Abstract
Context:
For the last 10 yr, continuous glucose monitoring (CGM) has brought up new insights into the accuracy of blood glucose analysis.
Objective:
Our objective was to determine how islet graft function was able to influence the various components of dysglycemia after islet transplantation (IT).
Design and Setting:
We conducted a single-arm open-labeled study with a 3-yr follow-up in a referral center (ClinicalTrial.gov identifiers NCT00446264 and NCT01123187).
Patients:
Twenty-three consecutive patients with type 1 diabetes (14 islet alone, nine islet after kidney) received IT within 3 months using the Edmonton protocol.
Intervention:
Intervention included 72-h CGM before and 3, 6, 9, 12, 24, and 36 months after transplantation.
Main Outcome Measure:
Graft function was estimated via β-score, a previously validated index (range 0–8) based on treatment requirements, C-peptide, blood glucose, and glycated hemoglobin.
Results:
At the 3-yr visit, graft function persisted in 19 patients (82%), and 10 (43%) remained insulin independent. Glycated hemoglobin decreased in the whole cohort from 8.3% (7.3–9.0%) at baseline to 6.7% (5.9–7.7%) at 3 yr [median (interquartile range), P < 0.01]. Mean glucose, glucose sd, and time spent with glycemia above 10 mmol/liter (hyperglycemia) and below 3 mmol/liter (hypoglycemia) were significantly lower after IT (P < 0.05 vs. baseline). The four CGM outcomes were related to β-score (P < 0.001). However, partial function (β-score >3) was sufficient to abrogate hypoglycemia; suboptimal function (β-score >5) was necessary to significantly improve mean glucose, glucose sd, and hyperglycemia; and optimal function (β score >7) was necessary to normalize them.
Conclusion:
The four components of dysglycemia were not equally affected by the degree of islet graft function, which could have important implications for future development of β-cell replacement. A β-score above 3 dramatically reduced the occurrence of hypoglycemia.
Replacement of β-cells is gradually emerging as a realistic treatment for selected patients with type 1 diabetes, especially those with severe glycemic lability, recurrent hypoglycemia, and hypoglycemia unawareness (1). By restoring endogenous insulin secretion, intraportal islet transplantation (IT) decreases exogenous insulin requirements and reduces glycated hemoglobin (HbA1c) as well as the frequency and severity of hypoglycemic episodes. However, the definition of success of islet transplantation remains a matter of debate. Initially, the primary goal of IT has been to maintain normal glucose control and insulin independence. But real-life experience has shown that success could also be measured in terms of lower frequency of hypoglycemic episodes and positive effects on vascular complications or quality of life. Moreover, technological advances such as continuous glucose monitoring (CGM) have contributed to better delineate the normal glucose imbalance and thus to refine the objectives of β-cell replacement since the assessment of glucose variability and hypoglycemia unawareness have become easier to apprehend (2, 3). Indeed, lower glucose variability has been shown in several cross-sectional studies comparing IT recipients with diabetic controls (4–6). However, the effect of β-cell replacement on the other components of dysglycemia, especially mean glucose level, is not firmly established over the long term. In this longitudinal study, we assessed the daily glucose profile via CGM of 23 consecutive IT recipients for 3 yr to determine how islet graft function was able to influence each component of the glucose profile.
Patients and Methods
Study design
Subjects were participants in two clinical trials exploring the outcome of IT using the Edmonton immunosuppression protocol in nonuremic patients with labile type 1 diabetes (NCT 00446264) and uremic patients with previous kidney transplantation (NCT 01123187). All patients receiving IT at our referral center between May 2003 and December 2007 were enrolled in this ancillary single-arm open-label study with a 3-yr follow-up. Briefly, IT consisted of two or three sequential infusions of fresh allogeneic islets within 3 months, with the aim of attaining adequate metabolic control (HbA1c ≤6.5%) without exogenous insulin. Immunosuppression was induced by IL-2 receptor antibodies and maintained with sirolimus and tacrolimus. The study protocol was approved by the institutional review board, and signed informed consent was obtained from each patient.
Patients
Patient characteristics and transplantation procedures have been previously detailed (7, 8). A total of 23 subjects, 11 males and 12 females, 14 nonuremic and nine uremic, were enrolled. Median age was 44 yr [interquartile range (IQR) = 37–52] with a diabetes duration of 28 yr (IQR = 23–34). These patients received a total islet mass of 12,615 islet-equivalent/kg (IQR = 10,933–15,606) in two (n = 10) or three (n = 13) infusions, within 62 d (IQR = 42–88). Graft function was confirmed by detectable serum C-peptide in each patient after IT. No patient was lost to follow-up.
Glucose profile
Daily glucose profile was assessed using a CGM system (Medtronic MiniMed, Northridge, CA) for 3 consecutive days at baseline and 3, 6, 9, 12, 24, and 36 months after the first IT. Glucose profile outcomes included mean glucose (MG), glucose sd around the mean value (GSD), percentage of time spent with glucose below 3 mmol/liter [hypoglycemia (HYPO)], and percentage of time spent with glucose above 10 mmol/liter [hyperglycemia (HYPER)]. The CGM device was put on and calibrated just before the patient left the hospital during one outpatient follow-up visit to assess the metabolic imbalance during usual daily life activities and diet. The system was blinded to the patient.
Graft function
Graft function was estimated with the β-score, a previously validated composite index, ranging from 0 (no graft function) to 8 (excellent graft function). The β-score was calculated as described by Ryan et al. (9) by giving two points for normal fasting glucose (<5.5 mmol/liter), HbA1c (<6.1%), stimulated and/or basal C-peptide (>0.3 nmol/liter or 0.9 ng/ml), and absence of insulin or oral hypoglycemic agent use. No point was awarded if fasting glucose was in the diabetic range (>7 mmol/liter), HbA1c was higher than 6.9%, C-peptide secretion was undetectable on stimulation (below 0.06 nmol/liter or 0.2 ng/ml), or daily insulin use was more than 0.24 U/kg. One point was given for intermediate values.
Criteria for discontinuing or resuming exogenous insulin
Subjects were considered insulin independent if they were able to titrate off insulin therapy for at least 2 wk and all of the following criteria were met: HbA1c no higher than 6.5%; fasting capillary glucose level no higher than 140 mg/dl (7.8 mmol/liter) more than three times in the past week (based on measuring capillary glucose levels a minimum of seven times in a 7-d period); 2-h postprandial capillary glucose no higher than 180 mg/dl (10.0 mmol/liter) more than three times in the past week (based on measuring capillary glucose levels a minimum of 21 times in a 7-d period); fasting serum glucose level no higher than 126 mg/dl (7.0 mmol/liter); and fasting or stimulated C-peptide levels of at least 0.5 ng/ml (0.16 nmol/liter).
The criteria for resuming insulin were as follows: HbA1c over 6.5%; fasting capillary glucose level over 140 mg/dl (7.8 mmol/liter) more than three times in the past week; 2-h postprandial capillary glucose over 180 mg/dl (10.0 mmol/liter) more than three times in the past week; fasting serum glucose level over 126 mg/dl (7.0 mmol/liter); and fasting or stimulated C-peptide levels below 0.5 ng/ml (0.16 nmol/liter).
Oral antidiabetic drugs were authorized before resuming insulin.
Data analysis
Continuous variables were expressed as median (IQR). All outcomes were analyzed according to the intention-to-treat principle irrespective of graft function. Matched values were compared with the Friedman test and Dunns' multiple nonparametric comparison test. Associations between quantitative variables were analyzed using Spearman correlation coefficient [95% confidence interval (CI)]. A P level <0.05 was considered as significant.
Results
Graft function
HbA1c decreased in the whole cohort from 8.3% (7.3–9.0%) at baseline to 6.7% (5.9–7.7%) at 3 yr [median (IQR), P < 0.01]. At the 3-yr visit, four of the 23 patients (8%) (two uremic, two nonuremic) had no graft function (interpreted as β-score of 0), whereas serum C-peptide remained detectable in the 19 other patients (82%). In nine of the 23 patients (39%), HbA1c rose above 6.5% during follow-up, requiring the resumption of small doses of exogenous insulin. Overall, 10 of the 23 patients (43%) remained insulin independent 3 yr after IT, with a median HbA1c of 5.8% (IQR = 5.2–6.2). Graft function remained optimal in seven patients with a β-score at 7 (n = 2) or 8 (n = 5) at 3 yr.
Glucose profile
The four CGM outcomes were significantly improved at the 6-month visit and remained so throughout the 3-yr study (Table 1). A decrease in the β-score was observed in 12 patients between 6 months and 3 yr and was associated with a slight but nonsignificant rise of GSD and HYPER in the entire cohort at the 3-yr visit.
Table 1.
Overall metabolic outcome
| Baseline (n = 23)a | 6 months (n = 23)a | 36 months (n = 23)a | ß-score 8 (n = 39)b | |
|---|---|---|---|---|
| HbA1c (%) | 8.3 (7.3–9) | 6.0 (5.7–6.4)c | 6.7 (5.9–7.7)c | 5.6 (5.0–5.8)c |
| Insulin requirements (IU/kg · d) | 0.63 (0.40–0.75) | 0 (0–0)c | 0 (0–0.28)c | 0 (0–0)c |
| MG (mmol/liter) | 8.0 (7.3–11.6) | 6.0 (5.5–7.8)c | 6.2 (5.8–7.3)d | 5.7 (5.3–6.2)c |
| GSD (mmol/liter) | 3.2 (2.3–4.3) | 0.9 (0.7–1.3)c | 1.8 (0.8–2.8)d | 0.8 (0.6–1.1)c |
| HYPER (% time >10 mmol/liter) | 33 (19–51) | 0 (0–5)c | 6 (0–15)d | 0 (0–0)c |
| HYPO (% time <3 mmol/liter) | 5 (1–8) | 0 (0–3.5)d | 0 (0–2)d | 0 (0–0)c |
Results are shown as median (IQR).
Analysis per intention to treat of CGM outcomes in the 23 patients throughout the 3-yr study.
Combined analysis of CGM outcomes of the 39 tests associated with excellent graft function (ß-score 8) at any given time following IT.
P < 0.01 vs. baseline.
P < 0.05 vs. baseline.
Association between glucose profile and graft function
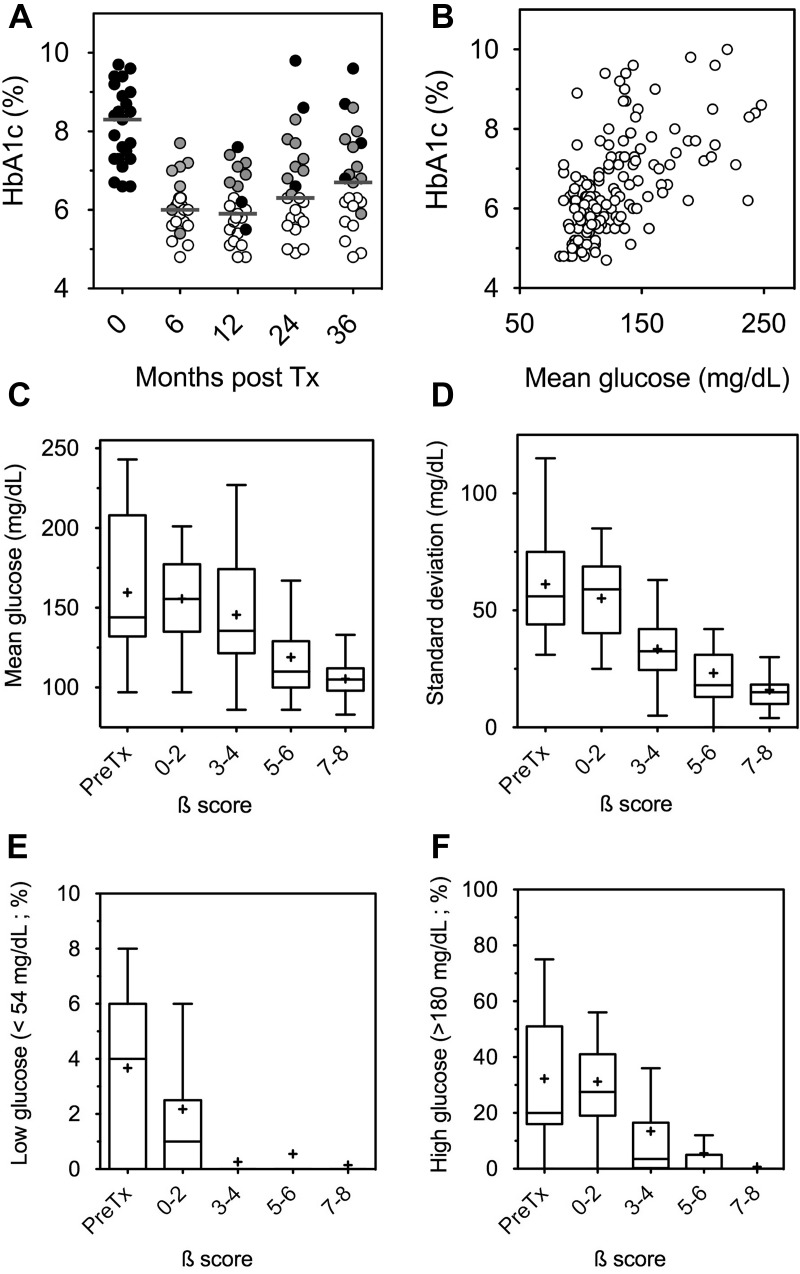
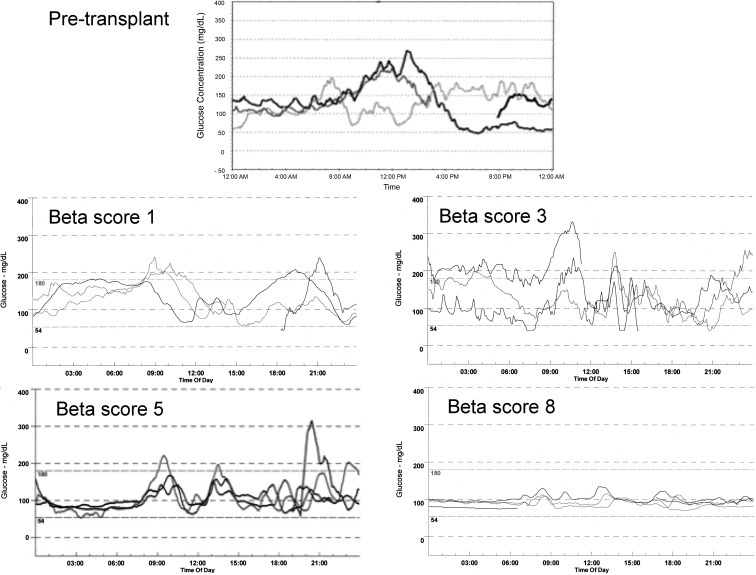
To explore the association between glucose profile and graft function, we analyzed all complete CGM and β-score pairings available during the 3-yr follow-up in the entire cohort. MG and HbA1c were significantly correlated [r = 0.58 (95% CI = 0.46–0.67), P < 0.0001], but marked individual discrepancies remained frequent (Fig. 1, A and B). We also found a significant negative correlation between β-score and GSD [r = −0.71 (95% CI = −0.79–−0.62); P < 0.0001], HYPER [r = −0.71 (95% CI = −0.79–−0.62); P < 0.0001], MG [r = −0.65 (95% CI = −0.73–−0.54); P < 0.0001] and, to a lesser extent, HYPO [r = −0.45 (95% CI = −0.58–−0.31), P < 0.0001]. As illustrated in Fig. 1 (C–F), partial graft function (β-score exceeding 3) was sufficient to abrogate HYPO, but optimal function (β-score exceeding 7) was necessary to fully correct MG, GSD, and HYPER. The results of the 39 tests associated with a β-score of 8 are summarized in Table 1. MG, GSD, HYPER, and HYPO differed significantly between the different subgroups represented by pretransplantation values and the subgroups of patients with a β-score in the 0–2, 3–4, 5–6, and 7–8 ranges 3 yr after transplantation (P < 0.0001). MG (P < 0.001), GSD (P < 0.001), and HYPER (P < 0.05) also significantly differed between the groups with partial function (β-score = 3–4) and optimal function (β-score = 7–8). There was no difference for HYPO between these two groups. Individual examples of CGM profiles according to β-score are given in Fig. 2.
Fig. 1.
Outcomes of continuous glucose measurement. A, HbA1c values in the 23 patients during the 3-yr follow-up. The gray bar indicates the median value. Open symbols indicate optimal function (insulin independence with HbA1c ≤6.5%), gray symbols indicate partial graft function, and black symbols indicate no graft function. B, Association between HbA1c and mean glucose throughout the 3-yr follow-up of the entire cohort [n = 154; Spearman's correlation coefficient r = 0.58 (95% CI = 0.46–0.67); P < 0.001]. C–F, Reciprocal association between graft function (β-score; see Patients and Methods) and the various outcomes of continuous glucose measurement throughout the 3-yr follow-up in the entire cohort (n = 23) (β-score 0–2, n = 17; β-score 3–4, n = 19; β-score 5–6, n = 20; β-score 7–8, n = 55). Tukey box plots indicate median, IQR (boxes), and upper and lower range (whiskers). MG, GSD, and time spent with HYPER and HYPO were significantly lower after IT (P < 0.05 vs. baseline). Tx, Transplantation.
Fig. 2.
Examples of CGM profiles before transplant (top) and 3 yr after transplant in the various groups of graft function: middle right, β-score 1–2; middle left, β-score 3–4; bottom left, β-score 5–6; bottom right, β-score 7–8.
Discussion
This longitudinal study provides direct evidence of the sustained impact of heterotopic β-cell replacement on all components of the daily glucose profile in type 1 diabetes patients. Results also showed that the four components of dysglycemia were not equally affected by the degree of islet graft function, which could have important implications for future development of β-cell replacement.
Previous reports of IT outcome after the first posttransplant year have been mainly based on exogenous insulin requirements, C-peptide levels, fasting glucose, and HbA1c. Here, we demonstrated that MG, an independent predictor of the long-term cardiovascular risk in type 1 diabetes (10), was markedly reduced for at least 3 yr after IT. We also found that MG significantly correlated with islet graft function but only moderately correlated with HbA1c. Factors known to disrupt HbA1c, including anemia or its treatment with iron or erythropoietin, are frequent after IT and likely explain the discrepancies observed between MG and HbA1c paired values in some of our patients (11, 12). Our results suggest that the objective and noninvasive assessment of MG with CGM could be a valuable complement when monitoring the outcome of β-cell replacement. Indeed, CGM allows a continuous monitoring of blood glucose levels including postprandial and overnight periods, enabling the assessment of blood glucose variability, which is one of the main features of brittle diabetes and the detection of non-felt hypoglycemia, which is one of the main indications of islet transplantation. (Non-felt hypoglycemia are hypoglycemia not detected by the patient because of the loss of symptoms. The patient does not perceive or detect low levels of blood glucose, and therefore is more at risk of sudden loss of consciousness.) Moreover, it is performed outside the hospital during usual daily life activities and is blinded to the patient. More to the point, posttransplantation CGM provides the evaluation of the natural history of the functional β-cell mass and islet graft loss, probably reproducing in an accelerated way what happens in β-cell dysfunction of type 1 and type 2 diabetes (13).
Importantly, MG values associated with excellent graft function (β-score of 8) closely matched values measured with the same device in healthy adults (14, 15) and in pancreas transplant recipients (16). Note that slight variations of MG values according to age, gender, and body mass index have been reported (17). Nevertheless, this small series of islet-transplanted patients did not allow reliable assessment of these parameters. One limitation of this single-arm study was the lack of a control group. In a previous controlled study (8), we found that the mean of seven self-reported capillary measurements of blood glucose was significantly lower 1 yr after IT than 1 yr after ip insulin infusion, an alternative innovative therapy for severe type 1 diabetes. MG values measured by Kessler et al. (4) with CGM in patients treated with ip insulin infusion were also markedly higher than those measured in the present study, 3 yr after IT. Whether this sustained improvement in glucose control outweighs the risks associated with chronic immunosuppression and allogeneic sensitization can at this time only be speculated. It is noteworthy that the only available prospective study comparing IT with optimized insulin therapy showed a slower progression of retinopathy and nephropathy after IT (18), as already established for pancreas transplantation.
Although their influence on long-term risk is more controversial, glucose variability and absence of predictability are daily issues confronting patients with type 1 diabetes and their physicians. In this study, GSD and HYPER were the most refractory components of dysglycemia after IT. Even slight impairment of graft function was sufficient to affect these two parameters (Figs. 1 and 2). Conversely, HYPO virtually disappeared when the β-score exceeded 3, in line with previous reports in patients with partial graft function (1) and type 1 diabetes patients with residual β-cell function (19), even if asymptomatic hypoglycemic events have been reported in subjects with normal glucose tolerance (20). Moreover, recent studies have shown the potential deleterious effect of hypoglycemia on vascular function and atherosclerosis, emphasizing the benefits provided by even partial graft function (21, 22).
In summary, this study provides direct evidence that heterotopic β-cell replacement clearly improves each component of type 1 diabetes dysglycemia, including MG, over the long term. Taken together, the results also suggest that the target level of graft function should be adjusted to the assigned goal of β-cell replacement. Current procedures of IT appear sufficient to avoid severe hypoglycemia in most cases. If adequate graft function can be achieved, β-cell replacement has the potential for also correcting other components of type 1 diabetes dysglycemia.
Acknowledgments
We are indebted to Rimed Ezzouaoui, Thomas Hubert, Isanga Aluka, Sandrine Belaïch, Nathalie Delalleau, Bruno Lukowiak, Ericka Moerman, Alexandre Patrice, Valerie Pawlowski (islet lab), and Helene Verstavel and Anne Sophie Vanceulebroek (metabolic evaluation).
This study was supported by grants from the 7th Framework Program of the European Commission (β-Cell Therapy), the French Ministry of Health (PHRC 2001), Fond Européen de Developpement Régional, Conseil Régional Nord Pas de Calais, Groupement Inter Hospitalier G4 (Amiens, Caen, Lille, Rouen), and Agence Nationale de La Recherche (ANR-10-LABX-46). Our team is also part of the Collaborative Islet Transplant Registry supported by the Juvenile Diabetes Research Foundation and the National Institutes of Health.
ClinicalTrial.gov identifiers are NCT00446264 and NCT01123187.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CGM
- Continuous glucose monitoring
- CI
- confidence interval
- GSD
- glucose sd around the mean value
- HbA1c
- glycated hemoglobin
- HYPER
- hyperglycemia
- HYPO
- hypoglycemia
- IQR
- interquartile range
- MG
- mean glucose
- IT
- islet transplantation.
References
- 1. Barton FB, Rickels MR, Alejandro R, Hering BJ, Wease S, Naziruddin B, Oberholzer J, Odorico JS, Garfinkel MR, Levy M, Pattou F, Berney T, Secchi A, Messinger S, Senior PA, Maffi P, Posselt A, Stock PG, Kaufman DB, Luo X, Kandeel F, Cagliero E, Turgeon NA, Witkowski P, Naji A, et al. 2012. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35:1436–1445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vantyghem MC, Press M. 2006. Management strategies for brittle diabetes. Ann Endocrinol (Paris) 67:287–296 [DOI] [PubMed] [Google Scholar]
- 3. Klonoff DC, Buckingham B, Christiansen JS, Montori VM, Tamborlane WV, Vigersky RA, Wolpert H. 2011. Continuous glucose monitoring: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab 96:2968–2979 [DOI] [PubMed] [Google Scholar]
- 4. Kessler L, Passemard R, Oberholzer J, Benhamou PY, Bucher P, Toso C, Meyer P, Penfornis A, Badet L, Wolf P, Colin C, Morel P, Pinget M; GRAGIL Group 2002. Reduction of blood glucose variability in type 1 diabetic patients treated by pancreatic islet transplantation: interest of continuous glucose monitoring. Diabetes Care 25:2256–2262 [DOI] [PubMed] [Google Scholar]
- 5. Paty BW, Senior PA, Lakey JR, Shapiro AM, Ryan EA. 2006. Assessment of glycemic control after islet transplantation using the continuous glucose monitor in insulin-independent versus insulin-requiring type 1 diabetes subjects. Diabetes Technol Ther 8:165–173 [DOI] [PubMed] [Google Scholar]
- 6. Gorn L, Faradji RN, Messinger S, Monroy K, Baidal DA, Froud T, Mastrototaro J, Ricordi C, Alejandro R. 2008. Impact of islet transplantation on glycemic control as evidenced by a continuous glucose monitoring system. J Diabetes Sci Technol 2:221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vantyghem MC, Kerr-Conte J, Arnalsteen L, Sergent G, Defrance F, Gmyr V, Declerck N, Raverdy V, Vandewalle B, Pigny P, Noel C, Pattou F. 2009. Primary graft function, metabolic control, and graft survival after islet transplantation. Diabetes Care 32:1473–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Vantyghem MC, Marcelli-Tourvieille S, Fermon C, Duhamel A, Raverdy V, Arnalsteen L, Kerr-Conte J, Noel C, Fontaine P, Pattou F. 2009. Intraperitoneal insulin infusion versus islet transplantation: comparative study in patients with type 1 diabetes. Transplantation 87:66–71 [DOI] [PubMed] [Google Scholar]
- 9. Ryan EA, Paty BW, Senior PA, Lakey JR, Bigam D, Shapiro AM. 2005. Beta-score: an assessment of β-cell function after islet transplantation. Diabetes Care 28:343–347 [DOI] [PubMed] [Google Scholar]
- 10. Kilpatrick ES, Rigby AS, Atkin SL. 2008. Mean blood glucose compared with HbA1c in the prediction of cardiovascular disease in patients with type 1 diabetes. Diabetologia 51:365–371 [DOI] [PubMed] [Google Scholar]
- 11. Ng JM, Cooke M, Bhandari S, Atkin SL, Kilpatrick ES. 2010. The effect of iron and erythropoietin treatment on the A1C of patients with diabetes and chronic kidney disease. Diabetes Care 33:2310–2313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sancho A, Pastor MC, Cañas L, Morales Indiano C, Ardèvol M, Aguerrevere S, Juega J, Romero R, Lauzurica R. 2011. Posttransplantation anemia: relationship with inflammatory markers, oxidation, and prohepcidin levels. Transplant Proc 43:2196–2198 [DOI] [PubMed] [Google Scholar]
- 13. Hirsch D, Odorico J, Danobeitia JS, Alejandro R, Rickels MR, Hanson M, Radke N, Baidal D, Hullett D, Naji A, Ricordi C, Kaufman D, Fernandez L. 2012. Early metabolic markers that anticipate loss of insulin independence in type 1 diabetic islet allograft recipients. Am J Transplant 12:1275–1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Zhou J, Li H, Ran X, Yang W, Li Q, Peng Y, Li Y, Gao X, Luan X, Wang W, Jia W. 2009. Reference values for continuous glucose monitoring in Chinese subjects. Diabetes Care 32:1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Fox LA, Beck RW, Xing D. 2010. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care 33:1297–1299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Rodríguez LM, Knight RJ, Heptulla RA. 2010. Continuous glucose monitoring in subjects after simultaneous pancreas-kidney and kidney-alone transplantation. Diabetes Technol Ther 12:347–351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Derosa G, Salvadeo SA, Mereu R, D'Angelo A, Ciccarelli L, Piccinni MN, Ferrari I, Gravina A, Maffioli P, Tinelli C. 2009. Continuous glucose monitoring system in free-living healthy subjects: results from a pilot study. Diabetes Technol Ther 11:159–169 [DOI] [PubMed] [Google Scholar]
- 18. Thompson DM, Meloche M, Ao Z, Paty B, Keown P, Shapiro RJ, Ho S, Worsley D, Fung M, Meneilly G, Begg I, Al Mehthel M, Kondi J, Harris C, Fensom B, Kozak SE, Tong SO, Trinh M, Warnock GL. 2011. Reduced progression of diabetic microvascular complications with islet cell transplantation compared with intensive medical therapy. Transplantation 91:373–378 [DOI] [PubMed] [Google Scholar]
- 19. Sherr J, Tamborlane WV, Xing D, Tsalikian E, Mauras N, Buckingham B, White NH, Arbelaez AM, Beck RW, Kollman C, Ruedy K; Diabetes Research in Children Network (DirecNet) Study Group 2012. Achievement of target A1C levels with negligible hypoglycemia and low glucose variability in youth with short-term type 1 diabetes and residual β-cell function. Diabetes Care 35:817–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang C, Lv L, Yang Y, Chen D, Liu G, Chen L, Song Y, He L, Li X, Tian H, Jia W, Ran X. 2012. Glucose fluctuations in subjects with normal glucose tolerance, impaired glucose regulation and newly-diagnosed type 2 diabetes mellitus. Clin Endocrinol (Oxf) 76:810–815 [DOI] [PubMed] [Google Scholar]
- 21. Castaldo E, Sabato D, Lauro D, Sesti G, Marini MA. 2011. Hypoglycemia assessed by continuous glucose monitoring is associated with preclinical atherosclerosis in individuals with impaired glucose tolerance. PLoS One 6:e28312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peña AS, Couper JJ, Harrington J, Gent R, Fairchild J, Tham E, Baghurst P. 2012. Hypoglycemia, but not glucose variability, relates to vascular function in children with type 1 diabetes. Diabetes Technol Ther 14:457–462 [DOI] [PMC free article] [PubMed] [Google Scholar]