Abstract
We used a pale-green maize (Zea mays L.) mutant that fails to accumulate ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco) to test the working hypothesis that the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase (PEPC) by its Ca2+-insensitive protein-serine/threonine kinase (PEPC kinase) in the C4 mesophyll cytosol depends on cross-talk with a functional Calvin cycle in the bundle sheath. Wild-type (W22) and bundle sheath defective2-mutable1 (bsd2-m1) seeds were grown in a controlled environment chamber at 100 to 130 μmol m−2 s−1 photosynthetic photon flux density, and leaf tissue was harvested 11 d after sowing, following exposure to various light intensities. Immunoblot analysis showed no major difference in the amount of polypeptide present for several mesophyll- and bundle-sheath-specific photosynthetic enzymes apart from Rubisco, which was either completely absent or very much reduced in the mutant. Similarly, leaf net CO2-exchange analysis and in vitro radiometric Rubisco assays showed that no appreciable carbon fixation was occurring in the mutant. In contrast, the sensitivity of PEPC to malate inhibition in bsd2-m1 leaves decreased significantly with an increase in light intensity, and there was a concomitant increase in PEPC kinase activity, similar to that seen in wild-type leaf tissue. Thus, although bsd2-m1 mutant plants lack an operative Calvin cycle, light activation of PEPC kinase and its target enzyme are not grossly perturbed.
In illuminated C4 leaf tissue, PEPC (EC 4.1.1.31) is primarily involved in photosynthetic carbon fixation. This cytosolic C4 enzyme has been shown to be phosphorylated and thus up-regulated by a reversibly light-activated, Ca2+-independent protein-Ser/Thr kinase (PEPC kinase). (For recent reviews on the regulation of plant PEPC by reversible protein phosphorylation, see Nimmo, 1993; Chollet et al., 1996; and Vidal and Chollet, 1997.) To determine how light causes increased PEPC kinase activity in C4 leaf tissue, the effect of several chemical inhibitors of photosynthesis, such as dl-glyceraldehyde and DCMU, on the phosphorylation of PEPC was examined in detached sorghum and maize (Zea mays) leaves (Bakrim et al., 1992; Jiao and Chollet, 1992). The results implicated a functional Calvin cycle as part of the PEPC kinase light-signal transduction chain, and were the “prelude” to subsequent cellular studies involving isolated C4 mesophyll protoplasts and cells.
Early experiments using isolated sorghum mesophyll protoplasts indicated that Ca2+ and alkaline conditions in the mesophyll cytosol were necessary for PEPC kinase activity to increase upon illumination (Pierre et al., 1992). Later work used isolated mesophyll cells and protoplasts from a different NADP-ME-type C4 plant (Digitaria sanguinalis), and showed that some component of the Calvin cycle, likely 3-PGA, was also a necessary signaling element (Duff et al., 1996; Giglioli-Guivarc'h et al., 1996). These in situ observations are consistent with the whole-leaf studies summarized above (Bakrim et al., 1992; Jiao and Chollet, 1992).
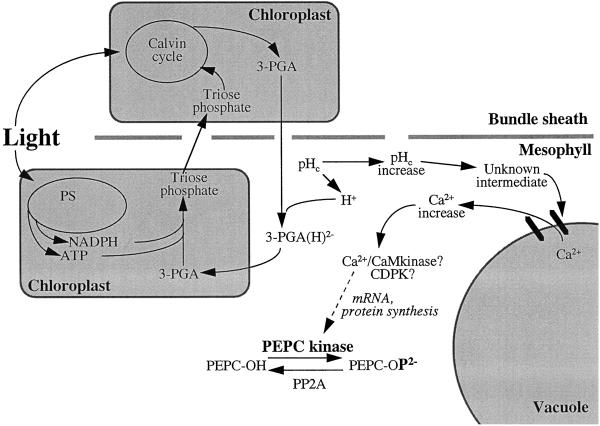
The present working model for C4 PEPC kinase up-regulation in the light was formulated mainly from these collective findings. It was proposed that 3-PGA (originating from the Calvin cycle in the bundle sheath) enters the mesophyll cytosol, where it is partially protonated to its dianionic (2−) form, and then enters the mesophyll chloroplast by the Pi translocator in a light-dependent manner (Fig. 1). Protonation of 3-PGA in the mesophyll cell cytosol would cause an increase in cytosolic pH. This, combined with a subsequent increase in cytosolic [Ca2+] (presumed to come from the vacuole), would ultimately cause an increase in PEPC kinase protein synthesis and, thus, an increase in PEPC kinase activity (Giglioli-Guivarc'h et al., 1996; Vidal and Chollet, 1997). Results from another group using an in vitro translation technique to study apparent changes in PEPC kinase mRNA levels in whole maize leaves showed that the light-induced increase in PEPC kinase activity on a protein level was correlated with an increase in translatable PEPC kinase mRNA (Hartwell et al., 1996). These findings are also incorporated into the model.
Figure 1.
Current working model of the organization of signaling elements in the C4 PEPC phosphorylation cascade upon illumination (modified from Vidal and Chollet, 1997). CaM, Calmodulin; CDPK, calmodulin-like domain (Ca2+ dependent) protein kinase; 3-PGA(H)2−, partially protonated (2−) form of 3-PGA; pHc, cytosolic pH; PP2A, type 2A protein phosphatase; PS, photosystems.
Consistent with this working model, it is generally assumed that in C4 leaf tissue there must be some form of cross-talk between the mesophyll and bundle-sheath cells to ensure efficient metabolic regulation during C4 photosynthesis (Hatch, 1987; Nelson and Langdale, 1992). For this reason alone, experiments using whole-leaf tissue to elucidate the chain of events leading from illumination of a C4 leaf to increased PEPC kinase activity are in many ways preferable to those involving isolated mesophyll cells or protoplasts, because no separation of the two photosynthetic cell types is involved. The bundle sheath defective2-mutable1 (bsd2-m1) mutation of maize disrupts C4 differentiation in bundle-sheath cells, such that Rubisco protein is absent and the bundle-sheath chloroplast structure is aberrant (Roth et al., 1996). Despite the absence of a functional Calvin cycle, the mesophyll cells of bsd2-m1 mutant leaves appear no different from those of the wild type (Roth et al., 1996). In certain genetic backgrounds, bsd2-m1 is a uniformly pale-green mutant that is unable to fix atmospheric CO2 and, thus, depends on its seed reserves until these are depleted and the seedling dies (within 15 d of planting) (Roth et al., 1996; R. Roth, S. Rolfe, L.H. Smith, J.A. Langdale, and R. Chollet, unpublished observations). We have used bsd2-m1 mutant seedlings in a noninvasive manner to further address the question of whether some component or derivative of the Calvin cycle is indispensable for the light activation of C4 PEPC kinase activity in maize leaf tissue.
MATERIALS AND METHODS
Chemicals
All chemicals were from Sigma, unless stated otherwise. [γ-32P]ATP (3000 Ci/mmol) and [14C]NaHCO3 (58 Ci/mol) were purchased from Amersham. Polyclonal antibodies raised against maize (Zea mays) leaf NADP-ME and sorghum leaf NADP-MDH were kindly provided by Drs. S. Madhavan (University of Nebraska-Lincoln) and J. Vidal (Université de Paris-Sud, France), respectively.
Plant Material and Growth Conditions
bsd2-m1 mutant seed was obtained as described by Roth et al. (1996). The bsd2-m1 allele was isolated by W.F. Sheridan (University of North Dakota, Grand Forks) from a maize line containing active Mutator transposable elements. Sectored plants in which green tissue is present along with chlorotic mutant tissue are able to grow beyond the seedling stage and were used to cross and backcross bsd2-m1 into the inbred line W22. Heterozygous progeny of the second cross into W22 were self-pollinated, and the resultant progeny were used for the experiments described in this study. Only uniformly pale-green bsd2-m1 seedlings were used.
Seedlings were grown in soil in a growth chamber maintained at 28°C with a 16-h light (100–130 μmol m−2 s−1 [400–700 nm])/8-h dark cycle. Third leaves were harvested 11 d after sowing just before the end of the dark period, 2 h into the normal low-light photoperiod, or after an additional 2 h under a more moderate light regime (400–450 μmol m−2 s−1). W22 seedlings (seeds obtained from the University of Wisconsin-Madison) were used as internal controls for the low-light growth conditions, and these were grown under conditions identical to those used for bsd2-m1 mutant plants. Additional external controls were also used in some experiments; these consisted of greenhouse-grown wild-type tissue (cv Golden Bantam) placed into the growth chamber at the start of the 8-h dark period before leaf harvest. All leaf tissue was harvested into liquid N2 and stored at −70°C until used for extraction.
Gas-Exchange Analysis
Net CO2-exchange analysis was performed with an IR gas analyzer (model 6200, Li-Cor, Lincoln, NE) under the low-light (approximately 100 μmol m−2 s−1) and moderate-light (approximately 400 μmol m−2 s−1) growth conditions for bsd2-m1 and W22 leaf tissue and under greenhouse-light conditions (approximately 1300 μmol m−2 s−1) for the external, greenhouse-grown control only, according to the manufacturer's instructions.
Preparation of Leaf Extracts for Enzyme Assays
Leaf tissue was extracted according to the method of Jiao et al. (1991), with the following exceptions: samples were ground in liquid N2 in a prechilled mortar to a fine powder, and were then transferred to a second prechilled mortar containing 3 volumes of the appropriate extraction buffer and mixed thoroughly. Buffer A (100 mm Tris-HCl, pH 8.0, 20% [v/v] glycerol, 10 mm MgCl2, 14 mm 2-mercaptoethanol, and 1 mm EDTA) was used for preparation of PEPC kinase, Rubisco, and NADP-ME (extracted at 4°C). Buffer A, containing 1 μm MC-LR, an inhibitor of type 1/2A protein phosphatase, and 10 μg/mL chymostatin was used for extraction of PEPC (extracted at 4°C). Buffer A plus 2 mm pyruvate was used for extraction of PPDK (extracted at a cool room temperature of not less than 10°C). Buffer B (100 mm Tris-HCl, pH 8.0, 1 mm EDTA, and 14 mm 2-mercaptoethanol) was used for preparation of NADP-MDH (extracted at 4°C). The leaf homogenates were filtered through a 100-μm nylon mesh and microcentrifuged at 13,000g for 2 min. The crude supernatant fluid was immediately used to assay PPDK and NADP-MDH activity or, after rapid desalting on a 200-μL Sephadex G-25 column preequilibrated with buffer C (100 mm Tris-HCl, pH 7.5, 10 mm MgCl2, and 20% [v/v] glycerol), to assay PEPC (buffer C plus 1 μm MC-LR and 10 μg/mL chymostatin), PEPC kinase, Rubisco, and NADP-ME.
Enzyme Assays
PEPC, PEPC kinase, PPDK, and NADP-MDH were assayed at 30°C according to the method of Jiao et al. (1991), with the following exceptions: (a) the various PEPC substrates used for PEPC kinase assays were purified maize leaf PEPC (dark form) and sorghum recombinant Ser-8, S8T, S8Y, and S8D C4 PEPCs, purified as described by Li et al. (1997); and (b) the Ca2+-independent protein-Ser/Thr kinase was assayed in the presence of 0.5 mm EGTA and 0.1 μm MC-LR. Dried SDS-PAGE minigels were analyzed by phosphorimaging (model Storm 860, Molecular Dynamics, Sunnyvale, CA). NADP-ME was assayed spectrophotometrically according to the method of Ashton et al. (1990) at 340 nm and at 30°C.
RuBP-dependent fixation of 14C into acid-stable products by Rubisco was assayed as follows: 10 μL of desalted leaf extract was preincubated in a serum-stoppered vial for 10 min at 30°C in assay medium containing 100 mm Tris-HCl, pH 8.0, 10 mm MgCl2, 0.1 mm EDTA, 0.5 mg mL−1 BSA, and 25 mm [14C]NaHCO3 (4 μCi), in a total volume of 500 μL. RuBP (0.5 mm) or buffer alone was then injected to start the reaction. The assays were terminated after 60 s at 30°C by the addition of 100 μL of acetic acid. The contents were dried at 80°C, and 500 μL of distilled water was added to each vial, along with 5 mL of a biodegradable scintillant. 14C incorporated into acid-stable products was determined as disintegrations per minute in a liquid-scintillation counter. Malate sensitivity of PEPC was assayed spectrophotometrically at 340 nm and at 30°C under suboptimal conditions (pH 7.3, 2.5 mm PEP) in the presence and absence of 0.5 mm l-malate (see Bakrim et al., 1992; Jiao and Chollet, 1992; Duff et al., 1996).
Immunoblot Analysis
Using desalted centrifuged leaf extracts prepared as for the PEPC assays, leaf soluble protein was electrophoresed on 10% (w/v) SDS-PAGE minigels (Laemmli, 1970) and blotted onto nitrocellulose membranes using a Mini-Protean II apparatus (Bio-Rad) according to the manufacturer's instructions. The blots were blocked for 1 h with Tris-Tween milk (100 mm Tris-HCl, pH 7.2, 0.2% [v/v] Tween 20, and 5% [w/v] dried skim-milk powder) and then incubated with primary antibody for 1 h using a 1:1000 dilution of rabbit IgG raised against soybean root nodule PEPC (Zhang et al., 1995), maize leaf PPDK (Budde and Chollet, 1986), tobacco Rubisco (Rejda et al., 1981), and maize leaf NADP-ME, and a 1:500 dilution of rabbit IgG raised against sorghum leaf NADP-MDH. The blots were washed thoroughly with Tris-Tween (100 mm Tris-HCl, pH 7.2, and 0.2% [v/v] Tween 20) and incubated in an alkaline phosphatase-conjugated secondary antibody (a 1:3000 dilution of goat anti-rabbit IgG [H+L]; Bio-Rad) for 1 h. After thorough washing with Tris-Tween, proteins were detected with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium reagent (SigmaFAST tablets).
Miscellaneous Assays
Soluble protein content was determined for all crude and desalted leaf extracts according to the method of Bradford (1976), using prepared dye reagent (Bio-Rad) and BSA as a standard. Total chlorophyll content was determined according to the method of Wintermans and de Mots (1965), using the filtered crude extracts.
RESULTS
bsd2-m1 Mutants Lack a Functional Calvin Cycle
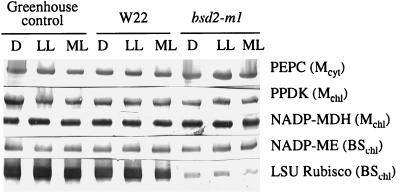
Total soluble protein and chlorophyll contents of bsd2-m1 leaves were reduced compared with W22, about 50% and 33%, respectively, of wild-type levels. For example, the average chlorophyll content of the bsd2-m1 samples was 0.4 mg/g leaf fresh weight, compared with 1.2 mg/g fresh weight for the W22 leaves. Immunoblot analysis showed that the polypeptides corresponding to PEPC, PPDK, NADP-MDH, and NADP-ME were present in comparable amounts in greenhouse-grown tissue and in chamber-grown W22 and bsd2-m1 plants at each light intensity examined when equal amounts of total soluble protein were analyzed by SDS-PAGE (Fig. 2). These findings are in agreement with recent data published by Roth et al. (1996). For immunoblots probed with antibodies raised against the crystalline Rubisco holoenzyme from tobacco, the component large- and small-subunit polypeptides were either completely absent or were present in very low amounts in leaf extracts of bsd2-m1 compared with greenhouse-grown control or W22 plants (Fig. 2).
Figure 2.
Immunoblot analysis of total soluble proteins extracted from greenhouse-grown control maize and chamber-grown W22 and bsd2-m1 leaves harvested after approximately 8 h of darkness (D), after 2 h of low-light (LL) illumination (approximately 100–130 μmol m−2 s−1), and after a further 2 h of illumination at a more moderate light (ML) intensity (approximately 400–450 μmol m−2 s−1). It should be noted that the trace amounts of Rubisco large subunit depicted for bsd2-m1 in this figure were observed infrequently and were not functional in in vitro, 14C-based Rubisco assays (see Table II and Discussion). Mcyt, Mesophyll-cell specific, located in the cytosol; Mchl, mesophyll-cell specific, located in the chloroplast; BSchl, bundle-sheath-cell specific, located in the chloroplast; LSU, large subunit.
Traces of Rubisco polypeptides were seen approximately 25% of the time. Because of this finding, we had to be sure that the mutant was not capable of fixing atmospheric carbon in the light. Thus, we determined net CO2 exchange by the mutant and controls under both low and moderate light intensities (approximately 100 and 400 μmol m−2 s−1, respectively), and also under greenhouse light for the corresponding control only (approximately 1300 μmol m−2 s−1).
When net CO2 uptake by bsd2-m1 leaves was determined under low light, a negative value resulted (Table I). Therefore, at the low light intensity at which the bsd2-m1 and W22 seedlings were grown, there was no net carbon fixation by the mutant. Under a more moderate light intensity, however, a small but positive value was obtained from the bsd2-m1 leaf tissue, amounting to less than 5% of the corresponding W22 control value. Whether this low apparent rate was a true indication of carbon fixation by Rubisco occurring in bsd2-m1 was determined by in vitro RuBP-dependent 14C assays performed with desalted extracts from the same leaves used for CO2-exchange analysis. The results from these sensitive radiometric assays showed that the bsd2-m1 sample had no acid-stable 14C products and was not fixing carbon (Table II). As expected, extracts from W22 were able to fix carbon in a RuBP-dependent manner, with a rate of about 0.4 μmol min−1 mg−1 soluble leaf protein.
Table I.
Net CO2 fixation by leaves of greenhouse-grown control maize and chamber-grown W22 and bsd2-m1 leaves at various incident light intensities
| PPFD | Net CO2 Fixation
|
||
|---|---|---|---|
| Control | W22 | bsd2-m1 | |
| μmol m−2 s−1 | |||
| 100 | 1.24 | 0.94 | −0.04a |
| 400 | 6.04 | 3.81 | 0.17 |
| 1300 | 20 | ||
Each value is the mean of two measurements on three leaves per plant type. se values were no more than 10%.
Negative value indicates net CO2 evolution in the light.
Table II.
Carboxylase activities and the change in sensitivity of PEPC to malate inhibition in W22 and bsd2-m1 leaves harvested after 8 h of darkness (D), after 2 h of low light (LL), and after a further 2 h in moderate light (ML)
| Plant Material | Carboxylase Activity
(LL)
|
Inhibition by l-Malatec
|
|||
|---|---|---|---|---|---|
| Rubiscoa | PEPCb | D | LL | ML | |
| μmol min−1 mg−1 protein | % | ||||
| W22 | 0.37 | 1.4 | 70 ± 8.3 | 49 ± 5.2 | 45 ± 5.3 |
| bsd2-m1 | 0 | 1.6 | 64 ± 9.1 | 48 ± 4.9 | 40 ± 3.7 |
Percentage inhibition by 0.5 mm l-malate was determined on a minimum of three leaves from two different batches of plants. se values were no more than 15% for the carboxylase activities, and were as indicated for inhibition by l-malate. See the legend to Figure 2 for other details.
RuBP-dependent, CO2/Mg2+-activated activity (pH 8.0, 25 mm NaHCO3, 0.5 mm RuBP).
Assayed at optimal conditions (pH 8.0, 5 mm PEP).
Assayed at pH 7.3 and 2.5 mm PEP, with or without 0.5 mm l-malate.
C4 Enzyme Activities
Once it had been established that bsd2-m1 leaf tissue neither contained functional Rubisco nor fixed appreciable atmospheric carbon, even in leaves that showed trace amounts of Rubisco protein by western-blot analysis, other photosynthetic enzymes were assayed to determine whether their activity was also affected by the mutation. First, there were no major differences in apparent polypeptide contents when equal amounts of total soluble protein were analyzed by immunoblotting (Fig. 2). This was true not only for mesophyll cytosolic (PEPC) and chloroplastic (PPDK, NADP-MDH) enzymes, but also for NADP-ME, which, like Rubisco, is localized in bundle-sheath chloroplasts (Hatch, 1987). Second, there was no marked difference in enzyme activity (per milligram of soluble protein) at near-Vmax assay conditions for PEPC (Table II), PPDK, NADP-MDH, and NADP-ME between control and mutant tissues (data not shown). In addition, for PPDK and NADP-MDH, which are both reversibly light-activated chloroplastic enzymes in C4 plants (Hatch, 1987), there was no difference in activation state (light versus dark activity) between either of the controls and bsd2-m1 (light/dark activity ratios of about 8 and 15 for PPDK and NADP-MDH, respectively).
Changes in the Sensitivity of PEPC to l-Malate Inhibition and in PEPC Kinase Activity in bsd2-m1
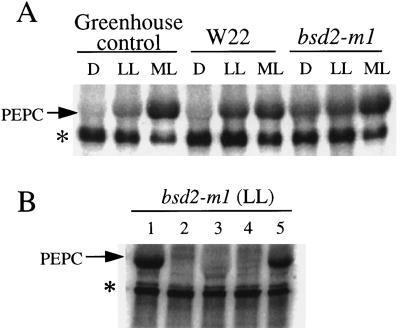
PEPC activity did not change appreciably under any of the three light regimes used in these experiments when assayed under suboptimal conditions (2.5 mm PEP, pH 7.3; data not shown). However, the enzyme became significantly less sensitive to inhibition by l-malate with an increase in light intensity in both W22 and bsd2-m1 leaves (Table II). This light-induced decrease in sensitivity of PEPC to malate inhibition implies an increase in PEPC kinase activity and a concomitant increase in the phosphorylation state of the maize target enzyme at Ser-15 (Jiao et al., 1991; McNaughton et al., 1991; Bakrim et al., 1992; Jiao and Chollet, 1992; Chollet et al., 1996). To document this point, PEPC kinase assays were performed using desalted leaf extracts from all plant types exposed to the three light intensities. Figure 3A shows the results of a representative set of such in vitro kinase assays performed in the presence of EGTA and MC-LR. It is clear that in the dark there was essentially no PEPC kinase activity in the greenhouse-grown control leaf tissue or in W22, although some kinase activity was detected in bsd2-m1 leaves harvested in the dark. As the light intensity increased to approximately 130 μmol m−2 s−1, there was a slight increase in kinase activity for the greenhouse-grown control, but a much larger increase in the low-light-grown W22 leaf tissue.
Figure 3.
In vitro assays of PEPC kinase activity. A, Determination of PEPC kinase activity in desalted crude extracts from greenhouse-grown control maize and chamber-grown W22 and bsd2-m1 leaves harvested after 8 h of darkness (D), after 2 h of low light (LL), and after a further 2 h of illumination at a more moderate light (ML) intensity. Ten micrograms of total soluble protein was assayed for PEPC kinase activity in the presence of [γ-32P]ATP/Mg (8 μCi), 0.5 mm EGTA, 0.1 μm MC-LR, and 10 μg of purified dephospho (dark form) wild-type maize PEPC. The arrow denotes the approximately 110-kD PEPC polypeptide on this representative phosphorimage. An unknown contaminating phosphoprotein may also be seen (denoted by the asterisk). This phosphoprotein was neither detected by Coomassie blue staining of the gel before phosphorimaging (data not shown) nor derived from the exogenous PEPC substrate (see lane 2 in B). B, Substrate and phosphorylation-site specificity of bsd2-m1 kinase activity. Low-light (LL)-illuminated bsd2-m1 leaves were extracted and assayed for PEPC kinase activity (10 μg of desalted soluble protein) as described for A, with or without 10 μg of purified exogenous recombinant wild-type (Ser-8) or phosphorylation-site mutant sorghum C4 PEPC in the in vitro phosphorylation medium. Lane 1, Plus Ser-8 PEPC; lane 2, minus exogenous PEPC (control); lane 3, plus S8D PEPC; lane 4, plus S8Y PEPC; and lane 5, plus S8T PEPC. The arrow denotes the position of the PEPC polypeptide on this representative phosphorimage.
At the highest light intensity examined (approximately 400 μmol m−2 s−1), the largest increase in kinase activity for the greenhouse-grown control occurred, with a much smaller increase in kinase activity for W22. Notably, for extracts prepared from bsd2-m1 after darkness or after exposure to low or moderate light, there was an increase in kinase activity with increasing light intensity (Fig. 3A). On average, the PEPC kinase activity in darkened bsd2-m1 samples amounted to approximately 25% of that detected in moderate light, and the kinase activity in low-light samples was approximately 80% of that in moderate light, as determined by phosphorimager analysis. In comparison, the PEPC kinase activity in the W22 leaf samples ranged from <1% (darkness) to approximately 85% (low light) of that in moderate light. These trends are generally consistent with the related changes seen in the sensitivity of PEPC to malate inhibition (Table II).
To ensure that “authentic” PEPC kinase activity was being measured in these in vitro phosphorylation experiments, we repeated the kinase assays using desalted leaf extracts from low-light-exposed bsd2-m1 in the presence and absence of various sorghum recombinant C4 PEPCs as substrate to document that the Ca2+-independent PEPC kinase activity we observed was specific for Ser/Thr residues at position 8. Figure 3B shows the results of a representative set of kinase assays using the different PEPC substrates. It is clear that the kinase activity from bsd2-m1 leaf tissue phosphorylates wild-type sorghum C4 PEPC (Ser-8) and the recombinant S8T form to a similar extent, but that it cannot phosphorylate the recombinant S8D or S8Y PEPCs. In these important respects the Ca2+-insensitive PEPC kinase activity in bsd2-m1 behaves identically to the kinase activity from wild-type maize leaves (Li et al., 1997).
DISCUSSION
In this study we set out to examine the light activation of PEPC kinase and its target enzyme in the Rubisco-deficient maize mutant bsd2-m1 (Roth et al., 1996). Because bsd2-m1 leaf tissue is sensitive to high PPFD (Roth et al., 1996), the seedlings were grown under a low-light regime (approximately 130 μmol m−2 s−1). Wild-type W22 seedlings were grown under these same conditions to serve as an internal control. In certain genetic backgrounds, bsd2-m1 is a uniformly pale-green mutant with reduced contents of total soluble protein and chlorophyll compared with the wild type. However, when total soluble leaf protein was subjected to SDS-PAGE and analyzed by immunoblotting, the only major difference between W22 and bsd2-m1 extracts was in the amount of Rubisco protein (Fig. 2). This bundle-sheath-specific stromal enzyme was frequently undetectable in bsd2-m1 leaves (Roth et al., 1996; also see above), which likely accounts for much of the approximately 50% decrease in total soluble protein in the tissue.
In bsd2-m1 leaves both the large- and small-subunit transcripts of Rubisco are present (Roth et al., 1996) and translated, but the polypeptides are unstable in planta (R. Roth and J.A. Langdale, unpublished data). This would account for the lack of both net CO2 fixation by bsd2-m1 (Table I) and RuBP-dependent carbon fixation in extracts from bsd2-m1 leaf tissue (Table II), even when trace amounts of the subunit polypeptides were detected on an immunoblot (e.g. Fig. 2), generally only 25% of the time. In addition, the comparative enzyme protein and activity findings summarized in Figure 2, Table II, and above are consistent with previous observations on decreased Rubisco levels (approximately 5%–50% of wild type) in certain hcf mutants of maize (Edwards et al., 1988) and in a C4 Flaveria species transformed with an antisense RNA construct targeted to RbcS (Furbank et al., 1996). In all cases, the level of C4 pathway enzymes was not correlated with that of Rubisco, including another bundle-sheath stromal enzyme, NADP-ME.
Although bsd2-m1 lacks functional Rubisco and, therefore, an operative Calvin cycle, PEPC becomes less sensitive to malate inhibition with an increase in light intensity, and correspondingly there is an increase in Ca2+-independent protein-Ser/Thr kinase activity (see Table II, Fig. 3, and comments above). These data are seemingly inconsistent with the current working hypothesis (Vidal and Chollet, 1997; Fig. 1) that light activation of C4 PEPC kinase involves metabolic cross-talk between the neighboring bundle-sheath and mesophyll cells because some component of the Calvin cycle (most likely 3-PGA) is necessary for alkalinization of the mesophyll cytosol, thereby initiating the chain of events resulting in up-regulation of the kinase and the concomitant phosphorylation of PEPC.
Use of bsd2-m1 mutant plants has allowed us to examine changes in PEPC kinase activity in planta after illumination in a seemingly “isolated” mesophyll tissue, without having to disrupt C4 leaf structure before the start of the experiment. However, our results may not necessarily indicate what actually occurs in normal C4 leaf tissue but, rather, may simply reflect the functional redundancy or flexibility seen in so many aspects of plant metabolism. For example, it is conceivable that in the mutant 3-PGA is provided by the mobilization of starch/Suc reserves during early seedling growth rather than directly from the Calvin cycle. A similar nonphotosynthetic origin of this presumed signaling element may also possibly occur in darkened leaves performing starch degradation/malate accumulation during CAM and in legume root nodules for the up-regulation of PEPC kinase activity (see Carter et al., 1991; Hartwell et al., 1996; Zhang and Chollet, 1997). Indeed, there is a precedent for such an involvement of reserve materials in providing signaling metabolites during the light activation of maize leaf Suc-P synthase under nonphotosynthetic conditions in an N2 atmosphere (Huber et al., 1987).
One other notable finding has come from this study. Previous experiments showed that light activation of PEPC in sorghum leaf tissue required a minimum light intensity of approximately 350 μmol m−2 s−1 (Bakrim et al., 1992). In our study, we had to grow bsd2-m1 plants under low light to prevent photooxidative damage of leaf tissue. When illuminated at approximately 130 μmol m−2 s−1, PEPC kinase activity increased in both bsd2-m1 and similarly grown W22 plants (see Fig. 3A and remarks above). Therefore, under a low-light growth regime, the sensitivity of PEPC to malate inhibition (Table II) and PEPC kinase activity (Fig. 3A) decreases and increases, respectively, as light intensity increases from darkness to this low PPFD. In contrast, the light activation of PEPC kinase in the greenhouse-grown control seedlings was most evident at the more moderate light intensity of approximately 400 μmol m−2 s−1 (Fig. 3A), in agreement with the results of Bakrim et al. (1992). This brings yet another factor into the problem of elucidating the regulation of PEPC kinase activity in C4 (and probably C3; Li et al., 1996) leaves: the relative light intensity during growth and kinase activation.
Additional experiments will need to be conducted with bsd2-m1 to determine whether alkalinization of the mesophyll cytosol and/or increases in [3-PGA] indeed occur in the light, thus verifying two basic tenets of the model depicted in Figure 1. Although related in situ experiments have been performed with isolated C4 leaf mesophyll protoplasts and cells (Pierre et al., 1992; Duff et al., 1996; Giglioli-Guivarc'h et al., 1996), these should be repeated using bsd2-m1 cellular preparations to determine how the mutant is able to up-regulate PEPC kinase and its target enzyme in the light in the absence of a metabolite signal originating directly from the Calvin cycle. It would also be worthwhile to further investigate how the up-/down-regulation of PEPC kinase and, thus, PEPC in C4 and C3 leaves varies under different light regimes used during growth.
ACKNOWLEDGMENTS
We thank Audrey Carl and, most especially, Shirley Condon for their excellent technical assistance, and Drs. S. Madhavan and J. Vidal for their generous gift of antibodies.
Abbreviations:
- MC-LR
microcystin-LR
- NADP-MDH
NADP-specific malate dehydrogenase
- NADP-ME
NADP-specific malic enzyme
- PEPC
PEP carboxylase
- 3-PGA
3-phosphoglyceric acid
- PPDK
pyruvate,orthophosphate dikinase
- RuBP
ribulose-1,5-bisphosphate
Footnotes
This work was supported in part by the National Science Foundation (grant nos. MCB-9315928 and MCB-9727236) and is published as no. 12,197 in the University of Nebraska Agricultural Research Division journal series.
LITERATURE CITED
- Ashton AR, Burnell JN, Furbank RT, Jenkins CLD, Hatch MD. Enzymes of C4 photosynthesis. In: Lea PJ, editor. Methods in Plant Biochemistry, Vol 3. San Diego, CA: Academic Press; 1990. pp. 39–72. [Google Scholar]
- Bakrim N, Echevarria C, Cretin C, Arrio-Dupont M, Pierre JN, Vidal J, Chollet R, Gadal P. Regulatory phosphorylation of Sorghum leaf phosphoenolpyruvate carboxylase: identification of the protein-serine kinase and some elements of the signal-transduction cascade. Eur J Biochem. 1992;204:821–830. doi: 10.1111/j.1432-1033.1992.tb16701.x. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Budde RJA, Chollet R. In vitro phosphorylation of maize leaf phosphoenolpyruvate carboxylase. Plant Physiol. 1986;82:1107–1114. doi: 10.1104/pp.82.4.1107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter PJ, Nimmo HG, Fewson CA, Wilkins MB. Circadian rhythms in the activity of a plant protein kinase. EMBO J. 1991;10:2063–2068. doi: 10.1002/j.1460-2075.1991.tb07737.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chollet R, Vidal J, O'Leary MH. Phosphoenolpyruvate carboxylase: a ubiquitous, highly regulated enzyme in plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:273–298. doi: 10.1146/annurev.arplant.47.1.273. [DOI] [PubMed] [Google Scholar]
- Duff SMG, Giglioli-Guivarc'h N, Pierre JN, Vidal J, Condon SA, Chollet R. In situ evidence for the involvement of calcium and bundle sheath-derived photosynthetic metabolites in the C4 phosphoenolpyruvate-carboxylase kinase signal-transduction chain. Planta. 1996;199:467–474. [Google Scholar]
- Edwards GE, Jenkins CLD, Andrews J. CO2 assimilation and activities of photosynthetic enzymes in high chlorophyll fluorescence mutants of maize having low levels of ribulose 1,5-bisphosphate carboxylase. Plant Physiol. 1988;86:533–539. doi: 10.1104/pp.86.2.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furbank RT, Chitty JA, von Caemmerer S, Jenkins CLD. Antisense RNA inhibition of RbcS gene expression reduces Rubisco level and photosynthesis in the C4 plant Flaveria bidentis. Plant Physiol. 1996;111:725–734. doi: 10.1104/pp.111.3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giglioli-Guivarc'h N, Pierre JN, Brown S, Chollet R, Vidal J, Gadal P. The light-dependent transduction pathway controlling the regulatory phosphorylation of C4 phosphoenolpyruvate carboxylase in protoplasts from Digitaria sanguinalis. Plant Cell. 1996;8:573–586. doi: 10.1105/tpc.8.4.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell J, Smith LH, Wilkins MB, Jenkins GI, Nimmo HG. Higher plant phosphoenolpyruvate carboxylase kinase is regulated at the level of translatable mRNA in response to light or a circadian rhythm. Plant J. 1996;10:1071–1078. [Google Scholar]
- Hatch MD. C4 photosynthesis: a unique blend of modified biochemistry, anatomy and ultrastructure. Biochim Biophys Acta. 1987;895:81–106. [Google Scholar]
- Huber SC, Ohsugi R, Usuda H, Kalt-Torres W. Light modulation of maize leaf sucrose phosphate synthase. Plant Physiol Biochem. 1987;25:515–523. [Google Scholar]
- Jiao JA, Chollet R. Light activation of maize phosphoenolpyruvate carboxylase protein-serine kinase activity is inhibited by mesophyll and bundle sheath-directed photosynthesis inhibitors. Plant Physiol. 1992;98:152–156. doi: 10.1104/pp.98.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao JA, Echevarria C, Vidal J, Chollet R. Protein turnover as a component in the light/dark regulation of phosphoenolpyruvate carboxylase protein-serine kinase activity in C4 plants. Proc Natl Acad Sci USA. 1991;88:2712–2715. doi: 10.1073/pnas.88.7.2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Li B, Pacquit V, Jiao JA, Duff SMG, Maralihalli GB, Sarath G, Condon SA, Vidal J, Chollet R. Structural requirements for phosphorylation of C4-leaf phosphoenolpyruvate carboxylase by its highly regulated protein-serine kinase: a comparative study with synthetic-peptide substrates and mutant target proteins. Aust J Plant Physiol. 1997;24:443–449. [Google Scholar]
- Li B, Zhang XQ, Chollet R. Phosphoenolpyruvate carboxylase kinase in tobacco leaves is activated by light in a similar but not identical way as in maize. Plant Physiol. 1996;111:497–505. doi: 10.1104/pp.111.2.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaughton GAL, MacKintosh C, Fewson CA, Wilkins MB, Nimmo HG. Illumination increases the phosphorylation state of maize leaf phosphoenolpyruvate carboxylase by causing an increase in the activity of a protein kinase. Biochim Biophys Acta. 1991;1093:189–195. doi: 10.1016/0167-4889(91)90122-e. [DOI] [PubMed] [Google Scholar]
- Nelson T, Langdale JA. Developmental genetics of C4 photosynthesis. Annu Rev Plant Physiol Plant Mol Biol. 1992;43:25–47. [Google Scholar]
- Nimmo HG. The regulation of phosphoenolpyruvate carboxylase by reversible phosphorylation. Soc Exp Biol Semin Ser. 1993;53:161–170. [Google Scholar]
- Pierre JN, Pacquit V, Vidal J, Gadal P. Regulatory phosphorylation of phosphoenolpyruvate carboxylase in protoplasts from Sorghum mesophyll cells and the role of pH and Ca2+ as possible components of the light-transduction pathway. Eur J Biochem. 1992;210:531–537. doi: 10.1111/j.1432-1033.1992.tb17451.x. [DOI] [PubMed] [Google Scholar]
- Rejda JM, Johal S, Chollet R. Enzymic and physicochemical characterization of ribulose 1,5-bisphosphate carboxylase/oxygenase from diploid and tetraploid cultivars of perennial ryegrass. Arch Biochem Biophys. 1981;210:617–624. doi: 10.1016/0003-9861(81)90228-9. [DOI] [PubMed] [Google Scholar]
- Roth R, Hall LN, Brutnell TP, Langdale JA. bundle sheath defective2, a mutation that disrupts the coordinated development of bundle sheath and mesophyll cells in the maize leaf. Plant Cell. 1996;8:915–927. doi: 10.1105/tpc.8.5.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal J, Chollet R. Regulatory phosphorylation of C4 PEP carboxylase. Trends Plant Sci. 1997;2:230–237. [Google Scholar]
- Wintermans JFGM, de Mots A. Spectrophotometric characteristics of chlorophylls a and b and their pheophytins in ethanol. Biochim Biophys Acta. 1965;109:448–453. doi: 10.1016/0926-6585(65)90170-6. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Chollet R. Phosphoenolpyruvate carboxylase protein kinase from soybean root nodules: partial purification, characterization, and up/down-regulation by photosynthate supply from the shoots. Arch Biochem Biophys. 1997;343:260–268. doi: 10.1006/abbi.1997.0190. [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li B, Chollet R. In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol. 1995;108:1561–1568. doi: 10.1104/pp.108.4.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]