Abstract
Context:
During the pubertal transition, LH secretion initially increases only during sleep; however, its relationship to sleep stage is unknown.
Objectives:
Our objective was to determine whether the initiation of LH pulses is related to a specific sleep stage in pubertal children.
Design and Setting:
Frequent blood sampling and polysomnographic studies were performed in a Clinical Research Center.
Subjects:
Fourteen studies were performed in nine healthy pubertal children, ages 9.9–15.6 yr.
Interventions:
Subjects underwent one to two overnight studies with polysomnography and blood sampling for LH at 10-min intervals.
Results:
Alignment of polysomnographic records and LH pulses demonstrated that LH pulses (n = 58) occurred most frequently during slow-wave sleep (SWS) (1.1 pulse/h, n = 30) compared with all other sleep stages or periods of wake after sleep onset (P < 0.001). There was also a significant increase in the amount of SWS in the 15 min preceding and the 5 min following each pulse compared with the amount of SWS seen across the study night (P < 0.01).
Conclusions:
During puberty, the majority of LH pulses that occur after sleep onset are preceded by SWS, suggesting that SWS is intimately involved in the complex control of pubertal onset. These studies raise concerns about the potential hormonal repercussions of the increasing prevalence of sleep disturbances in adolescents.
The onset of puberty is heralded by a dramatic rise in GnRH and consequent LH secretion. In the 1970s, Boyar et al. (1) made the seminal observation in a group of seven pubertal boys that augmented LH secretion initially occurs during nighttime sleep. The dependence of LH secretion on sleep, rather than on time of day, was later proven by sleep-reversal studies in pubertal boys (2). Work by a number of investigators has since confirmed the relationship between gonadotropin secretion and sleep during puberty (3–10). Although the association between increased LH secretion and sleep has been known for nearly 40 yr, the physiological relationship between the sleep and reproductive systems in normal pubertal children remains unknown.
Both sleep and circadian signals are powerful and specific modulators of hormone secretion. Although hormones such as prolactin and TSH respond to the overall sleep-wake state, others such as GH and renin are sensitive to specific sleep stages or to transitions between nonrapid eye movement (NREM) and rapid eye movement (REM) sleep (11).
For example, a large peak in GH secretion is seen in association with the first period of slow-wave sleep (SWS) in both children and adults (12, 13). The importance of this sleep stage in the control of GH secretion has been confirmed by interventional studies that delayed the onset of sleep or pharmacologically increased the amount of SWS (reviewed in Ref. 14). The nocturnal pattern of renin secretion is also influenced by the sleep cycle with levels rising with NREM sleep onset and declining with REM sleep onset (15). Finally, sleep-related inhibition of LH secretion in reproductive-aged women is specifically related to deep sleep with LH pulses occurring during brief episodes of wakefulness (16). Thus, although a precedent exists for the association of hormone secretion with specific sleep stages, this potential interface has not been investigated for reproductive hormones in pubertal children.
The current studies were designed to explore the effect of sleep on GnRH secretion during puberty by examining LH pulses in relation to specific sleep stages, assessed by polysomnography (PSG). Through simultaneous frequent blood sampling and PSG, we demonstrated a novel temporal association between SWS and LH pulses suggesting that SWS in particular, rather than the overall sleep state, is responsible for augmented LH secretion during sleep in puberty.
Subjects and Methods
Subjects
Subjects were pubertal children and included five boys, ages 11.8–15.6 yr, with a testicular volume of 4–15 ml, and four premenarcheal girls, ages 9.9–12.8 yr, with Tanner stage II–III breasts (17, 18). All physical examinations were performed by the same investigator, and pubertal onset was confirmed by morning LH levels. Subjects were originally recruited for a clinical research study designed to determine the effects of obstructive sleep apnea (OSA) on nocturnal reproductive hormone secretion. Subjects were recruited based on PSG-confirmed OSA (19) treated with continuous positive airway pressure (CPAP) or symptoms strongly suggestive of OSA. However, at the time of the study, a subset of subjects (n = 9) no longer met criteria for OSA, defined as an apnea-hypopnea index (AHI) greater than 5 (20), or had borderline OSA (AHI = 5) when studied off CPAP. These nine subjects are the focus of this report. Subjects were euthyroid, were not on any medication known to interfere with sleep, growth, or puberty, and did not have a history of precocious puberty or premature adrenarche. Subjects took iron supplements (approximately 30 mg ferrous gluconate/kg·d) for the duration of the study and for 1 month thereafter to prevent anemia.
The study was approved by the Partners Human Research Committee. Signed informed assent and consent was obtained from each subject and his/her parent, respectively.
Experimental protocol
Subjects were admitted to the Clinical Research Center of the Massachusetts General Hospital for one or two overnight studies consisting of frequent blood sampling and PSG. Studies were spaced 1–2 months apart in compliance with federal regulations governing blood sampling in children. Six subjects were on home CPAP therapy for treatment of OSA at the time of the study. Because the resolution of OSA in these subjects was unknown at the time of their enrollment, subjects on CPAP were studied twice, in random order, either on CPAP, using their prescribed settings, or off CPAP. All other subjects participated in a single overnight study visit.
PSG was performed according to standard methodology (19) using an electroencephalogram (frontal, central, and occipital leads), electrooculogram, electromyogram, electrocardiogram, and pulse oximetry recordings (GRASS Technologies PSG systems, TWin version 4.3 software). An iv catheter was inserted upon admission, and a long line was connected so that blood sampling could be performed outside of the sleeping room. All subjects ate dinner before lights out. Caffeine was prohibited. Lights were turned off between 2030 and 2230 h, based on subject and parent reports of habitual bedtime. Blood samples (3–5 ml) were drawn at 10-min intervals for 8 h 20 min using a blood-sparing technique, as previously described (21), beginning 20 min before lights out. Subjects were monitored remotely during the entire study by video camera. Sampling was interrupted in one subject (subject 2) for approximately 1 h due to temporary loss of iv access. Hemoglobin levels were assessed on admission and at the completion of sampling and remained within the normal range for age (mean decrease 1 g/dl, range 0–1.3 g/dl).
All blood samples were analyzed for LH using a chemiluminescent microparticle immunoassay (Architect; Abbott Laboratory Diagnostics, Chicago, IL), which has a minimum detectable concentration of 0.07 IU/liter. LH values are expressed in international units per liter, as equivalents of the Pituitary Second International Reference Standard 80/552.
Data analysis
The sleep recordings were visually scored by a registered PSG technician according to American Academy of Sleep Medicine criteria (19) in 30-sec epochs as stages of NREM [N1, N2, and N3 (N3 is referred to hereafter as SWS)], REM, or wake. All arousals, apneas, hypopneas, and oxygen desaturations (<90%) were recorded. The AHI was defined as the average number of obstructive apneas and hypopneas per hour of sleep and the arousal index (AI) as the number of arousals per hour of sleep. Sleep latency (minutes) was defined as the period of time between lights out and the first epoch of sleep. Sleep efficiency was defined as the percentage of the time spent asleep after lights out. Paired t tests were used to compare the percentage of time each subject spent in each sleep stage in studies on CPAP compared with off CPAP.
Pulsatile LH secretion was analyzed using a validated modification of the Santen and Bardin method of pulse detection (22, 23). Only LH pulses occurring after sleep onset (defined as the first epoch of any sleep stage) were considered. To compare LH pulsatility across different sleep stages, sleep records were aligned to the onset of the LH pulse using the preceding nadir.
To examine the association between LH pulse initiation and specific sleep stages, the data were first expressed as the number of pulses observed during each sleep stage, normalized for the amount of time spent in each sleep stage during the 8 h of blood sampling following lights out. The average frequency of LH pulses in each sleep stage and in the presence or absence of CPAP was compared using a linear mixed model to calculate least squares means including fixed stage and treatment (CPAP) effects and a random subject effect. Correction for multiple comparisons was performed using Dunnett's or Bonferroni's method, where applicable. To explore further the observation that LH pulses were seen most frequently during SWS, a similar model was used to compare the percentage of time spent in SWS in 5-min periods before and after each pulse nadir (−15 to −10, −10 to −5, −5–0, 0–5, and 5–10 min) as previously described (16) with the percentage of time spent in SWS across the study night for each subject. LH pulse amplitude was also compared between stages N2 and N3 using a linear mixed model.
Data are expressed as mean ± sd unless otherwise indicated, and P < 0.05 is considered significant.
Results
Baseline characteristics
Before enrollment, six subjects had been diagnosed with OSA via PSG (secondary to adenotonsillar and/or nasal turbinate hypertrophy, micrognathia, deviated septum, small upper airways, or obesity) and five of six had been using CPAP for the past 3 months to 1.5 yr. The remaining three subjects were suspected of having OSA based on a history of snoring, nocturnal enuresis, daytime fatigue, and/or inattention. All subjects were pubertal according to physical exam and confirmed by a detectable morning LH level (Table 1).
Table 1.
Subject characteristics
| Subject | Age (yr) | Sex | Race | BMI (kg/m2) | BMI percentilea | Pubertal stageb | Morning LH (IU/liter) |
|---|---|---|---|---|---|---|---|
| 1 | 12.3 | F | C | 26.0 | 95 | BII | 4.7 |
| 2 | 14.4 | M | AA | 40.0 | >97 | 12 ml | 1.5 |
| 3 | 11.8 | M | C | 15.0 | 5 | 4 ml | 0.5 |
| 4 | 13.4 | M | C | 18.2 | 44 | 5 ml | 1.6 |
| 5 | 11.8 | M | C | 17.3 | 44 | 5 ml | 2.2 |
| 6 | 12.8 | F | C | 25.4 | 94 | BII | 0.9 |
| 7 | 11.8 | F | Hisp | 35.3 | >97 | BII | 4.3 |
| 8 | 15.6 | M | C | 20.1 | 47 | 15 ml | 3.4 |
| 9 | 9.9 | F | C | 22.1 | 93 | BIII | 1.0 |
AA, African-American; BMI, body mass index; C, Caucasian; F, female; Hisp, Hispanic; M, male.
Age- and sex-adjusted body mass index percentile over 85 is classified as overweight and over 95 is classified as obese in children (56).
Tanner breast stage (B) or testicular volume. All girls were premenarcheal. Although 12- to 15-ml testes fall within the normal range of testicular size in adult males, review of growth charts for subjects 2 and 8 indicated a pubertal growth velocity, confirming that they had not yet completed puberty.
Sleep parameters
Analysis of the sleep recordings indicated that eight subjects did not meet criteria for OSA when studied off CPAP, whereas one subject met borderline criteria [AHI of 5.4 (normal < 5)]. As a group, the nine patients had an average sleep latency of 51.3 ± 39.0 min, somewhat longer than what has been reported for adolescents referred for PSG due to suspicion of OSA (24), whereas sleep efficiency (83.8 ± 10.3%) was similar to results from previous studies in this age group (25). All subjects demonstrated normal sleep macroarchitecture for age with 26.5 ± 5.9% of total sleep time spent in SWS. There were no intra-subject differences in the sleep parameters in the six subjects studied on CPAP and off CPAP (Table 2).
Table 2.
Arousals, respiratory events, and sleep architecture of subjects studied on or off CPAP
| CPAP | Arousals and respiratory events |
Sleep architecture |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AI (no./h TST), NR = 9 ± 3 |
AHI (no./hr TST), NR = 0.1 ± 0.1 |
SpO2 nadir (%), NR = 93 ± 4 |
N1 (% TST), NR = 5 ± 3 |
N2 (% TST), NR = 42 ± 8 |
N3 (% TST), NR = 26 ± 8 |
REM (% TST), NR = 20 ± 5 |
||||||||
| On | Off | On | Off | On | Off | On | Off | On | Off | On | Off | On | Off | |
| Subject | ||||||||||||||
| 1 | 20.7 | 42.9 | 4.1 | 5.4 | 86 | 90 | 9.8 | 13.7 | 42.6 | 52.6 | 27.6 | 19.5 | 20.1 | 14.3 |
| 2 | 13.6 | 15.3 | 0.2 | 2.2 | 94 | 90 | 9.7 | 6.0 | 55.0 | 45.1 | 24.4 | 18.2 | 24.4 | 18.0 |
| 3 | 7.2 | 4.7 | 0 | 0 | 93 | 94 | 2.8 | 0.8 | 44.4 | 40.1 | 29.4 | 36.5 | 23.4 | 22.6 |
| 4 | 8.0 | 14.1 | 0 | 1.2 | 95 | 92 | 16.1 | 4.7 | 47.8 | 38.5 | 18.2 | 34.9 | 18.0 | 10.0 |
| 5 | 8.1 | 7.4 | 0 | 0.1 | 95 | 97 | 3.0 | 7.3 | 42.3 | 49.6 | 29.5 | 29.0 | 25.2 | 14.1 |
| 6 | 5.4 | 0 | 95 | 2.6 | 46.3 | 32.7 | 18.4 | |||||||
| 7 | 10.0 | 0.4 | 88 | 10.1 | 51.7 | 28.4 | 9.7 | |||||||
| 8 | 8.7 | 0.1 | 93 | 6.5 | 60.6 | 9.4a | 23.6 | |||||||
| 9 | 11.1 | 0.1 | 94 | 6.2 | 37.7 | 24.3 | 19.2 | |||||||
| P valueb | 0.1 | 0.1 | 0.8 | 0.9 | 0.8 | 0.9 | 0.7 | |||||||
Normal ranges (NR) (mean ± sd) for healthy children (43) are shown. SpO2, Oxygen saturation; TST, total sleep time.
Note that subject 8 had less N3 than normal because PSG began after he had been asleep for 2 h, likely missing the first episode of N3 of the night.
P value for paired t test.
LH pulse dynamics and their relation to sleep stage
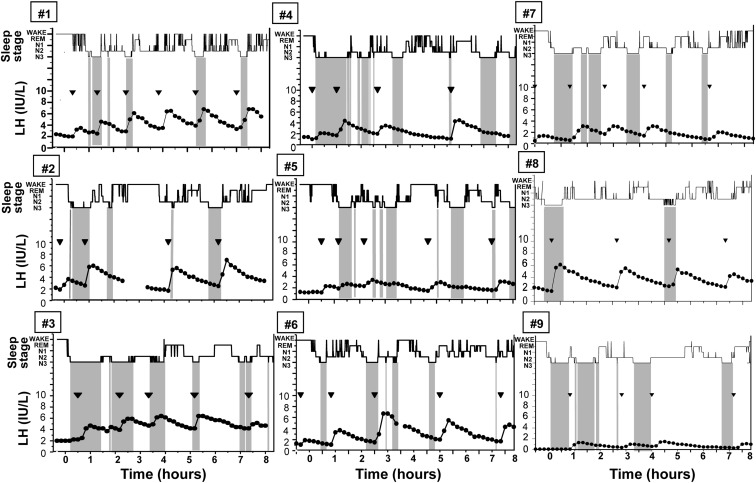
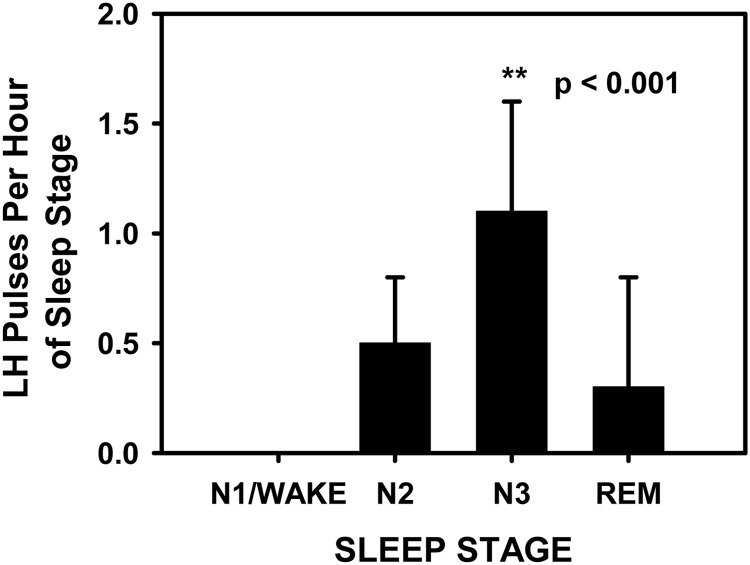
Subjects demonstrated an LH pulse frequency of four to six pulses during the 8 h of frequent sampling (Fig. 1). The subject in whom blood sampling was interrupted (subject 2) was awake for the majority of this time, and review of hormonal data indicated that it was unlikely that an LH pulse was missed because there was no change in the slope of LH as it descended from a preceding peak before loss of the iv. Indexing of the onset of each LH pulse (n = 58) to the sleep stage records revealed that 52% (30 of 58) of LH pulses initiated during SWS, 36% (20 of 58) during N2, 10% (six of 58) during REM, and 2% (one of 58) during N1. No pulses initiated during periods of wake after sleep onset. Controlling for the time spent in each sleep stage during the night, LH pulse frequency during SWS was significantly greater than during all other sleep stages and wake after sleep onset (P < 0.001; Fig. 2). The distribution of LH pulses among the different sleep stages was similar in subjects studied on CPAP compared with off CPAP (P = 0.4). There was no difference in the amplitude of LH pulses occurring during N2 and SWS (P = 0.3). There were insufficient pulses during N1, REM, and periods of wake after sleep onset to compare LH pulse amplitude across these stages.
Fig. 1.
Representation of sleep stages [wake, REM, N1, N2, and N3 (SWS), in descending order] and LH values in the nine subjects (studied off CPAP) demonstrates the close association between SWS (highlighted by the vertical gray bars) and LH pulse onset (marked by inverted triangles). Note that alignment of the raw PSG data and LH levels, rather than visual inspection, was used in the statistical analyses to determine the sleep stage associated with the onset of each LH pulse.
Fig. 2.
LH pulse frequency (mean ± sd) was significantly greater (P < 0.001) during SWS (N3) than during any other sleep stage or during periods of wake after sleep onset.
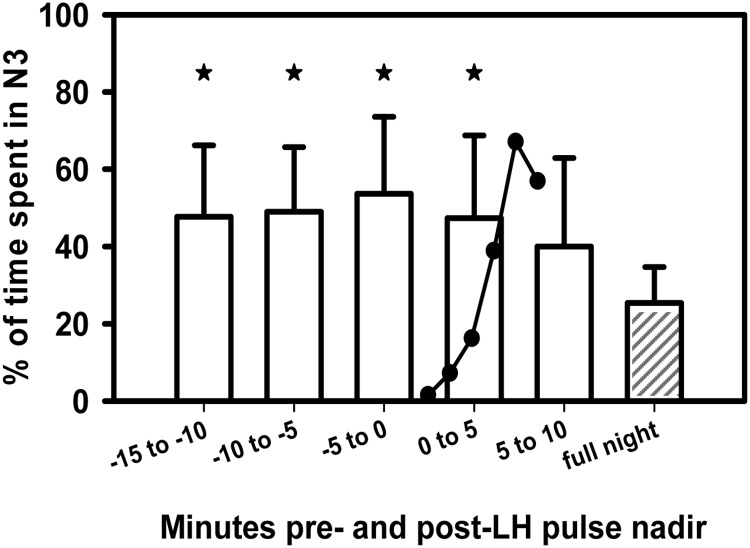
Having demonstrated an association between SWS and LH pulse onset, we examined the distribution of SWS in the 10–15 min surrounding an LH pulse compared with the rest of the study night to explore the temporal link between SWS and LH pulse onset. There was a significant increase in the percentage of time spent in SWS in the 0–15 min preceding and the 0–5 min interval following each pulse compared with the percentage of time spent in SWS across the study night (P < 0.01; Fig. 3). Ninety-three percent of the time, the presence of SWS in the 0–5 min interval after a pulse represented the continuation of a period of SWS beginning before pulse onset. SWS predominance was no longer significant 5–10 min after a pulse. The relationship between SWS and LH pulse onset was unaffected by the use of CPAP (P = 0.2). The association of SWS with LH pulse onset suggests that SWS may play a role in the initiation of LH pulses during puberty.
Fig. 3.
There was an increased amount of SWS in the 15 min preceding and the 5 min following each pulse compared with the amount of SWS seen across the study night (shaded bar). *, P < 0.01. The cartoon depicts the typical shape of an LH pulse at its onset. Data represent the average (+1 sd) percent time spent in SWS among all subjects. Comparisons were made using a linear mixed model adjusting for repeated measures in each subject.
Discussion
Pubertal onset is heralded by a dramatic increase in pulsatile LH secretion that is initially restricted to sleep (1). Studies of pubertal children of both genders and across a range of ages, weights, and races have documented an association between sleep and increased LH secretion (3–10). These observations indicate that the interface between sleep and the reproductive axis is a consistent and important part of normal pubertal maturation. These observations further suggest that sleep augments GnRH/LH secretion during the developmentally programmed reactivation of the reproductive axis. In the current study, we demonstrated that SWS, rather than sleep in general, is associated with the nocturnal increase in LH secretion during puberty.
Analyzing the relationship between LH pulse onset and sleep stage is challenging because these two variables have very different signal characteristics. Blood sampling every 10 min permits assessment of the acute secretion of LH, its rise, and its pharmacokinetic decline due to clearance from serum. The time of onset of each secretory episode (with a resolution of 10 min) is of primary interest with respect to its potential link with sleep stages. Sleep stages were analyzed as categorical variables across three NREM stages, REM, and wake within sleep, with a resolution of 30 sec. Our initial approach used a straightforward analysis of the number of LH pulses per hour as a function of sleep stage. The striking increase in the frequency of LH pulses associated with SWS shows a highly significant departure from the null hypothesis of no interaction that would have been manifest as a uniform frequency distribution across all sleep stages.
In the current study, 52% of all pulses that occurred after sleep onset were associated with SWS despite the fact that SWS was limited to only 26% of the night. In the one other study that used sleep recording in conjunction with blood sampling in pubertal subjects, visual inspection of the data, without statistical analysis, suggested that LH pulses were associated with NREM sleep (1). The current study expanded upon these observations by incorporating a greater number of sleep studies and by taking advantage of the increased precision afforded by digital sleep recording, thereby demonstrating the importance of SWS in the control of LH secretion during puberty.
The high probability of SWS occurring in the 15 min preceding the onset of an LH pulse suggests that SWS is involved in GnRH pulse initiation during puberty. The temporal relationship of SWS to LH pulse onset may reflect a direct influence of hypothalamic NREM-active neurons on GnRH neurons or an indirect effect via kisspeptin neurons that have recently been implicated in the control of GnRH secretion (26). Support for the latter hypothesis derives from frequent sampling studies in nonhuman primates demonstrating that, like GnRH, kisspeptin secretion is augmented at night during puberty (27, 28).
An alternative hypothesis is that both NREM-active neurons and GnRH neurons are stimulated by a common upstream signal. Both GH secretion and SWS are stimulated by GHRH (29, 30). This provides a potential mechanism for the close association between GH secretion and SWS demonstrated in observational and interventional studies in children (13) and adults (31–33). In rodents, a subset of GHRH neurons project to (34) and activate (35) cells of the ventrolateral preoptic nucleus, an area rich in γ-aminobutyric acid (GABA)- and galaninergic neurons that is important in generating NREM sleep (36). The human homolog of the ventrolateral preoptic nucleus, the intermediate nucleus, also contains a population of GABAergic (37) and galaninergic cells (38, 39) that are active during NREM sleep (40), although there have been no human studies examining the connection between GHRH neurons and the intermediate nucleus. We hypothesize that a parallel system exists in the reproductive axis. A hypothalamic signal that stimulates both SWS and GnRH secretion may first develop at the time of puberty or may become apparent only during reactivation of GnRH neurons in the early stages of puberty.
The concept of signaling between NREM-active neurons and GnRH neurons, both located in the hypothalamus, is consistent with the traditional understanding that the initiation of puberty begins at the level of the hypothalamus (with an increase in GnRH quantity) and precedes any changes in pituitary responsiveness to GnRH (41, 42). Studies in nonhuman primates have indeed demonstrated augmented secretion of nocturnal GnRH from the median eminence during puberty (28, 41), further supporting the role of GnRH in driving sleep-related LH secretion during puberty in children. Although SWS appears to play a major role in sleep-related pulsatile GnRH secretion in puberty, the occurrence of individual LH pulses in the absence of SWS (particularly during stage N2) in the current study suggests that SWS may act as an amplifier of GnRH neuronal activity.
The transition from puberty to mature reproductive function is marked by a loss of the diurnal pattern of LH secretion (44, 45). Sleep-specific augmentation of LH secretion reappears in adulthood during recovery from the inhibition of pulsatile GnRH secretion seen in anorexia nervosa (46), hypothalamic amenorrhea (47), and during the postpartum period (48), suggesting that sleep plays a critical role in the reactivation of reproductive function in a variety of physiological and pathological settings. Intriguingly, deep sleep inhibits pulsatile LH secretion in women in the early follicular phase of established menstrual cycles, whereas LH pulses occur in association with brief episodes of wakefulness (16). Taken together, these observations imply that sleep-related signals play a significant role in the maintenance of reproductive axis integrity not only during puberty but also during the entire reproductive lifespan in women.
Although several recent studies without PSG monitoring demonstrated an attenuation of the nocturnal rise in mean LH and decreased LH pulse amplitude during sleep in obese compared with normal-weight girls (49, 50), the current studies did not demonstrate an effect of obesity on nocturnal LH secretion during puberty. The five overweight or obese subjects in the current study had robust LH secretion during sleep and demonstrated the same pattern of LH pulse onset in association with SWS as the lean subjects. The complete absence of LH pulses during episodes of wakefulness after sleep onset in the current studies suggests that the obese girls in other studies may have had fewer pulses than the lean girls because they spent more time awake during the night. Frequent sampling studies with simultaneous PSG monitoring in obese and lean pubertal girls will be necessary to determine the effect of obesity on LH independent of differences in sleep architecture. Additional studies designed to investigate the connection between SWS and LH secretion from early puberty to late puberty and that address potential differences between boys and girls would also be of interest.
Given the barriers to conducting research in healthy pediatric subjects (51), it was only feasible to study children presumed to have OSA. However, the current findings are relevant to the connection between sleep and reproductive physiology in healthy pubertal children because all subjects included in this report demonstrated normal or near-normal sleep characteristics, including sleep macroarchitecture, oxygen saturation, AIs, and AHIs when studied off CPAP. In addition, the relationship of LH secretion to sleep stage was not different in studies on CPAP compared with off CPAP in these subjects. The normal PSG results in the subjects with a history of OSA suggest that either their OSA had resolved since the time of their original diagnosis or that the absence of CPAP during a single study night was insufficient to reproduce OSA. Indeed, resolution of both airway edema and muscle fatigue have been reported with long-term CPAP treatment in adults (52, 53), although this has not yet been investigated in children.
Cross-correlation has been widely used in the sleep and circadian literature (33, 54). However, we chose not to use this method of analysis due to a number of concerns. As noted by Gronfier and Brandenberger (55), large but infrequent synchronous peaks between two signals (e.g. SWS and LH) have the potential to inflate the cross-correlation coefficient despite the presence of smaller, but more frequent asynchronous peaks. This is particularly problematic when the number of events is small, as in the current study. Our analysis, by contrast, is strictly based on temporal coincidences and is not influenced by LH pulse amplitude. In addition to the LH amplitude effects, cross-correlation would have required deconvolution of the LH data and either interpolation of LH data or filtering of PSG data to permit data strings at common time points. These analyses have the potential of introducing significant error associated with the selection of clearance kinetics and basal secretory rates, in addition to the choice of interpolation and filtering algorithms. Finally, raw cross-correlations are strongly affected by the intrinsic autocorrelation of the underlying pair of signals. Although statistical procedures have been developed to control for autocorrelation, these approaches are inadvisable when analyzing the more complex sleep and hormonal data obtained in the current studies.
Thus, we have shown that during the pubertal window when the reproductive axis is more active during sleep, there is a close association between SWS and LH pulse initiation. Our results provide evidence that SWS represents a critical component in the hypothalamic signal cascade that reactivates the GnRH pulse generator at the time of puberty and raise new questions about the anatomic and physiological basis underlying the connection between the sleep and GnRH neuronal systems. These studies also raise concerns that the disordered or restricted sleep that is increasingly prevalent in the adolescent population may compromise normal pubertal development.
Acknowledgments
We thank Karen Gannon and the Clinical Research Center staff for their support in conducting these studies and Doug Hayden, Ph.D., for statistical support.
N.D.S. received fellowship support from the National Institutes of Health (5T32 HD062315), the Charles A. King Trust, Genentech, the Pediatric Endocrine Society, the American Medical Association, the Thrasher Research Fund, the Endocrine Fellows Foundation, and the Scholars in Clinical Science Program of Harvard Catalyst (The Harvard Clinical and Translational Science Center Award UL1 RR 025758), and financial contributions from Harvard University and its affiliated academic healthcare centers. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard Catalyst, Harvard University and its affiliated academic healthcare centers, the National Center for Research Resources, or the National Institutes of Health.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- AHI
- Apnea-hypopnea index
- AI
- arousal index
- CPAP
- continuous positive airway pressure
- NREM
- nonrapid eye movement
- OSA
- obstructive sleep apnea
- PSG
- polysomnography
- REM
- rapid eye movement
- SWS
- slow-wave sleep.
References
- 1. Boyar R, Finkelstein J, Roffwarg H, Kapen S, Weitzman E, Hellman L. 1972. Synchronization of augmented luteinizing hormone secretion with sleep during puberty. N Engl J Med 287:582–586 [DOI] [PubMed] [Google Scholar]
- 2. Kapen S, Boyar RM, Finkelstein JW, Hellman L, Weitzman ED. 1974. Effect of sleep-wake cycle reversal on luteinizing hormone secretory pattern in puberty. J Clin Endocrinol Metab 39:293–299 [DOI] [PubMed] [Google Scholar]
- 3. Apter D, Bützow TL, Laughlin GA, Yen SS. 1993. Gonadotropin-releasing hormone pulse generator activity during pubertal transition in girls: pulsatile and diurnal patterns of circulating gonadotropins. J Clin Endocrinol Metab 76:940–949 [DOI] [PubMed] [Google Scholar]
- 4. Dunkel L, Alfthan H, Stenman UH, Selstam G, Rosberg S, Albertsson-Wikland K. 1992. Developmental changes in 24-hour profiles of luteinizing hormone and follicle-stimulating hormone from prepuberty to midstages of puberty in boys. J Clin Endocrinol Metab 74:890–897 [DOI] [PubMed] [Google Scholar]
- 5. Landy H, Boepple PA, Mansfield MJ, Charpie P, Schoenfeld DI, Link K, Romero G, Crawford JD, Crigler JF, Jr, Blizzard RM. 1990. Sleep modulation of neuroendocrine function: developmental changes in gonadotropin-releasing hormone secretion during sexual maturation. Pediatr Res 28:213–217 [DOI] [PubMed] [Google Scholar]
- 6. Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. 1999. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, and testosterone secretion before the onset of male puberty. J Clin Endocrinol Metab 84:29–37 [DOI] [PubMed] [Google Scholar]
- 7. Mitamura R, Yano K, Suzuki N, Ito Y, Makita Y, Okuno A. 2000. Diurnal rhythms of luteinizing hormone, follicle-stimulating hormone, testosterone, and estradiol secretion before the onset of female puberty in short children. J Clin Endocrinol Metab 85:1074–1080 [DOI] [PubMed] [Google Scholar]
- 8. Oerter KE, Uriarte MM, Rose SR, Barnes KM, Cutler GB., Jr 1990. Gonadotropin secretory dynamics during puberty in normal girls and boys. J Clin Endocrinol Metab 71:1251–1258 [DOI] [PubMed] [Google Scholar]
- 9. Penny R, Olambiwonnu NO, Frasier SD. 1977. Episodic fluctuations of serum gonadotropins in pre- and post-pubertal girls and boys. J Clin Endocrinol Metab 45:307–311 [DOI] [PubMed] [Google Scholar]
- 10. Wu FC, Butler GE, Kelnar CJ, Huhtaniemi I, Veldhuis JD. 1996. Ontogeny of pulsatile gonadotropin releasing hormone secretion from midchildhood, through puberty, to adulthood in the human male: a study using deconvolution analysis and an ultrasensitive immunofluorometric assay. J Clin Endocrinol Metab 81:1798–1805 [DOI] [PubMed] [Google Scholar]
- 11. Czeisler CA, Klerman EB. 1999. Circadian and sleep-dependent regulation of hormone release in humans. Recent Prog Horm Res 54:97–130; discussion 130–132 [PubMed] [Google Scholar]
- 12. Takahashi Y, Kipnis DM, Daughaday WH. 1968. Growth hormone secretion during sleep. J Clin Invest 47:2079–2090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Underwood LE, Azumi K, Voina SJ, Van Wyk JJ. 1971. Growth hormone levels during sleep in normal and growth hormone deficient children. Pediatrics 48:946–954 [PubMed] [Google Scholar]
- 14. Van Cauter E, Plat L, Copinschi G. 1998. Interrelations between sleep and the somatotropic axis. Sleep 21:553–566 [PubMed] [Google Scholar]
- 15. Schulz H, Brandenberger G, Gudewill S, Hasse D, Kiss E, Löhr K, Pollmächer T, Follenius M. 1992. Plasma renin activity and sleep-wake structure of narcoleptic patients and control subjects under continuous bedrest. Sleep 15:423–429 [DOI] [PubMed] [Google Scholar]
- 16. Hall JE, Sullivan JP, Richardson GS. 2005. Brief wake episodes modulate sleep-inhibited luteinizing hormone secretion in the early follicular phase. J Clin Endocrinol Metab 90:2050–2055 [DOI] [PubMed] [Google Scholar]
- 17. Marshall WA, Tanner JM. 1969. Variations in pattern of pubertal changes in girls. Arch Dis Child 44:291–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Marshall WA, Tanner JM. 1970. Variations in the pattern of pubertal changes in boys. Arch Dis Child 45:13–23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Iber C, Ancoli-Israel S, Chesson A, Quan SF. 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology, and technical specifications. Westchester, IL: American Academy of Sleep Medicine [Google Scholar]
- 20. Marcus CL, Omlin KJ, Basinki DJ, Bailey SL, Rachal AB, Von Pechmann WS, Keens TG, Ward SL. 1992. Normal polysomnographic values for children and adolescents. Am Rev Respir Dis 146:1235–1239 [DOI] [PubMed] [Google Scholar]
- 21. Adams JM, Taylor AE, Schoenfeld DA, Crowley WF, Jr, Hall JE. 1994. The midcycle gonadotropin surge in normal women occurs in the face of an unchanging gonadotropin-releasing hormone pulse frequency. J Clin Endocrinol Metab 79:858–864 [DOI] [PubMed] [Google Scholar]
- 22. Hayes FJ, McNicholl DJ, Schoenfeld D, Marsh EE, Hall JE. 1999. Free α-subunit is superior to luteinizing hormone as a marker of gonadotropin-releasing hormone despite desensitization at fast pulse frequencies. J Clin Endocrinol Metab 84:1028–1036 [DOI] [PubMed] [Google Scholar]
- 23. Santen RJ, Bardin CW. 1973. Episodic luteinizing hormone secretion in man. Pulse analysis, clinical interpretation, physiologic mechanisms. J Clin Invest 52:2617–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Accardo JA, Shults J, Leonard MB, Traylor J, Marcus CL. 2010. Differences in overnight polysomnography scores using the adult and pediatric criteria for respiratory events in adolescents. Sleep 33:1333–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verhulst SL, Schrauwen N, Haentjens D, Van Gaal L, De Backer WA, Desager KN. 2007. Reference values for sleep-related respiratory variables in asymptomatic European children and adolescents. Pediatr Pulmonol 42:159–167 [DOI] [PubMed] [Google Scholar]
- 26. Seminara SB, Crowley WF., Jr 2008. Kisspeptin and GPR54: discovery of a novel pathway in reproduction. J Neuroendocrinol 20:727–731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guerriero KA, Keen KL, Terasawa E. 2012. Developmental increase in kisspeptin-54 release in vivo is independent of the pubertal increase in estradiol in female rhesus monkeys (Macaca mulatta). Endocrinology 153:1887–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keen KL, Wegner FH, Bloom SR, Ghatei MA, Terasawa E. 2008. An increase in kisspeptin-54 release occurs with the pubertal increase in luteinizing hormone-releasing hormone-1 release in the stalk-median eminence of female rhesus monkeys in vivo. Endocrinology 149:4151–4157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kerkhofs M, Van Cauter E, Van Onderbergen A, Caufriez A, Thorner MO, Copinschi G. 1993. Sleep-promoting effects of growth hormone-releasing hormone in normal men. Am J Physiol 264:E594–E598 [DOI] [PubMed] [Google Scholar]
- 30. Obal F, Jr, Alfoldi P, Cady AB, Johannsen L, Sary G, Krueger JM. 1988. Growth hormone-releasing factor enhances sleep in rats and rabbits. Am J Physiol 255:R310–R316 [DOI] [PubMed] [Google Scholar]
- 31. Holl RW, Hartman ML, Veldhuis JD, Taylor WM, Thorner MO. 1991. Thirty-second sampling of plasma growth hormone in man: correlation with sleep stages. J Clin Endocrinol Metab 72:854–861 [DOI] [PubMed] [Google Scholar]
- 32. Ocampo-Lim B, Guo W, DeMott-Friberg R, Barkan AL, Jaffe CA. 1996. Nocturnal growth hormone (GH) secretion is eliminated by infusion of GH-releasing hormone antagonist. J Clin Endocrinol Metab 81:4396–4399 [DOI] [PubMed] [Google Scholar]
- 33. Van Cauter E, Kerkhofs M, Caufriez A, Van Onderbergen A, Thorner MO, Copinschi G. 1992. A quantitative estimation of growth hormone secretion in normal man: reproducibility and relation to sleep and time of day. J Clin Endocrinol Metab 74:1441–1450 [DOI] [PubMed] [Google Scholar]
- 34. Toppila J, Alanko L, Asikainen M, Tobler I, Stenberg D, Porkka-Heiskanen T. 1997. Sleep deprivation increases somatostatin and growth hormone-releasing hormone messenger RNA in the rat hypothalamus. J Sleep Res 6:171–178 [DOI] [PubMed] [Google Scholar]
- 35. Peterfi Z, McGinty D, Sarai E, Szymusiak R. 2010. Growth hormone-releasing hormone activates sleep regulatory neurons of the rat preoptic hypothalamus. Am J Physiol Regul Integr Comp Physiol 298:R147–R156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Sherin JE, Shiromani PJ, McCarley RW, Saper CB. 1996. Activation of ventrolateral preoptic neurons during sleep. Science 271:216–219 [DOI] [PubMed] [Google Scholar]
- 37. Gao B, Moore RY. 1996. The sexually dimorphic nucleus of the hypothalamus contains GABA neurons in rat and man. Brain Res 742:163–171 [DOI] [PubMed] [Google Scholar]
- 38. Bonnefond C, Palacios JM, Probst A, Mengod G. 1990. Distribution of Galanin mRNA Containing Cells and Galanin Receptor Binding Sites in Human and Rat Hypothalamus. Eur J Neurosci 2:629–637 [DOI] [PubMed] [Google Scholar]
- 39. Gai WP, Geffen LB, Blessing WW. 1990. Galanin immunoreactive neurons in the human hypothalamus: colocalization with vasopressin-containing neurons. J Comp Neurol 298:265–280 [DOI] [PubMed] [Google Scholar]
- 40. Nofzinger EA, Buysse DJ, Miewald JM, Meltzer CC, Price JC, Sembrat RC, Ombao H, Reynolds CF, Monk TH, Hall M, Kupfer DJ, Moore RY. 2002. Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 125:1105–1115 [DOI] [PubMed] [Google Scholar]
- 41. Watanabe G, Terasawa E. 1989. In vivo release of luteinizing hormone releasing hormone increases with puberty in the female rhesus monkey. Endocrinology 125:92–99 [DOI] [PubMed] [Google Scholar]
- 42. Roth JC, Kelch RP, Kaplan SL, Grumbach MM. 1972. FSH and LH response to luteinizing hormone-releasing factor in prepubertal and pubertal children, adult males and patients with hypogonadotropic and hypertropic hypogonadism. J Clin Endocrinol Metab 35:926–930 [DOI] [PubMed] [Google Scholar]
- 43. Katz ES, D'Ambrosio CM. 2010. Pediatric obstructive sleep apnea syndrome. Clin Chest Med 31:221–234 [DOI] [PubMed] [Google Scholar]
- 44. Klingman KM, Marsh EE, Klerman EB, Anderson EJ, Hall JE. 2011. Absence of circadian rhythms of gonadotropin secretion in women. J Clin Endocrinol Metab 96:1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Boyar R, Perlow M, Hellman L, Kapen S, Weitzman E. 1972. Twenty-four hour pattern of luteinizing hormone secretion in normal men with sleep stage recording. J Clin Endocrinol Metab 35:73–81 [DOI] [PubMed] [Google Scholar]
- 46. Katz JL, Boyar R, Roffwarg H, Hellman L, Weiner H. 1978. Weight and circadian luteinizing hormone secretory pattern in anorexia nervosa. Psychosom Med 40:549–567 [DOI] [PubMed] [Google Scholar]
- 47. Perkins RB, Hall JE, Martin KA. 1999. Neuroendocrine abnormalities in hypothalamic amenorrhea: spectrum, stability, and response to neurotransmitter modulation. J Clin Endocrinol Metab 84:1905–1911 [DOI] [PubMed] [Google Scholar]
- 48. Liu JH, Park KH. 1988. Gonadotropin and prolactin secretion increases during sleep during the puerperium in nonlactating women. J Clin Endocrinol Metab 66:839–845 [DOI] [PubMed] [Google Scholar]
- 49. Bordini B, Littlejohn E, Rosenfield RL. 2009. Blunted sleep-related luteinizing hormone rise in healthy premenarcheal pubertal girls with elevated body mass index. J Clin Endocrinol Metab 94:1168–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. McCartney CR, Prendergast KA, Blank SK, Helm KD, Chhabra S, Marshall JC. 2009. Maturation of luteinizing hormone (gonadotropin-releasing hormone) secretion across puberty: evidence for altered regulation in obese peripubertal girls. J Clin Endocrinol Metab 94:56–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rosenfield RL. 2008. Improving balance in regulatory oversight of research in children and adolescents: a clinical investigator's perspective. Ann NY Acad Sci 1135:287–295 [DOI] [PubMed] [Google Scholar]
- 52. Carrera M, Barbé F, Sauleda J, Tomás M, Gómez C, Agustí AG. 1999. Patients with obstructive sleep apnea exhibit genioglossus dysfunction that is normalized after treatment with continuous positive airway pressure. Am J Respir Crit Care Med 159:1960–1966 [DOI] [PubMed] [Google Scholar]
- 53. Schotland HM, Insko EK, Schwab RJ. 1999. Quantitative magnetic resonance imaging demonstrates alterations of the lingual musculature in obstructive sleep apnea. Sleep 22:605–613 [DOI] [PubMed] [Google Scholar]
- 54. Simon C, Gronfier C, Schlienger JL, Brandenberger G. 1998. Circadian and ultradian variations of leptin in normal man under continuous enteral nutrition: relationship to sleep and body temperature. J Clin Endocrinol Metab 83:1893–1899 [DOI] [PubMed] [Google Scholar]
- 55. Gronfier C, Brandenberger G. 1998. Ultradian rhythms in pituitary and adrenal hormones: their relations to sleep. Sleep Med Rev 2:17–29 [DOI] [PubMed] [Google Scholar]
- 56. Barlow SE. 2007. Expert committee recommendations regarding the prevention, assessment, and treatment of child and adolescent overweight and obesity: summary report. Pediatrics 120(Suppl 4):S164–S192 [DOI] [PubMed] [Google Scholar]