Abstract
Context:
Data on thyroid function in the oldest old are sparse, and existing studies show conflicting evidence on the relationship between thyroid function and mortality in this age group.
Objective:
We describe longitudinal changes in thyroid function in a cohort of elderly individuals and determine the relationship between thyroid function and mortality.
Design, Setting, and Participants:
Eight hundred forty-three participants in the Cardiovascular Health Study All Stars Study who were not taking thyroid medications and had thyroid function testing in 2005–2006 (mean age 85 yr).
Main Outcome Measure:
Thyroid-stimulating hormone (TSH), free T4 (FT4), total T3, and thyroid peroxidase antibody status were measured in 1992–1993 and 2005–2006. Deaths were ascertained through February 2011.
Results:
There was a statistically significant 13% increase in TSH, 1.7% increase in FT4, and 13% decrease in total T3 over the 13-yr period. Two hundred eighty-seven deaths occurred over a median follow-up of 5.1 yr. There was no association between subclinical hypothyroidism[hazard ratio (HR) 0.97, 95% confidence interval (CI) 0.66–1.43], TSH level (HR per milliunits per liter 0.94, 95% CI 0.88–1.01), or persistent thyroid peroxidase antibody positivity (HR 1.09, 95% CI 0.62–1.92), and death. However, FT4 was positively associated with death (HR per nanograms per deciliter 2.57, 95% CI 1.32–5.02).
Conclusions:
TSH increased over time in these older individuals. This elevation was not associated with increased or decreased mortality, although higher FT4 levels were associated with death. These findings raise concern for treatment of mild elevations of TSH in advanced age. Further studies are needed to determine the potential benefit of treating age-related changes in thyroid function.
Elevations in thyroid-stimulating hormone (TSH) concentrations are found in up to 15% of the U.S. population over age 70 yr (1). Results from the Third National Health and Nutrition Survey (NHANES III) suggest an increase in mean TSH with age; however, these findings are based on cross-sectional data and include only a modest number of participants older than 80 yr old (1). Recently published longitudinal data from the Busselton Health Survey support an increase in TSH concentrations within individuals, with a mean change in TSH of 0.32 mU/liter over a 13-yr period (2). This TSH increase was most marked in those aged 60 yr or older in this study, although there were few participants above age 70 yr. Therefore, the natural history of thyroid function in the oldest old remains undescribed.
Furthermore, the clinical implications of age-associated changes in thyroid function, positive thyroid antibodies, and elevated TSH concentrations found in subclinical hypothyroidism (elevated TSH with normal free T4 concentrations) are not well understood. Studies of cohorts of individuals over the age of 65 have failed to find an association between subclinical hypothyroidism and mortality (3–5). A recent meta-analysis that included these data found no increase in all-cause mortality across the age spectrum (6). Importantly, data on thyroid function in the oldest old are sparse. In the Leiden 85+ Study, which included 599 participants who were aged 85 yr at enrollment, individuals with higher TSH levels had greater longevity than their euthyroid counterparts, suggesting a protective effect of subclinical hypothyroidism in this age group (7). These data have remained unchallenged due to the lack of cohorts with sufficient numbers of participants in this age group.
We sought to describe the longitudinal change in thyroid function and antibodies in older individuals over time, using data from the Cardiovascular Health Study (CHS) All Stars cohort, which is an extended follow-up of participants enrolled in the CHS. We also sought to determine the relationship between thyroid function and thyroid antibodies and mortality in the CHS All Stars.
Materials and Methods
Study participants
The CHS is a multicenter, population-based cohort study of risk factors for cardiovascular disease and stroke in older adults (8). The original cohort of 5201 participants was sampled from Medicare eligibility lists of community-dwelling ambulatory men and women aged 65 yr and older from four U.S. centers (Forsyth County, NC; Sacramento County, CA; Washington County, MD; and Allegheny County, PA) in 1989–1990. In 1992–1993 an additional 687 African-American participants were recruited. The institutional review board at each center approved the study and all participants provided informed consent. Clinic visits were repeated annually through 1999. Thyroid hormone medication use was assessed annually via medication bottle examination during study visits and subsequently by annual surveillance phone calls. In 2005–2006, all surviving participants of the cohort were re-recruited for the CHS All Stars Study (n = 1677) to examine changes in physical and cognitive function in the oldest old (9). The All Stars examination included medical history, medication inventory, and physical examination. Fasting phlebotomy was performed in 1059 participants, of whom 10 did not have sufficient serum for thyroid function testing, 205 were taking thyroid hormone medication, and one was taking antithyroid medication, yielding 843 All Stars participants for analysis.
Thyroid function measurements
Serum TSH, free T4 (FT4), total T3, and antithyroid peroxidase antibody concentrations (TPOAb) were measured in 2010 from banked samples obtained at the 1992–1993 and 2005–2006 study visits. Thyroid function assays were performed at the CHS Central Blood Analysis Laboratory at the University of Vermont (Burlington, VT) using chemiluminescent immunoassays on the Elecsys 2010 analyzer (Roche Diagnostics, Indianapolis, IN). TSH concentrations were measured with a third-generation assay with a functional sensitivity of 0.005 mU/liter and 2.1% intraassay and 3.1% interassay coefficients of variation (CV). Two values of TSH reported as greater than 50 mU/liter were recoded as equal to 50 mU/liter and one suppressed TSH was recoded to 0.005 mU/liter for the purpose of analysis. The FT4 assay had a functional sensitivity of 0.23 ng/dl (2.96 pmol/liter) and 1.7% intraassay and 3.3% interassay CV. A reference range of 0.7–1.7 ng/dl (9–22 pmol/liter) was used. The total T3 assay had a functional sensitivity of 19.5 ng/dl (0.3 nmol/liter), reference range of 84.4–201.3 ng/dl (1.3–3.1 nmol/liter), and 4.2% intrassay and 4.7% interassay CV. The TPOAb assay had a functional sensitivity of 5.0 U/liter, a threshold of 37 U/liter to define positivity (10) and 2.5% intrassay and 9.2% interassay CV.
Mortality
Information about deaths was obtained from medical records, death certificates, autopsy reports, and coroners' reports. Deaths were considered through February 28, 2011.
Statistical analysis
Participant characteristics at the All Stars visits were compared using χ2 or t tests. Paired t tests were used to compare changes in TSH, FT4, total T3, and TPOAb testing between the 1992–1993 and 2005–2006 visits in the subset of All Stars with testing at both visits (n = 657). Additional comparisons were performed using a thyroid disease-free reference population (n = 533) defined by excluding participants with overt hyper- or hypothyroidism or positive TPOAb at either visit (n = 124).
Age-, sex-, and race-adjusted Cox proportional hazards models were used to determine the relationship between TSH, FT4, total T3 levels, and TPOAb status at the 2005–2006 visit and risk of death in the 843 All Stars participants with thyroid function testing who were not taking thyroid medication. Additional models were performed comparing those with subclinical hypothyroidism (TSH 4.50–19.99 mU/liter with normal FT4) and euthyroid individuals (TSH 0.45–4.49 mU/liter). Proportional hazards assumptions were checked, and all models were tested for interaction with age, sex, and race. The relationship between 13-yr change in TSH, FT4, and total T3 levels and mortality was also examined. Kaplan-Meier plots were used to illustrate cumulative mortality comparing subclinical hypothyroid and euthyroid groups, TPOAb-positive and -negative groups, and quartiles of FT4 levels.
All statistical analyses were performed using STATA version 11 (StataCorp LP, College Station, TX).
Results
Participant characteristics
Characteristics of participants in the CHS All Stars cohort at the 2005–2006 visit are described in Table 1. All Stars participants who did not have banked serum available for thyroid function testing were slightly older (P < 0.001) and more likely to be female (P < 0.005) than those in whom thyroid testing was performed. The mean age of the 843 participants who had thyroid testing and were not taking thyroid medication at the All Stars examination was 85.3 ± 3.7 yr. They were more likely than thyroid medication users to be euthyroid and less likely to be female, white, or TPOAb positive (P < 0.05 for each). They also had a significantly lower mean FT4, higher T3, and lower mean TPOAb levels than those receiving thyroid medications (P < 0.01 for each), with no significant difference in TSH levels between thyroid medication users and nonusers (P = 0.6). Subclinical hypothyroidism was the most common endogenous thyroid function abnormality, affecting 10% of the cohort (n = 85). Endogenous subclinical hyperthyroidism was present in 1% (n = 11), and less than 1% had overt hypothyroidism (n = 6).
Table 1.
Participant characteristics of the CHS All Stars (2005–2006)
| With thyroid testing (n = 1049) |
No thyroid testing (n = 628) | ||
|---|---|---|---|
| No thyroid medication use (n = 843) | Thyroid medication use (n = 206) | ||
| Age (yr) | 85.3 (3.7) | 85.3 (3.3) | 86.2 (4.1) |
| Female sex | 513 (60.9) | 159 (77.2) | 444 (70.7) |
| Nonwhite race | 154 (18.3) | 22 (10.7) | 113 (18.0) |
| BMI (kg/m2) | 26.6 (4.5) | 27.3 (5.1) | 27.0 (4.5)a |
| TSH (mU/liter) | 2.99 (5.07) | 3.45 (4.47) | |
| FT4 (ng/dl) | 1.22 (0.01) | 1.41 (0.02) | |
| Total T3 (ng/dl) | 103.9 (19.5) | 90.9 (19.5) | |
| TPOAb (U/liter) | 27.8 (67.8) | 66.5 (133.8) | |
All values given as mean (sd) or n (%). Thyroid medication use defined as thyroid hormone replacement or antithyroid medication. To convert FT4 to nanomoles per liter, multiply by 12.87. To convert total T3 to nanomoles per liter, multiply by 0.0154. BMI, Body mass index.
Available only on n = 74 of those without thyroid testing.
Change in thyroid function between 1992–1993 and 2005–2006 in All Stars participants
When comparing thyroid function tests from 1992–1993 with those in 2005–2006 among individuals not taking thyroid medication at either visit, we found a 13% increase in TSH (0.34 mU/liter, P < 0.01) and a 1.7% increase in FT4 (0.02 ng/dl, P = 0.01) along with a 13% decrease in total T3 (−14.9 ng/dl, P < 0.01) in the 13 yr between assessments (Table 2). Changes were similar in a disease-free reference population, with increases in TSH of 12% (0.28 mU/liter, P < 0.01) and FT4 of 2.5% (0.03 ng/dl) and a decline in total T3 of 13% (−14.9 ng/dl).
Table 2.
Mean change in thyroid function between baseline (1992–1993) and CHS All-Stars (2005–2006) visits
| All participants (n = 657) |
Disease-free reference population (n = 533) |
|||||
|---|---|---|---|---|---|---|
| Baseline | Δ over time | % Δ | Baseline | Δ over time | % Δ | |
| TSH (mU/liter) | 2.6 (3.6) | 0.34 (2.3)a | +13% | 2.3 (1.4) | 0.28 (1.4)a | +12% |
| FT4 (ng/dl) | 1.2 (0.2) | 0.02 (0.18)a | +1.7% | 1.2 (0.2) | 0.03 (0.17)a | +2.5% |
| Total T3 (ng/dl) | 116.9 (19.5) | −14.9 (20.8)a | −13% | 116.9 (19.5) | −14.9 (19.5)a | −13% |
| TPOAb+ (%) | 8.6 | 0.52 | +6.0% | |||
Baseline values are mean (sd) unless otherwise noted. All participants includes participants with values from both baseline and All-Stars examination reporting no thyroid hormone use at either visit. Disease-free reference population additionally excludes participants with overt hypo- or hyperthyroidism or TPOAb positivity at either visit. To convert FT4 to nanomoles per liter, multiply by 12.87. To convert total T3 to nanomoles per liter, multiply by 0.0154.
P < 0.05.
Mean and median TSH were 2.61 and 2.29 mU/liter, respectively, and the 2.5th to 97.5th percentile was 0.54–6.34 mU/liter in the disease-free reference population. The mean and median TSH of the All Stars, as well as the magnitude of increase in TSH, were highest in those aged 85 yr and older (Table 3).
Table 3.
Comparison by age of TSH concentration (mU/liter) at the CHS All-Stars (2005–2006) visit and TSH change from 1992–1993 to 2005–2006 in the disease-free reference population
| Age (yr) (n) | Mean | Median | 2.5th percentile | 97.5th percentile | Δ (sd) 1992/1993–2005/2006 | Annual change |
|---|---|---|---|---|---|---|
| 75–79 (15) | 1.54 | 1.56 | 0.71 | 2.67 | −0.11 (0.8) | 0.004 (0.05) |
| 80–84 (264) | 2.46 | 2.20 | 0.60 | 6.16 | +0.17 (1.2) | 0.022 (0.08) |
| 85–89 (175) | 2.82 | 2.59 | 0.51 | 6.41 | +0.45 (1.7) | 0.048 (.15) |
| ≥90 (79) | 2.83 | 2.53 | 0.20 | 7.96 | +0.35 (1.5) | 0.037 (.12) |
The disease-free reference population excludes participants with overt hypo- or hyperthyroidism, with TPOAb positivity and thyroid medication use at either visit.
There was no significant difference in change in TSH by sex. The mean change in TSH for women and men was 0.24 and 0.33 mU/liter (P = 0.49), respectively. There was also no significant difference in change in FT4 or total T3 by sex (P > 0.05 for both). Most of the disease-free population (80%, n = 426) had a change in TSH between the two visits of less than 1 mU/liter. Twenty percent (n = 107) had an increase in TSH of greater than 1 mU/liter, and only 7% (n = 36) experienced a change in TSH of greater than 2 mU/liter over the 13-yr period.
Change in TPOAb status between 1992–1993 and 2005–2006
Among participants reporting no thyroid medication use at either visit, 9% (n = 56) were TPOAb positive at the All Stars examination. Over the 13-yr period between visits, 541 participants who were TPOAb negative at baseline remained negative, 18 individuals became TPOAb positive over time, 17 who were TPOAb positive at baseline became negative, and 38 who were positive at baseline remained TPOAb positive by the All-Stars visit.
Thyroid function, TPOAb status, and mortality
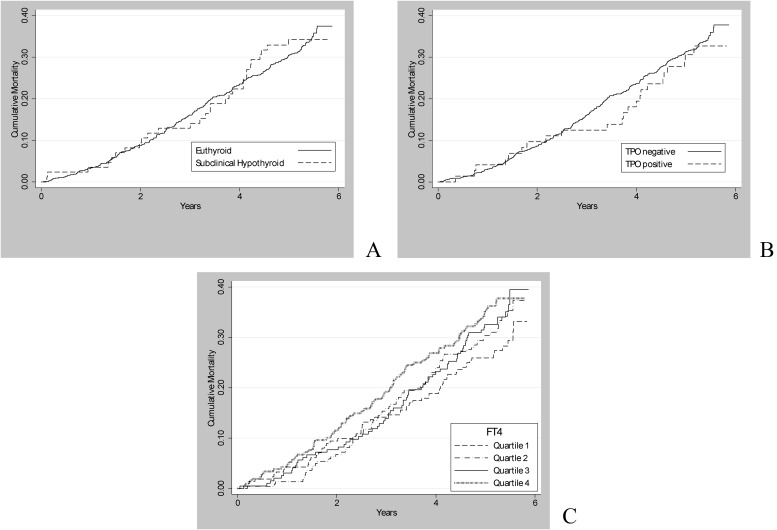
Among participants not taking thyroid medications at the All-Stars visit, there were 287 deaths (including 249 in euthyroid and 29 in subclinically hypothyroid individuals) over a median follow-up time of 5.1 (maximum 5.9) yr. Neither subclinical hypothyroidism nor TPOAb positivity at the All Stars visit was associated with mortality (Fig. 1). In age-, sex-, and race-adjusted models, we found no association between subclinical hypothyroidism and death [hazard ratio (HR) 0.97, 95% confidence interval (CI) 0.66–1.43] or TSH level and death (HR per milliunits per liter 0.94, 95% CI 0.88–1.01) (Table 4). However, there was a positive association between FT4 and mortality (HR per nanograms per deciliter 2.57, 95% CI 1.32–5.02), even after excluding those with FT4 levels outside the normal range (HR per nanograms per deciliter 2.51, 95% CI 1.19–5.33). Estimates were similar for cardiovascular (HR per nanograms per deciliter 2.78, 95% CI 0.94, 8.30) and noncardiovascular (HR per nanograms per deciliter 2.46, 95% CI 1.05–7.73) death. The T3 levels were not associated with mortality (HR per 10 ng/dl 0.96, 95% CI 0.90–1.03). When FT4 and T3 were simultaneously entered into the model, the adjusted HR were unchanged. No statistically significant interactions for age above or below 85 yr, sex, or race were found. There were no associations between 13-yr change in TSH, FT4, or total T3 levels and mortality (data not shown). There was also no association between new or persistent TPOAb positivity and death (for new TPOAb positivity: HR 1.08, 95% CI 0.51–2.30; for persistent TPOAb positivity: HR 1.09, 95% CI 0.62–1.92).
Fig. 1.
Cumulative incidence of death by thyroid status (A), TPOAb status (B), and FT4 level (C).
Table 4.
Thyroid function at the CHS All Stars (2005–2006) visit and risk of death
| HR (95% CI) | P value | |
|---|---|---|
| TSH (per mU/liter) | 0.94 (0.88–1.01) | 0.08 |
| FT4 (per ng/dl) | 2.57 (1.32–5.02) | 0.006 |
| Adjusted for total T3 | 2.47 (1.26–4.38) | 0.008 |
| Total T3 (per 10 ng/dl) | 0.96 (0.90–1.03) | 0.24 |
| Adjusted for FT4 | 0.95 (0.89–1.02) | 0.18 |
| Subclinical hypothyroida | 0.97 (0.66–1.43) | 0.89 |
| TPOAb positivity | 0.87 | |
| Persistent negative | 1.00 (reference) | |
| Resolved | 0.68 (0.25–1.84) | |
| New positivity | 1.08 (0.51–2.30) | |
| Persistent positivity | 1.09 (0.62–1.92) |
Model excludes participants reporting thyroid medication use at the CHS All Stars visit. Models were adjusted for age, sex, and race.
Euthyroid category is referent.
Discussion
We observed an increase in TSH and decrease in T3 levels of similar proportions over a 13-yr period along with a small increase in FT4 levels. These findings in an older population parallel those seen in a recent report in a younger population enrolled in the Busselton Health Survey (mean age 58 yr at follow-up), in which there was an increase in TSH over the same time frame and no change in FT4 (2). We also addressed the question of the effect of thyroid function at this advanced age on mortality. In contrast to the Leiden 85+ Study, we found no association, either protective or harmful, between higher TSH levels and mortality in this cohort of the oldest old. However, similar to this study, higher endogenous FT4 levels were associated with mortality, even after adjusting for T3 levels, suggesting that this finding was not due to individuals with low T3 syndromes.
The distribution of TSH values in the CHS All Stars cohort is similar to reported values in a comparable NHANES population of disease-free participants aged 80 yr and older (924 community dwelling individuals, approximately 50% of whom were women), although there are several differences (1). Among TPOAb-negative All Stars participants not taking thyroid medications and without overt hypo- or hyperthyroidism, the mean and median TSH (2.61 and 2.29 mU/liter) were slightly higher than that reported among disease-free NHANES III participants over 80 yr old (2.44 and 1.90 mU/liter). However, the 97.5th percentile for TSH was lower among the All-Stars than in similarly aged participants of NHANES III (6.34 vs. 7.49 mU/liter), suggesting less right-sided skew in the distribution of TSH in the All Stars. Consistent with this, fewer All-Stars participants had abnormally elevated TSH values (11 vs. 15%, with TSH values above the reference range of 4.5 mU/liter), although a similar proportion (12%) had TSH levels above the reference range in the Leiden 85+ Study (n = 588 participants, 66% women, aged 85 yr at enrollment) (7). The 97.5th percentile of TSH in the All Stars cohort (6.34 mU/liter for the disease-free reference population) was higher than the generally accepted upper limit of the reference range (4.5 mU/liter). When the usual adult reference range for TSH is applied to the oldest old, many more individuals may be classified as having abnormally high TSH. We have previously shown that those aged 80 yr and older are more than twice as likely to initiate thyroid hormone medication than those in younger age groups (11), despite an absence of data to support treatment of subclinical hypothyroidism in this age group. It has been suggested that mild elevations in TSH may reflect the natural history of thyroid function with aging, with recommendations to increase the upper limit of the reference range for TSH in older persons (1, 12). However, the use of the 95% confidence interval ties treatment to the population distribution, not to the clinical consequences associated with a specific TSH level.
The change in TSH observed over a 13-yr period in the CHS All-Stars (mean age 85 yr at follow-up) was similar to findings from the Busselton Health Survey (mean age 58 yr at follow-up) (2). Among a disease-free group of CHS All-Stars, the mean increase in TSH over a 13-yr period was 0.28 mU/liter. In the Busselton Health Survey, the mean increase in TSH over 13 yr of follow-up was 0.32 mU/liter. In the present study, the increase in TSH was seen in conjunction with a slight increase in FT4 levels and a decrease in total T3. Younger participants in the Busselton Study did not have a significant change in FT4 over time, although participants aged 50 yr and older did have a slight increase in FT4 levels (mean 0.5 pmol/liter) during follow-up. Our findings do not support an increase in thyroid failure with age because FT4 levels slightly increased, rather than decreased, in parallel with the increase in TSH. Instead, our data support either an age-associated alteration in TSH set point due to diminished sensitivity of the thyrotropes to negative feedback, a decrease in TSH bioactivity, or a decreased responsiveness of the thyroid gland to TSH, as has been suggested previously (2, 12).
Higher TSH levels have been associated with longevity in several cross-sectional studies, with higher TSH in centenarians vs. younger controls, and in nonagenarians with reported familial longevity (13, 14). However, the hypothesis that decreased thyroid function or higher TSH levels are associated with longevity has only been demonstrated prospectively in the Leiden 85+ study, in which higher TSH levels were associated with decreased mortality (7). In contrast to what was reported in the Leiden 85+ study, we did not find a protective effect of TSH in this cohort. Previous studies of older adults have similarly failed to find a protective effect of elevated TSH levels. In the Health, Aging, and Body Composition Study, the Cardiovascular Health Study, and the Osteoporotic Fractures in Men Study, subclinical hypothyroidism was neither positively nor negatively associated with mortality, although the mean age of participants in these studies (73–75 yr old) was younger than that of the CHS All-Stars or Leiden 85+ studies (3–5). Furthermore, in a recent meta-analysis by Rodondi et al. (6), subgroup analysis revealed no evidence of mortality differences among pooled participants over 80 yr old.
Similar to the Leiden study, we observed an association between higher FT4 levels and death. This association was present for both cardiovascular and noncardiovascular mortality. A study by Gammage et al. (15) also found an isolated association between higher FT4 levels (but not TSH) and increased prevalence of atrial fibrillation among participants with normal TSH values. Why higher FT4, but not lower TSH, predicts mortality or other adverse outcomes in some studies remains unexplained because TSH and FT4 should be concordant in individuals with intact pituitary function. One possible explanation for these discordant results is an alteration of pituitary sensitivity or the pituitary TSH set point in the elderly, in whom higher FT4 levels do not cause the same TSH suppression as in younger individuals. Therefore, FT4 may better represent clinical thyroid status in this population than is generally considered. It is also possible that higher FT4 levels are a marker of decreased 5′deiodination due to nonthyroidal illness and would be associated with lower T3 levels. In our study, however, the addition of T3 to our multivariable models did not attenuate our findings, suggesting the relationship with FT4 is independent of low-T3 syndromes.
We also observed a significant decrease in T3 levels over time. Lower T3 levels have previously been documented in healthy centenarians and may reflect a decrease in 5′deiodinase activity with aging (16). Animal models support this hypothesis; one study found a decrease in peripheral T3 concentrations with an associated decrease in hepatic and thyroidal type 1 5′deiodinase activity in old male rats. Interestingly, intrapituitary T3 and 5′deiodinase activity were higher than in young animals, suggesting that the changes in deiodinase activity with aging may be tissue specific (17).
Although several cross-sectional studies have reported a lower prevalence of thyroid autoantibodies among healthy centenarians compared with younger old individuals (70–80 yr olds) or the disabled elderly, there are limited prospective data on the association between TPOAb status and death (16, 18, 19). Our study found no association between new or persistent TPOAb positivity and death, which argues against a harmful effect of antithyroid antibodies. Our findings are consistent with those of previous studies examining the association between TPOAb and mortality. In both the Nagasaki Adult Health Study and the Whickham Survey, the association between subclinical hypothyroidism and ischemic heart disease was independent of the presence of antithyroid antibodies, and the Busselton Health Study found no association between baseline TPOAb positivity and cardiovascular death (20–22).
There are several strengths and weaknesses of our study. The CHS All Stars represent a large cohort of the oldest old, and one particular strength is the availability of complete thyroid function tests and TPOAb levels, even in euthyroid individuals, assayed using the same assay at a single assay run at both time points. In addition, follow-up for death is complete. Our study population also included a large proportion of female (61%) and black (18%) participants. One limitation of our study is its generalizability to all older people because participants who returned for the All-Stars visit were healthier than those who died, were lost to follow-up, or refused re-recruitment (9). We also lacked information about underlying thyroid conditions such as goiters or nodular disease, although we did have yearly assessment of thyroid medication use. We had excellent power to examine thyroid function tests continuously. However, although our point estimate suggests no association, we acknowledge limited power to exclude an association between subclinical hypothyroidism and mortality.
In conclusion, we present the first data demonstrating longitudinal changes in thyroid function in a reference cohort of the oldest old. Over a 13-yr period, these older individuals experienced an increase in TSH, a slight increase in FT4, and decrease in total T3. The percentage of individuals with TPOAb positivity remained stable over time. Although validating the age-related increase in TSH levels found cross-sectionally in NHANES, our FT4 findings do not suggest that this increase in TSH is due to an increase in thyroid disease. Although we found neither a positive nor negative impact of TSH on mortality, higher FT4 levels were associated with death in this cohort. Our findings suggest that reflexively treating mild elevations in TSH in those of advanced age is unnecessary. Further studies are needed to determine threshold levels of thyroid function that would benefit from intervention.
Acknowledgments
This work was supported by Contracts HHSN268201200036C, N01-HC-85239, and N01-HC-85079 through Contracts N01-HC-85086, N01-HC-35129, N01 HC-15103, N01 HC-55222, N01-HC-75150, and N01-HC-45133 and Grant HL080295 from the National Heart, Lung, and Blood Institute, with an additional contribution from the National Institute of Neurological Disorders and Stroke. Additional support was provided through Grants AG-023629, AG-15928, AG-20098, and AG-027058 from the National Institute on Aging. A full list of principal Cardiovascular Health Study investigators and institutions can be found at http://www.chs-nhlbi.org/pi.htm.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- CHS
- Cardiovascular Health Study
- CI
- confidence interval
- CV
- coefficient of variation
- FT4
- free T4
- HR
- hazard ratio
- NHANES III
- Third National Health and Nutrition Survey
- TPOAb
- thyroid peroxidase antibody
- TSH
- thyroid-stimulating hormone.
References
- 1. Surks MI, Hollowell JG. 2007. Age-specific distribution of serum thyrotropin and antithyroid antibodies in the US population: implications for the prevalence of subclinical hypothyroidism. J Clin Endocrinol Metab 92:4575–4582 [DOI] [PubMed] [Google Scholar]
- 2. Bremner AP, Feddema P, Leedman PJ, Brown SJ, Beilby JP, Lim EM, Wilson SG, O'Leary PC, Walsh JP. 2012. Age-related changes in thyroid function: a longitudinal study of a community-based cohort. J Clin Endocrinol Metab 97:1554–1562 [DOI] [PubMed] [Google Scholar]
- 3. Cappola AR, Fried LP, Arnold AM, Danese MD, Kuller LH, Burke GL, Tracy RP, Ladenson PW. 2006. Thyroid status, cardiovascular risk, and mortality in older adults. JAMA 295:1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Rodondi N, Newman AB, Vittinghoff E, de Rekeneire N, Satterfield S, Harris TB, Bauer DC. 2005. Subclinical hypothyroidism and the risk of heart failure, other cardiovascular events, and death. Arch Intern Med 165:2460–2466 [DOI] [PubMed] [Google Scholar]
- 5. Waring AC, Harrison S, Samuels MH, Ensrud KE, LeBlanc ES, Hoffman AR, Orwoll E, Fink HA, Barrett-Connor E, Bauer DC. 2012. Thyroid function and mortality in older men: a prospective study. J Clin Endocrinol Metab 97:862–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Rodondi N, den Elzen WP, Bauer DC, Cappola AR, Razvi S, Walsh JP, Asvold BO, Iervasi G, Imaizumi M, Collet TH, Bremner A, Maisonneuve P, Sgarbi JA, Khaw KT, Vanderpump MP, Newman AB, Cornuz J, Franklyn JA, Westendorp RG, Vittinghoff E, Gussekloo J. 2010. Subclinical hypothyroidism and the risk of coronary heart disease and mortality. JAMA 304:1365–1374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gussekloo J, van Exel E, de Craen AJ, Meinders AE, Frölich M, Westendorp RG. 2004. Thyroid status, disability and cognitive function, and survival in old age. JAMA 292:2591–2599 [DOI] [PubMed] [Google Scholar]
- 8. Fried LP, Borhani NO, Enright P, Furberg CD, Gardin JM, Kronmal RA, Kuller LH, Manolio TA, Mittelmark MB, Newman A, et al. 1991. The Cardiovascular Health Study: design and rationale. Ann Epidemiol 1:263–276 [DOI] [PubMed] [Google Scholar]
- 9. Strotmeyer ES, Arnold AM, Boudreau RM, Ives DG, Cushman M, Robbins JA, Harris TB, Newman AB. 2010. Long-term retention of older adults in the Cardiovascular Health Study: implications for studies of the oldest old. J Am Geriatr Soc 58:696–701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kratzsch J, Fiedler GM, Leichtle A, Brügel M, Buchbinder S, Otto L, Sabri O, Matthes G, Thiery J. 2005. New reference intervals for thyrotropin and thyroid hormones based on National Academy of Clinical Biochemistry criteria and regular ultrasonography of the thyroid. Clin Chem 51:1480–1486 [DOI] [PubMed] [Google Scholar]
- 11. Somwaru LL, Arnold AM, Cappola AR. 2011. Predictors of thyroid hormone initiation in older adults: results from the Cardiovascular Health Study. J Gerontol A Biol Sci Med Sci 66:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Surks MI, Boucai L. 2010. Age- and race-based serum thyrotropin reference limits. J Clin Endocrinol Metab 95:496–502 [DOI] [PubMed] [Google Scholar]
- 13. Atzmon G, Barzilai N, Hollowell JG, Surks MI, Gabriely I. 2009. Extreme longevity is associated with increased serum thyrotropin. J Clin Endocrinol Metab 94:1251–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rozing MP, Houwing-Duistermaat JJ, Slagboom PE, Beekman M, Frölich M, de Craen AJ, Westendorp RG, van Heemst D. 2010. Familial longevity is associated with decreased thyroid function. J Clin Endocrinol Metab 95:4979–4984 [DOI] [PubMed] [Google Scholar]
- 15. Gammage MD, Parle JV, Holder RL, Roberts LM, Hobbs FD, Wilson S, Sheppard MC, Franklyn JA. 2007. Association between serum free thyroxine concentration and atrial fibrillation. Arch Intern Med 167:928–934 [DOI] [PubMed] [Google Scholar]
- 16. Mariotti S, Barbesino G, Caturegli P, Bartalena L, Sansoni P, Fagnoni F, Monti D, Fagiolo U, Franceschi C, Pinchera A. 1993. Complex alteration of thyroid function in healthy centenarians. J Clin Endocrinol Metab 77:1130–1134 [DOI] [PubMed] [Google Scholar]
- 17. Donda A, Lemarchand-Béraud T. 1989. Aging alters the activity of 5′-deiodinase in the adenohypophysis, thyroid gland, and liver of the male rat. Endocrinology 124:1305–1309 [DOI] [PubMed] [Google Scholar]
- 18. Magri F, Muzzoni B, Cravello L, Fioravanti M, Busconi L, Camozzi D, Vignati G, Ferrari E. 2002. Thyroid function in physiological aging and in centenarians: possible relationships with some nutritional markers. Metabolism 51:105–109 [DOI] [PubMed] [Google Scholar]
- 19. Mariotti S, Barbesino G, Chiovato L, Marinò M, Pinchera A, Zuliani G, Mezzetti A, Fellin R. 1999. Circulating thyroid autoantibodies in a sample of Italian octo-nonagenarians: relationship to age, sex, disability, and lipid profile. Aging (Milano) 11:362–366 [DOI] [PubMed] [Google Scholar]
- 20. Walsh JP, Bremner AP, Bulsara MK, O'Leary P, Leedman PJ, Feddema P, Michelangeli V. 2005. Subclinical thyroid dysfunction as a risk factor for cardiovascular disease. Arch Intern Med 165:2467–2472 [DOI] [PubMed] [Google Scholar]
- 21. Imaizumi M, Akahoshi M, Ichimaru S, Nakashima E, Hida A, Soda M, Usa T, Ashizawa K, Yokoyama N, Maeda R, Nagataki S, Eguchi K. 2004. Risk for ischemic heart disease and all-cause mortality in subclinical hypothyroidism. J Clin Endocrinol Metab 89:3365–3370 [DOI] [PubMed] [Google Scholar]
- 22. Razvi S, Weaver JU, Vanderpump MP, Pearce SH. 2010. The incidence of ischemic heart disease and mortality in people with subclinical hypothyroidism: reanalysis of the Whickham Survey cohort. J Clin Endocrinol Metab 95:1734–1740 [DOI] [PubMed] [Google Scholar]