–1096 ng/mL) | 742 (12 yr) | 505 (10 yr) |
| IGFBP-3 (2.2–5.9 mg/liter) | 3.48 (16 yr) | 2.60 (12 yr) |
| Cortisol, baseline (mcg/dl) | 15.7 (22 yr) | 8 (18 yr) |
| Cortisol, 30 min after 1 μg cosyntropin | 20.0 | 22.5 |
sd scores are based on the standards of the U.S. Centers for Disease Control and Prevention (18). ND, Not done; SDS, sd score; GHBP, GH binding protein; IGFBP, IGF binding protein.
Age test carried out (in parentheses).
Other significant medical problems included congenital bilateral hip dysplasia requiring orthopedic surgical correction in infancy, subglottic stenosis also requiring surgical correction in infancy, and seizures starting at age 5 months, controlled by anticonvulsant therapy. She has severe microcephaly, with head circumference measuring 44.8 cm, (−6.8 sd). A magnetic resonance imaging (MRI) of the brain at age 20 yr showed the cerebral hemispheres affected more severely than the cerebellum, in addition to a slightly immature sulcation pattern (Fig. 1). There were no gross malformations. She has significant developmental delay. At age 22 yr, her intellectual skills are estimated to be at a preschool level; she has no knowledge of letters or numbers, has occasional difficulties in communicating her needs, and is not toilet trained.
Subject 2
The clinical phenotype of this subject was identical to her older sister, with severe prenatal and postnatal growth retardation, microcephaly, and development delay noted (Fig. 1 and Table 1). At age 5 yr, she was started on a trial of GH treatment, but with poor response, and treatment was discontinued at age 10 yr. Throughout childhood, her bone age was delayed, on average by 3 yr. Her final adult height is 106.7 cm (−8.63 sd). She had onset of pubic hair growth at age 12 yr, and at age 16 yr, like her sister, she had primary amenorrhea, Tanner 1 breast development, and Tanner 5 pubic hair growth. Serum LH and FSH were in the normal ranges, 8.9 and 5.4 mIU/ml, respectively, with a borderline low serum estradiol of 35 pg/ml. Pelvic ultrasound showed early ovarian development, with the right ovary measuring 3.4 cc and the left ovary measuring 2.5 cc; no follicles were visible. The uterus was prepubertal, measuring 3.6 cm in length. She was started on estrogen replacement therapy. Serum thyroid function tests showed borderline central hypothyroidism (Table 1), and, with worsening obesity, she also was started on thyroid hormone replacement. As in her sister, adrenal function was normal on stimulation testing (Table 1).
Other significant medical problems included congenital bilateral hip dysplasia and seizures starting at age 18 months, controlled by anticonvulsant therapy. She has severe microcephaly, with head circumference measuring 45.0 cm (−6.7 sd), and at age 18 yr, her intellectual skills, similar to her sister, are estimated at a preschool level.
Methods
Written informed consent for all studies, including permission to use photographs of the patients, was obtained from the subject's parents. This study was approved by the Institutional Review Board at Oregon Health and Science University (Portland, OR).
Biochemical assays
Free T4, TSH, cortisol, and serum GH were determined in-house (Oregon Health and Science University/Kaiser laboratory). Serum IGF-I, IGFBP-3, LH, FSH, and estradiol were analyzed at Esoterix Laboratory Services Inc. (Calabasas Hills, CA).
Genomic DNA and whole-genome typing
Genomic DNA was extracted from whole blood and from primary fibroblasts as previously described (19). Targeted gene sequencing of the IGF1 and IGF1R genes was as described (20). Whole-genome single-nucleotide polymorphism (SNP) genotyping was performed on the two subjects and both parents using the Omni-1 Quad genotyping platform. Identity by descent was estimated using PLINK version 1.07 (21). Blocks of missing genotype data were investigated using PLINK to search for areas of homozygous deletions. Whole-exome sequencing of both affected probands was performed at the Broad Institute. Hybrid selection was performed using Agilent's SureSelect human all exon kit version 2 (Agilent Technologies, Santa Clara, CA). The two samples were sequenced using the Illumina HiSeq 2000 platform (Illumina Inc., San Diego, CA), aligned the resulting reads to the hg19 reference genome with Burrows-Wheeler Aligner (22), applied Genome Analysis Toolkit (23) base quality score recalibration and indel (insertion-deletion) realignment, and performed SNP and indel discovery and genotyping across both samples simultaneously using variant quality score recalibration (24). Variants were annotated for functional effect using SnpEff 2.0.5 (http://snpeff.sourceforge.net/). Allele frequency data were obtained from the 1000 Genomes project (February 2012 release) (25) and the National Heart, Lung, and Blood Institute (NHLBI; Bethesda, MD) exome variant server (http://evs.gs.washington.edu/EVS/). Candidate variants in NIN, genomic DNA from whole blood and fibroblasts, were PCR amplified and sequenced using traditional Sanger sequencing. Primer sequences are available upon request.
Cell culture
Primary fibroblasts were established and cultured from skin biopsies of the parents and subject 1 as previously described (19). Cells were maintained in MEM-α+Glutamax medium (Gibco BRL, Lake Placid, NY) supplemented with 15% fetal calf serum (Gibco BRL) at 37 C in a 5% CO2 atmosphere.
Immunocytochemistry
Fibroblasts were seeded at 20% confluence, cultured overnight on 1.5 high-precision coverslips (Thermo Scientific, Waltham, MA), and fixed in methanol (5 min, 0 C). Coverslips were blocked in antibody dilution buffer [1× Tris-buffered saline, 0.15% Tween and 5% (vol/vol) normal donkey serum], incubated in primary antibody (mouse monoclonal anti-γ-tubulin and rabbit polyclonal antininein; Abcam, Cambridge, MA), rinsed with 1× PBS, incubated with the secondary antibodies (DyLight; Jackson Labs, Bar Harbor, ME), rinsed with blocking solution containing Hoechst 33342 (Invitrogen, Grand Island, NY) and then 1× PBS, mounted with Fluoromount-GT (Electron Microscopy Sciences, Hatfield, PA), and dried overnight at 4 C. Cells were imaged with an ORCA-ER (Hamamatsu, Bridgewater, NJ) camera mounted on an eclipse 90i wide-field microscope (Nikon, Melville, NY) with Apo Plan (20 × 0.75) Nikon objective running MetaMorph software (Molecular Devices, Sunnyvale, CA). For centrosome quantification, automatic thresholding and integration of ninein-stained puncta in multiple fluorescent micrograph fields was performed using FIJI (27) and combined for statistical analysis.
Long-term time-lapse imaging
Fibroblasts were seeded at 3–5% confluence in a six-well dish, cultured 16 h in medium supplemented with 2 mm thymidine (Sigma, St. Louis, MO). Thymidine was washed out with 2 ml fresh medium that we overlay with PCR-grade mineral oil and placed into the 37 C imaging chamber of a Nikon Ti automated inverted microscope with Perfect Focus. Two regions per well were imaged using a Hamamatsu ORCA-AG camera with a Plan Fluor Phase 1 (10 × 0.3) objective at a 15.20-mm working distance (Nikon Imaging Center, Harvard Medical School, Boston, MA). The 3 × 3 image montages were acquired every 10 min for up to 30 h and stitched using NIS Elements software (Nikon). The resulting image stacks were manually analyzed in FIJI (27) to determine the time of mitotic rounding and postmitotic spreading.
Western immunoblot analysis
Preparation of fibroblast cell lysates and subsequent Western immunoblot analysis were performed as described previously (28). For immunoblot analysis, the primary antibody used was purified rabbit polyclonal IgG against human ninein (dilution 1:500) from BioLegend (San Diego, CA), and the secondary antibody was antirabbit IgG (Amersham Pharmacia Biotech, Uppsala, Sweden).
Zebrafish husbandry and analysis
Zebrafish strains were maintained by standard raising conditions at Children's Hospital Animal Research Aquatics facility. Wild-type strains (AB) and transgenic line Tg (wnt1-GVP-UG) (29) were used for analysis of ninein function during development. Embryos for injection were obtained by timed pair matings.
In situ expression
Zebrafish embryonic ninein cDNA was PCR amplified and cloned into PGEM-EasyT (Promega, Madison, WI) using primers specific for the 3′ sequence: forward, ATGGAAACCCTTGTGATGGA; reverse, TTCATTCCATGCCAACTTCA. Purified plasmid containing zebrafish ninein was linearized, and the sense and antisense digoxygenin (dig)-labeled riboprobes were generated using Sp6 and T7 RNA polymerase and dig-deoxyuridine 5-triphosphate labeling kit (Roche, Indianapolis, IN). Probe hybridization was detected by the use of anti-dig antibody coupled to alkaline phosphatase and subsequent colormetric detection by enzymatic regulation of 4-nitro blue tetrazolium chloride/5-bromo-4-chloro-3-indoyl-phosphate, 4-toluidine salt substrate (Sigmafast, St. Louis, MO). Preparation of embryos and hybridization conditions were as described (30).
Experimental manipulation of early gene function in zebrafish
Splice blocking morpholinos (Gene Tools, Philomath, OR) were designed to disrupt splicing ninein exon 3 (nin_MO, GATATTATCTTTACCTGGTTGTCA), found in all three ninein isoforms (Zv9: ENSDARG00000060298); the morpholino is predicted to lead to exon skipping, a frame shift, and early termination of ninein. Splice blocking and the 5bp-mismatch control morpholino oligomers were diluted and injected into one-cell embryos at a concentration of 0.2 or 0.5 mm. Splicing efficiency in the nin_MO-affected embryos was verified by RT-PCR (forward, CGAGGGACCAATATGAGGAA; reverse, TCCTGTCATCGCTCTCTTGA) of larvae at 24–36 h post fertilization (hpf) and the 385-bp splicing products confirmed by capillary sequencing. Based on splicing efficiencies, 0.5 mm was used for all subsequent analyses.
Results
Microcephalic primordial dwarfism phenotype and IGF-I resistance are not due to defects in the IGF1 or IGF1R genes
The severe growth retardation starting in utero, microcephaly, mental retardation, and insensitivity to GH therapy concomitant with normal serum IGF-I concentrations suggested possible abnormalities with the IGF-I or IGF-I receptor peptides. Targeted sequencing of the IGF1 and the IGF1R genes (genomic and cDNA), however, indicated the genes were wild type. Evaluation of the IGF-I-IGF-I receptor signaling pathways in fibroblasts derived from subject 1, furthermore, indicated no obvious signaling abnormalities (data not shown).
Genomic genotyping and exome sequencing analysis
Because the targeted gene sequencing and analysis did not reveal a potential causative mutation, more comprehensive, genome-wide, genetic approaches were used. Whole-genome SNP genotyping suggested that the parents could be distantly related, with their proportional identity by descent estimated at approximately 3%. There were no detected regions of homozygous missing SNP in the probands to suggest the presence of a homozygous deletion.
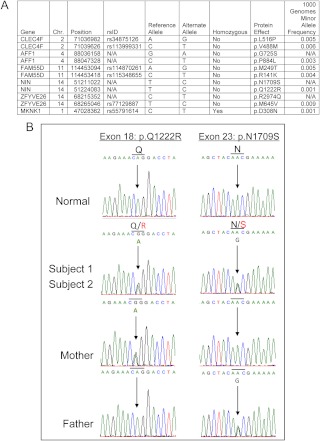
Given the fact that the two subjects were affected and both parents were unaffected, we hypothesized that this disorder could be autosomal recessive. Whole-exome sequencing of the two probands was undertaken and 34,606 shared variants were identified. Stringent unbiased filtering to remove all variants with minor allele frequency greater than 1% in the 1000 Genomes project, the NHLBI exome variant server, or in 50 HapMap control exomes, further narrowed down potential pathological variants to 616 rare autosomal SNP, from which six candidate genes were subsequently identified based on the presence of homozygous or more than one heterozygous rare nonsynonymous variants (Fig. 2A).
Fig. 2.
Identification of compound heterozygous variants in the NIN gene. A, Rare nonsynonymous variants in candidate genes found in both subjects from screening of whole-exome sequencing. CLEC4F, C-type lectin domain family 4 member F; AFF1, AF4/FMR2 family member 1; FAM55D, family with sequence similarity 55 member D; NIN, ninein; ZFYVE26, zinc finger FYVE domain containing 26 (also known as spastizin); MKNK1, MAPK interacting serine-threonine kinase 1; N/A, variant is either not present in dbSNP or in the 1000 Genomes data set. B, Electropherogram of rare and novel NIN variants in probands and parents.
NIN, a candidate gene for MPD
The extreme short stature and skeletal abnormalities of the probands, together with a lack of identifiable hormonal abnormality in the GH axis, suggest a dysfunction at the growth plate. We compared the list of six candidate genes with a list of genes showing differential expression in the growth plate in a previously published analysis (31). We noted that the rodent ortholog of one of the six genes, Nin, was differentially expressed in the growth plate. It was therefore of significance that NIN was one of the six genes identified by exome sequencing in our patients. NIN encodes Ninein, an approximately 243-kDa, cell cycle expressed centrosomal protein recently demonstrated in cell systems and rodent models to be involved in asymmetric mitotic spindle formation (15). Because dysfunctional centrosomes have been associated with MPD, we focused on NIN as the most probable candidate causal gene in our patients.
Our exome sequencing data indicated that our subjects were both heterozygous for two rare missense variants in NIN (p.Q1222R/p.N1709S to UniProtKB/Swiss-Prot Q8N4C6). Each of the substituted nucleotides is evolutionarily conserved. Sanger sequencing (genomic DNA and cDNA) confirmed that the probands were, in fact, compound heterozygotes in NIN, with each unaffected parent being heterozygous for a single variant (Fig. 2B). The p.N1709S is a novel variant that is not present in Single Nucleotide Polymorphism database, 1000 Genomes pilot data, or the NHLBI exome variant server. The p.Q1222R was present only in the 1000 Genomes pilot data with an overall minor allele frequency of 0.001 (0.005 in the Americas subcohort).
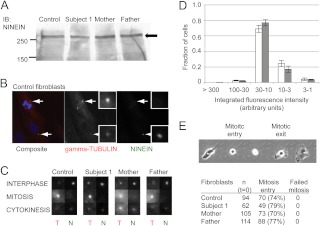
Normal ninein expression and mitotic functions of patient primary fibroblasts carrying NIN compound heterozygous mutations
To determine whether the identified NIN nonsynonymous variants affected Ninein expression, immunoblot analysis of total cell lysates from subject 1, and parent-derived fibroblasts were compared with those from normal control fibroblasts. As shown in Fig. 3A, the expression of Ninein was readily and comparably detected in all fibroblast lines. We next explored the possible functional effects of the compound heterozygous NIN variants by characterizing mitotic functions of the fibroblasts. In control fibroblasts, indirect immunofluorescence evaluations indicated that, consistent with published data (12, 14), Ninein targets to centrosomes during the interphase of the cell cycle (Fig. 3B, bottom arrow) but not during mitosis (Fig. 3B; top arrow). Similar trends in Ninein targeting were seen in fibroblasts from subject 1 and the parents through the cell cycle (Fig. 3C). A more quantitative analysis further demonstrated the amounts of Ninein targeted to interphase centrosomes in control and subject-derived cells were indistinguishable (Fig. 3D). Finally, by long-term time-lapse video microscopy, proliferation, measured as the proportion of individual cells entering mitosis, was comparable among all fibroblasts (Fig. 3E), and mitotic defects such as binucleate cells, prolonged cell rounding, or cytokinesis failure, were not observed.
Fig. 3.
Analysis of dermal fibroblasts for ninein expression and function. A, Antininein Western blot analysis of whole-cell lysates from control-, subject 1-, or the parent-derived fibroblasts. The positions of molecular weight markers in a separate lane are indicated in kilodaltons (left). Arrow indicates position of ninein (right). IB, Immunoblot. B, Localization of ninein and γ-tubulin in control fibroblasts. The composite image (left panel) is stained for DNA (blue), γ-tubulin (red), and ninein (green). The two arrows indicate centrosomes in a mitotic (top) and interphase (bottom) cell. Middle and right panels are gray-scale images in the γ-tubulin and ninein channels (labeled), with inset images ×4 magnified of the corresponding centrosomes. C, Representative magnified images of centrosomes in fibroblasts from control, subject 1, and parents, fixed during the interphase, mitosis, or cytokinesis and stained for ninein (N) and γ-tubulin (T). D, Quantitation and distribution of integrated ninein staining intensity at interphase centrosomes in control- (white bars, n = 248, four fields) and subject 1 (gray bars, n = 384, five fields)-derived fibroblasts. Integrated intensities were given numerical values and arbitrarily divided as indicated (x-axis), with the highest intensity having an arbitrary unit of greater than 300. E, Live-imaging analysis of the mitotic cycle in patient fibroblasts compared with control fibroblasts. Representative phase-contrast images of control fibroblasts taken every 20 min illustrate the onset of cell rounding (mitotic entry) and spreading (mitotic exit). Individual cells were imaged (every 10 min, 30 h) after release from S-phase arrest and scored for entry and successful exit from mitosis. The total number of cells (n) analyzed for each fibroblast line, and the proportion that successfully entered mitosis (number and percentage provided) or failed, are tabulated. No mitotic defects were identified.
Altogether, our results suggested that, at least in primary dermal fibroblasts, the compound heterozygous NIN defects did not disrupt Ninein expression or localization or obviously affect mitotic functions. These results, although contrary to the observed effects of other centrosomal genes on mitotic progression (e.g. Sil, Pcnt), are consistent with recent rodent developmental neuronal studies, in which in vivo knockdown of Nin also did not affect centrosome duplication, segregation, or cell division but caused specific premature depletion of radial glia progenitors (17).
Zebrafish ninein has a specific developmental function during early morphogenesis and formation of the brain
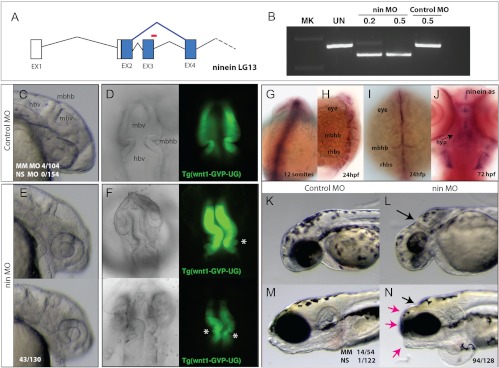
To further explore the functions of Ninein beyond cell division, we focused on the role(s) of ninein during early development by creating a zebrafish model in which expression of ninein was reduced by morpholino oligonucleotide (MO) methodology. Ninein has a single ortholog in the zebrafish with three predicted isoforms. Splice-blocking MO was designed to inhibit splicing of exon 3 of ninein, leading to a frame shift and early truncation of the predicted protein of all isoforms (Fig. 4A, red bar). Treatment of 0.2 and 0.5 mm Nin-MO led to specific, dose-dependent inhibition of splicing in 72 hpf embryos. The altered splicing of ninein was not seen in untreated or control morpholino-treated embryos (Fig. 4B).
Fig. 4.
Specific developmental role of ninein in the formation of the brain and shape of the skull. A, Splice blocking morpholino oligonucleotide (red, nin MO) was designed against ninein exon 3 and is predicted to cause altered splicing (blue), resulting in a frame shift in the coding sequence of the resulting message. B, RT-PCR of ninein message after injection of control or nin MO 24 hpf. C and E, Morphology of forming brain of the zebrafish at 24 hpf embryos injected with control or nin MO. D and F, Dorsal views of the forming anterior neural folds of 24-hpf embryos in wnt1:GFP transgenic fish [Tg(wnt1-GVP-UG)] highlight early neuroectodermal structures and initiation of differentiation in larvae injected with control (D) or nin MO (F; asterisk, aberrant folds found rostral to the MBHB). G–J, Whole-mount in situ analysis of ninein expression. G, Expression of ninein at 12 somite limited to neuroectodermal structures of the rostral and caudal neural tube. H and I, Expression of ninein in 24-hpf embryos resolves to mark anterior neural folds as well as the forming rhobomeres. J, Ventral view of 72-hpf zebrafish showing specific expression of ninein in the forming hypothalamus. K and L, Knockdown of ninein results in anterior deficiencies of the skull showing reduced eye and skull growth, specifically in the area of the MBHB (arrow). M and N, Morphology of 5-dpf larvae of control (M) and nin MO-treated groups showed specific shortening of the skull (red arrows) coincident with smaller eyes, otoliths, and anterior clefts at the site of the MBHB. hbv, Hindbrain ventricle; hyp, hypothalamus; mbhb, midbrain hindbrain isthmus fold; mbv, midbrain ventricle; rhbs, rhombomeres.
Developmental effects of embryos injected with control MO (Fig. 4, C and D) or nin-MO (Figs. 4, E and F) indicated that ninein knockdown led to specific defects in the specification and morphogenesis of the anterior neuroectoderm during early development of the larvae, leading to patterning defects in the isthmus organizer of the midbrain-hindbrain boundary (MBHB). Analysis of the morphogenic changes in these larvae showed consistent deficiencies in ventricle inflation and in the invagination of the isthmus of the MBHB (Fig. 4F; 50%, n = 54). These effects were highly specific to ninein knockdown, showing low variation in 5-bp ninein mismatch (4%, n = 104) and nonspecific (0%, n = 154) morpholino controls. Strikingly, the mirror image pattern of the neuroectoderm of the controls (Fig. 4D) is often lost in nin-MO, leading to parallel folds localized at the MBHB (Fig. 4F) and blebbing of the neuroectoderm (Fig. 4F, asterisk). Furthermore, marking the neuroectoderm of the midbrain and cerebellar rhombic lip with a transgenic line for wnt1 expression [Tg(wnt1-GVP-UG) (29)] clearly demonstrated altered morphologies of the MBHB and posterior neuroectodermal structures (Fig. 4, D and F).
Given the specific, early phenotype of ninein knockdown, we next evaluated the regulated expression of ninein transcripts during normal early zebrafish development (Fig. 4, G–J). Whole-mount in situ analysis using a ninein antisense probe against the 5′ end of ninein (incorporating all predicted isoforms) showed specific expression of ninein in the early neuroectoderm (Fig. 4G) and localizing to the lateral folds of the anterior neural fold and specific rhombomere expression at 24 hpf (Fig. 4H). The forming MBHB showed prominent expression (Fig. 4I). Ninein expression at this stage was not detected in mesenchymal or nonneural tissues throughout the developing larvae. At 72 hpf, expression of ninein remained localized, maintaining expression in the MBHB (data not shown) but now also marking the forming hypothalamus (Fig. 4J).
Ninein knockdown resulted in the deformity of the developing cranium, with obvious deformities at the site of the MBHB (Fig. 4, K and L). Injected larvae at 5 d post fertilization showed shortened frontonasal, jaw, and development of a small, squared skull (Fig. 4, M and N). This effect on late skull morphology was observed at high frequency (73%, n = 128) only with ninein knockdown. The deformity was absent when two other nonspecific MO were used, and the low frequency detected with the 5-bp-mismatch MO controls (26%, n = 54) was likely due to low level MO toxicity.
Altogether, we demonstrate a specific role for ninein in the early formation of the MBHB boundary and find that it is necessary for morphogenesis of the anterior neuroectoderm. The lasting effects of the morpholino knockdown include phenotypes such as reduced growth and altered patterning of the skull, consistent with general phenotypic characteristics of MPD, further validating NIN as an excellent candidate gene underlying our subjects' phenotype.
Discussion
We report, in this study, the first cases of MPD associated with compound heterozygous mutations in the NIN gene located on chromosome 14q22.1. Through a whole-exome sequence analysis of the affected patients and cross-referencing with a growth plate gene expression data set, we identified NIN as a high probability candidate gene. NIN was one of only six genes in which both siblings carried extremely rare nonsynonymous variants and the only one of the six that was highlighted by the growth plate gene expression analysis. Furthermore, the biological role of the NIN gene product, Ninein, highlighted this gene as a highly probable candidate. Ninein, a large regulated centrosomal protein (13), associates specifically with the mature mother centriole during asymmetric cell division (15–17). As an integral centrosomal protein, Ninein was an intriguing candidate because the clinical presentations of the two probands were consistent with MPD, resembling Seckel syndrome and MOPDII, diseases known to be associated with loss-of-function mutations affecting genes involved in centrosome function [PCNT and CENPJ (32)]. Through analysis of known protein-protein interactions highlighting the causal gene products (CENPJ, CEP152, PCNT, and CEP63) of MPD, we show that Ninein is associated with a broader cluster of genes involved in centrosome and microtubule function (Supplemental Fig. 1, published on The Endocrine Society's Journals Online web site at http://jcem.endojournals.org).
Our zebrafish studies demonstrate, for the first time, critical ninein function during early development. By knockdown of ninein, we show that it is essential for the early formation and patterning of the brain. In contrast to developmental analysis of centrosome protein components such as Cep152 (33), Orc1 (34), and Sil (35) that have a general role in cell proliferation and the regulation of cell death, the regulation and function of ninein are highly specific to the neuroectoderm and organization of the MBHB during early development. The knockdown of ninein results in deficiencies in brain and skull development that are reminiscent of phenotypes associated with MPD. Similar functional and cell type-specific effects in humans might explain the microcephalic features and severe intellectual deficiencies in our patients.
In addition to severe pre- and postnatal growth retardation and profound microcephaly, the probands presented with dramatic and significant developmental delay, seizures, and distinctive absence of pubertal breast and uterine development. MOPDII patients carrying PCNT mutations (Table 2), in contrast, have normal intelligence, undergo premature puberty, and, unlike our patients or patients carrying mutations in other centrosomal genes, appear highly vulnerable to severe, early-onset, type 2 diabetes (36). Seckel syndrome patients are genetically and phenotypically diverse (Table 2), featuring variable facial features, learning disabilities, and delayed puberty.
Table 2.
Comparison of growth, physical characteristics, radiographic findings, and intellect in subjects 1 + 2, MOPDII, and Seckel syndrome
| MOPD II | Seckel syndrome | Cases 1 and 2 | |
|---|---|---|---|
| Anthropometrics | |||
| Prenatal growth | IUGR | IUGR | IUGR |
| Postnatal growth | Growth retardation | Growth retardation | Growth retardation |
| Head size | Microcephaly, severe | Microcephaly, severe | Microcephaly, severe |
| Adult height | Short stature, severe | Short stature, severe | Short stature, severe |
| Physical characteristics | |||
| Facies | Asymmetry | Occassional asymmetry | No asymmetry |
| Eyes | Normal | Down-slanting palpebral fissures, strabismus, cataracts | Hypotelorism |
| Ears | Normal | Low-set, malformed, lack of lobule | Small |
| Nose | Long, prominent tip, hypoplastic alae nasi | Prominent | Prominent |
| Jaw | Micrognathia | Micrognathia | Normal |
| Teeth | Hypoplasia, partial anodontia, enamel hypoplasia | Partial anodontia | Normal |
| Extremities | Short lower arms | Normal | |
| Puberty | Premature puberty | Males: cryptorchidism Delayed puberty | Absent thelarche, menarche |
| Radiographic | |||
| Hand | General brachdactyly, diaphyseal constriction | Clinodactyly fifth fingers, absence of some phalangeal epiphyses | Normal phalangeal length, clinodactyly fifth fingers, abnormal carpal bones |
| Wrist | Flat shape distal radius and ulna | Hypoplasia of proximal radius with dislocation of radial head | Foreshortened ulna (Madelung deformity) |
| Thorax | 11 ribs | ||
| Spine | Scoliosis | Lumbar scoliosis | |
| Hip | Dysplasia | Dysplasia | |
| Extremities | Hypoplasia of proximal fibula; gap between first and second toes | ||
| Other features | |||
| Acanthosis nigricans-insulin resistance, type 2 diabetes | Reported | Not reported | Not observed |
| Intelligence | Normal | Mild-moderate learning disability | Significant learning disability |
| Seizures | Occasional | Present | |
| Gene mutations | PCNT | ATR, CENPJ, CEP152, CEP63, RBBP8 | NIN |
IUGR, Intrauterine growth retardation.
The complete absence of pubertal breast and uterine development in our subjects is striking and distinct from characterized MPD patients. The lack of elevation in gonadotropins suggests that this is not due to primary ovarian insufficiency but, rather, due to an abnormality in gonadotropin secretion or regulation. The etiology for this phenotype may be associated with NIN defects because ninein expression in the early developing hypothalamus was detected in the zebrafish, but further investigations are necessary to determine biological correlations and significance.
Human mutations in NIN have not been previously described, although antibodies against Ninein have been detected in sera from patients with autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus (37). The two novel heterozygous missense variants, p.Q1222R (exon 18) and p.N1709S (exon 23) identified in our patients, both altered evolutionarily conserved residues of Ninein. Given the nature of these alleles it is likely that these are hypomorphic and lead to decreased protein functions. In other centrosomal protein implicated in MPD, such as those encoded by CENPJ (32), PCNT (38, 39), CEP63 (40), and CEP152 (26), the pathological variants identified resulted in complete loss of expression and/or function.
Analysis of Ninein expression, localization, and cell cycle progression in primary dermal fibroblasts derived from subject 1 did not divulge a mechanism(s) of pathophysiology. However, unlike previous centrosomal proteins implicated in MPD, Ninein may have specific functions apart from mitotic spindle organization and proliferation. In addition to our zebrafish data, knockdown of Nin in mouse developmental studies did not affect centrosome duplication, segregation, or cell division in neuronal cells (17) but, in vivo, caused premature depletion of radial glia progenitors with concomitant increases in differentiating neurons (17). This suggests that, in humans, Ninein may play a specific, and essential, role(s) in development of neurons beyond its association with the mitotic spindle.
In conclusion, we have identified a novel clinical subtype of MPD in two sisters who have rare variants in NIN. Patient fibroblasts did not show alterations in centrosome cohesion, mitotic functions, or targeting of Ninein and other centrosomal protein. However, we show that reduction of ninein function during zebrafish development leads to specific deficiencies of brain development and, strikingly, specific cranial defects reminiscent of the microcephaly phenotype present in the patients. These findings are consistent with evidence from mouse knockdown experiments showing an essential role of ninein during neural development. NIN is a new candidate gene for MPD and offers a developmental basis for the generation of the complex phenotypes in our patients. Identification of more patients with MPD/Seckel syndrome, combined with careful genotype-phenotype analysis, should clarify the role(s) of NIN and related genes in human growth and development.
Acknowledgments
We thank the subjects and their family for participating in this work. We also thank Tim Mitchison, Tony Hyman, and Tim Yu for their thoughtful guidance; Daniel Mirel for his adept project management; and Peng Fang and Michael Derr for the IGF-I signaling studies.
This work was supported by National Institutes of Health Grant 5K12HD052896 (to A.D.), March of Dimes Grant 6-FY09-507 (to J.N.H.), The Intramural Research Program of the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health (to J.C.L. and J.B.) as well as The Nikon Imaging Center at Harvard Medical School. T.H.P. is supported by The Danish Council for Independent Research Medical Sciences (FSS).
Disclosure Summary: The authors have no conflicts of interest to disclose.
Footnotes
- dig
- Digoxygenin
- hpf
- hours post fertilization
- MBHB
- midbrain-hindbrain boundary
- MO
- morpholino oligonucleotide
- MOPDII
- microcephalic osteodysplastic primordial dwarfism type II
- MPD
- microcephalic primordial dwarfism
- MRI
- magnetic resonance imaging
- NHLBI
- National Heart, Lung, and Blood Institute
- NR
- normal range
- SNP
- single-nucleotide polymorphism.
References
- 1. Majewski F, Goecke T. 1982. Studies of microcephalic primordial dwarfism I: approach to a delineation of the Seckel syndrome. Am J Med Genet 12:7–21 [DOI] [PubMed] [Google Scholar]
- 2. Seckel H. 1960. Bird-headed dwarfs: studies in developmental anthropology including human proportions. Springfield, IL: Charles C. Thomas [Google Scholar]
- 3. Hall JG, Flora C, Scott CI, Jr, Pauli RM, Tanaka KI. 2004. Majewski osteodysplastic primordial dwarfism type II (MOPD II): natural history and clinical findings. Am J Med Genet A 130A:55–72 [DOI] [PubMed] [Google Scholar]
- 4. Majewski F, Ranke M, Schinzel A. 1982. Studies of microcephalic primordial dwarfism II: the osteodysplastic type II of primordial dwarfism. Am J Med Genet 12:23–35 [DOI] [PubMed] [Google Scholar]
- 5. Bongers EM, Opitz JM, Fryer A, Sarda P, Hennekam RC, Hall BD, Superneau DW, Harbison M, Poss A, van Bokhoven H, Hamel BC, Knoers NV. 2001. Meier-Gorlin syndrome: report of eight additional cases and review. Am J Med Genet 102:115–124 [DOI] [PubMed] [Google Scholar]
- 6. Gorlin RJ, Cervenka J, Moller K, Horrobin M, Witkop CJ., Jr 1975. Malformation syndromes. A selected miscellany. Birth Defects Orig Artic Ser 11:39–50 [PubMed] [Google Scholar]
- 7. Klingseisen A, Jackson AP. 2011. Mechanisms and pathways of growth failure in primordial dwarfism. Genes Dev 25:2011–2024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Driscoll M, Ruiz-Perez VL, Woods CG, Jeggo PA, Goodship JA. 2003. A splicing mutation affecting expression of ataxia-telangiectasia and Rad3-related protein (ATR) results in Seckel syndrome. Nat Genet 33:497–501 [DOI] [PubMed] [Google Scholar]
- 9. Netchine I, Azzi S, Le Bouc Y, Savage MO. 2011. IGF1 molecular anomalies demonstrate its critical role in fetal, postnatal growth and brain development. Best Pract Res Clin Endocrinol Metab 25:181–190 [DOI] [PubMed] [Google Scholar]
- 10. Woods KA, Camacho-Hübner C, Savage MO, Clark AJ. 1996. Intrauterine growth retardation and postnatal growth failure associated with deletion of the insulin-like growth factor I gene. N Engl J Med 335:1363–1367 [DOI] [PubMed] [Google Scholar]
- 11. Nigg EA, Stearns T. 2011. The centrosome cycle: centriole biogenesis, duplication and inherent asymmetries. Nat Cell Biol 13:1154–1160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chen CH, Howng SL, Cheng TS, Chou MH, Huang CY, Hong YR. 2003. Molecular characterization of human ninein protein: two distinct subdomains required for centrosomal targeting and regulating signals in cell cycle. Biochem Biophys Res Commun 308:975–983 [DOI] [PubMed] [Google Scholar]
- 13. Hong YR, Chen CH, Chuo MH, Liou SY, Howng SL. 2000. Genomic organization and molecular characterization of the human ninein gene. Biochem Biophys Res Commun 279:989–995 [DOI] [PubMed] [Google Scholar]
- 14. Ou YY, Mack GJ, Zhang M, Rattner JB. 2002. CEP110 and ninein are located in a specific domain of the centrosome associated with centrosome maturation. J Cell Sci 115:1825–1835 [DOI] [PubMed] [Google Scholar]
- 15. Knoblich JA. 2010. Asymmetric cell division: recent developments and their implications for tumour biology. Nat Rev Mol Cell Biol 11:849–860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Piel M, Meyer P, Khodjakov A, Rieder CL, Bornens M. 2000. The respective contributions of the mother and daughter centrioles to centrosome activity and behavior in vertebrate cells. J Cell Biol 149:317–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang X, Tsai JW, Imai JH, Lian WN, Vallee RB, Shi SH. 2009. Asymmetric centrosome inheritance maintains neural progenitors in the neocortex. Nature 461:947–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. National Center for Health Statistics 2000. CDC Growth Charts. http://www.cdc.gov/growthcharts/percentile_data_files.htm
- 19. Kofoed EM, Hwa V, Little B, Woods KA, Buckway CK, Tsubaki J, Pratt KL, Bezrodnik L, Jasper H, Tepper A, Heinrich JJ, Rosenfeld RG. 2003. Growth hormone insensitivity associated with a STAT5b mutation. N Engl J Med 349:1139–1147 [DOI] [PubMed] [Google Scholar]
- 20. Fang P, Schwartz ID, Johnson BD, Derr MA, Roberts CT, Jr, Hwa V, Rosenfeld RG. 2009. Familial short stature caused by haploinsufficiency of the insulin-like growth factor i receptor due to nonsense-mediated messenger ribonucleic acid decay. J Clin Endocrinol Metab 94:1740–1747 [DOI] [PubMed] [Google Scholar]
- 21. Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, Maller J, Sklar P, de Bakker PI, Daly MJ, Sham PC. 2007. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet 81:559–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Li H, Durbin R. 2009. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25:1754–1760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McKenna A, Hanna M, Banks E, Sivachenko A, Cibulskis K, Kernytsky A, Garimella K, Altshuler D, Gabriel S, Daly M, DePristo MA. 2010. The Genome Analysis Toolkit: a MapReduce framework for analyzing next-generation DNA sequencing data. Genome Res 20:1297–1303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. DePristo MA, Banks E, Poplin R, Garimella KV, Maguire JR, Hartl C, Philippakis AA, del Angel G, Rivas MA, Hanna M, McKenna A, Fennell TJ, Kernytsky AM, Sivachenko AY, Cibulskis K, Gabriel SB, Altshuler D, Daly MJ. 2011. A framework for variation discovery and genotyping using next-generation DNA sequencing data. Nat Genet 43:491–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. 1000 Genomes Project Consortium 2010. A map of human genome variation from population-scale sequencing. Nature 467:1061–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Kalay E, Yigit G, Aslan Y, Brown KE, Pohl E, Bicknell LS, Kayserili H, Li Y, Tüysuz B, Nürnberg G, Kiess W, Koegl M, Baessmann I, Buruk K, Toraman B, Kayipmaz S, Kul S, Ikbal M, Turner DJ, Taylor MS, Aerts J, Scott C, Milstein K, Dollfus H, Wieczorek D, Brunner HG, Hurles M, Jackson AP, Rauch A, Nürnberg P, Karaguzel A, Wollnik B. 2010. CEP152 is a genome maintenance protein disrupted in Seckel syndrome. Nat Genet 43:23–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Walter T, Shattuck DW, Baldock R, Bastin ME, Carpenter AE, Duce S, Ellenberg J, Fraser A, Hamilton N, Pieper S, Ragan MA, Schneider JE, Tomancak P, Hériché JK. 2010. Visualization of image data from cells to organisms. Nat Methods 7:S26–S41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hwa V, Little B, Kofoed EM, Rosenfeld RG. 2004. Transcriptional regulation of insulin-like growth factor-I by interferon-γ requires STAT-5b. J Biol Chem 279:2728–2736 [DOI] [PubMed] [Google Scholar]
- 29. Volkmann K, Chen YY, Harris MP, Wullimann MF, Köster RW. 2010. The zebrafish cerebellar upper rhombic lip generates tegmental hindbrain nuclei by long-distance migration in an evolutionary conserved manner. J Comp Neurol 518:2794–2817 [DOI] [PubMed] [Google Scholar]
- 30. Shulte-Merker S. 2002. Looking at embryos. In: Nuesslein-Volhard C, Dahm R, eds. Zebrafish. Oxford, UK: Oxford University Press; 39–58 [Google Scholar]
- 31. Lui JC, Andrade AC, Forcinito P, Hegde A, Chen W, Baron J, Nilsson O. 2010. Spatial and temporal regulation of gene expression in the mammalian growth plate. Bone 46:1380–1390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Al-Dosari MS, Shaheen R, Colak D, Alkuraya FS. 2010. Novel CENPJ mutation causes Seckel syndrome. J Med Genet 47:411–414 [DOI] [PubMed] [Google Scholar]
- 33. Blachon S, Gopalakrishnan J, Omori Y, Polyanovsky A, Church A, Nicastro D, Malicki J, Avidor-Reiss T. 2008. Drosophila asterless and vertebrate Cep152 are orthologs essential for centriole duplication. Genetics 180:2081–2094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Bicknell LS, Walker S, Klingseisen A, Stiff T, Leitch A, Kerzendorfer C, Martin CA, Yeyati P, Al Sanna N, Bober M, Johnson D, Wise C, Jackson AP, O'Driscoll M, Jeggo PA. 2011. Mutations in ORC1, encoding the largest subunit of the origin recognition complex, cause microcephalic primordial dwarfism resembling Meier-Gorlin syndrome. Nat Genet 43:350–355 [DOI] [PubMed] [Google Scholar]
- 35. Pfaff KL, Straub CT, Chiang K, Bear DM, Zhou Y, Zon LI. 2007. The zebra fish cassiopeia mutant reveals that SIL is required for mitotic spindle organization. Mol Cell Biol 27:5887–5897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Huang-Doran I, Bicknell LS, Finucane FM, Rocha N, Porter KM, Tung YC, Szekeres F, Krook A, Nolan JJ, O'Driscoll M, Bober M, O'Rahilly S, Jackson AP, Semple RK. 2011. Genetic defects in human pericentrin are associated with severe insulin resistance and diabetes. Diabetes 60:925–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Howng SL, Chou AK, Lin CC, Lin ZA, Wang CJ, Loh JK, Lieu AS, Yen JH, Lee CI, Hong YR. 2011. Autoimmunity against hNinein, a human centrosomal protein, in patients with rheumatoid arthritis and systemic lupus erythematosus. Mol Med Rep 4:825–830 [DOI] [PubMed] [Google Scholar]
- 38. Griffith E, Walker S, Martin CA, Vagnarelli P, Stiff T, Vernay B, Al Sanna N, Saggar A, Hamel B, Earnshaw WC, Jeggo PA, Jackson AP, O'Driscoll M. 2008. Mutations in pericentrin cause Seckel syndrome with defective ATR-dependent DNA damage signaling. Nat Genet 40:232–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Rauch A, Thiel CT, Schindler D, Wick U, Crow YJ, Ekici AB, van Essen AJ, Goecke TO, Al-Gazali L, Chrzanowska KH, Zweier C, Brunner HG, Becker K, Curry CJ, Dallapiccola B, Devriendt K, Dörfler A, Kinning E, Megarbane A, Meinecke P, Semple RK, Spranger S, Toutain A, Trembath RC, Voss E, Wilson L, Hennekam R, de Zegher F, Dörr HG, Reis A. 2008. Mutations in the pericentrin (PCNT) gene cause primordial dwarfism. Science 319:816–819 [DOI] [PubMed] [Google Scholar]
- 40. Sir JH, Barr AR, Nicholas AK, Carvalho OP, Khurshid M, Sossick A, Reichelt S, D'Santos C, Woods CG, Gergely F. 2011. A primary microcephaly protein complex forms a ring around parental centrioles. Nat Genet 43:1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]